Abstract
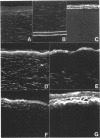
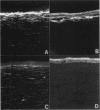
An indirect immunohistochemical technique was used to monitor the expression of cellular fibronectin (cFN) and tenascin (TN) in the rabbit cornea after photorefractive keratectomy (PRK) in a 1 year follow up study. Rabbits received a 5.0 D myopic PRK, and were killed 3 days, 1, 3, 6, or 12 months after the operation. In most corneas, secondary epithelial defects appeared after the primary healing (mean 6.3 (SD 1.2) days). Corneal haze appeared a few weeks after PRK and was observed throughout the follow up. Three days after wounding an immunoreaction for cFN was observed as a bright narrow subepithelial line, but no immunoreaction for TN could be seen in the anterior third of the corneal stroma. However, at 1-6 months a similar location of immunoreactions for both cFN and TN was observed. Both were found in the anterior stroma at depths of 30-50 microns. At 12 months, only a trace of cFN immunoreaction but no TN immunoreaction could be discerned. Our results suggest that subepithelial scar tissue contains both cFN and TN up to 12 months.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azar D. T., Spurr-Michaud S. J., Tisdale A. S., Moore M. B., Gipson I. K. Reassembly of the corneal epithelial adhesion structures following human epikeratoplasty. Arch Ophthalmol. 1991 Sep;109(9):1279–1284. doi: 10.1001/archopht.1991.01080090105032. [DOI] [PubMed] [Google Scholar]
- Campos M., Cuevas K., Shieh E., Garbus J. J., McDonnell P. J. Corneal wound healing after excimer laser ablation in rabbits: expanding versus contracting apertures. Refract Corneal Surg. 1992 Sep-Oct;8(5):378–381. [PubMed] [Google Scholar]
- Chiquet-Ehrismann R., Mackie E. J., Pearson C. A., Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986 Oct 10;47(1):131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Picasso M., Ponassi M., Sun M. Z., Balza E. Tenascin and fibronectin distribution in human normal and pathological synovium. J Rheumatol. 1992 Sep;19(9):1439–1447. [PubMed] [Google Scholar]
- Erickson H. P., Bourdon M. A. Tenascin: an extracellular matrix protein prominent in specialized embryonic tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Fantes F. E., Hanna K. D., Waring G. O., 3rd, Pouliquen Y., Thompson K. P., Savoldelli M. Wound healing after excimer laser keratomileusis (photorefractive keratectomy) in monkeys. Arch Ophthalmol. 1990 May;108(5):665–675. doi: 10.1001/archopht.1990.01070070051034. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons T. D., Fagerholm P., Härfstrand A., Schenholm M. Hyaluronic acid in the rabbit cornea after excimer laser superficial keratectomy. Invest Ophthalmol Vis Sci. 1992 Oct;33(11):3011–3016. [PubMed] [Google Scholar]
- Fujikawa L. S., Foster C. S., Gipson I. K., Colvin R. B. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J Cell Biol. 1984 Jan;98(1):128–138. doi: 10.1083/jcb.98.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartry D. S., Kerr Muir M. G., Marshall J. Excimer laser photorefractive keratectomy. 18-month follow-up. Ophthalmology. 1992 Aug;99(8):1209–1219. doi: 10.1016/s0161-6420(92)31821-4. [DOI] [PubMed] [Google Scholar]
- Gaster R. N., Binder P. S., Coalwell K., Berns M., McCord R. C., Burstein N. L. Corneal surface ablation by 193 nm excimer laser and wound healing in rabbits. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):90–98. [PubMed] [Google Scholar]
- Gipson I. K., Spurr-Michaud S., Tisdale A., Keough M. Reassembly of the anchoring structures of the corneal epithelium during wound repair in the rabbit. Invest Ophthalmol Vis Sci. 1989 Mar;30(3):425–434. [PubMed] [Google Scholar]
- Hanna K. D., Pouliquen Y., Waring G. O., 3rd, Savoldelli M., Cotter J., Morton K., Menasche M. Corneal stromal wound healing in rabbits after 193-nm excimer laser surface ablation. Arch Ophthalmol. 1989 Jun;107(6):895–901. doi: 10.1001/archopht.1989.01070010917041. [DOI] [PubMed] [Google Scholar]
- Howeedy A. A., Virtanen I., Laitinen L., Gould N. S., Koukoulis G. K., Gould V. E. Differential distribution of tenascin in the normal, hyperplastic, and neoplastic breast. Lab Invest. 1990 Dec;63(6):798–806. [PubMed] [Google Scholar]
- Jones F. S., Burgoon M. P., Hoffman S., Crossin K. L., Cunningham B. A., Edelman G. M. A cDNA clone for cytotactin contains sequences similar to epidermal growth factor-like repeats and segments of fibronectin and fibrinogen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2186–2190. doi: 10.1073/pnas.85.7.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Marshall J., Trokel S. L., Rothery S., Krueger R. R. Long-term healing of the central cornea after photorefractive keratectomy using an excimer laser. Ophthalmology. 1988 Oct;95(10):1411–1421. doi: 10.1016/s0161-6420(88)32997-0. [DOI] [PubMed] [Google Scholar]
- McCachren S. S., Lightner V. A. Expression of human tenascin in synovitis and its regulation by interleukin-1. Arthritis Rheum. 1992 Oct;35(10):1185–1196. doi: 10.1002/art.1780351011. [DOI] [PubMed] [Google Scholar]
- Pearson C. A., Pearson D., Shibahara S., Hofsteenge J., Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. EMBO J. 1988 Oct;7(10):2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroutsos G., Guimaraes R., Giraud J., Pouliquen Y. Antibiotics and corneal epithelial wound healing. Arch Ophthalmol. 1983 Nov;101(11):1775–1778. doi: 10.1001/archopht.1983.01040020777023. [DOI] [PubMed] [Google Scholar]
- Pällysaho T., Tervo K., Kivelä T., Virtanen I., Tarkkanen A., Tervo T. Cellular fibronectin and tenascin in an orbital nylon prosthesis removed because of infection caused by Staphylococcus aureus. Graefes Arch Clin Exp Ophthalmol. 1993 Feb;231(2):61–65. doi: 10.1007/BF00920213. [DOI] [PubMed] [Google Scholar]
- Rawe I. M., Zabel R. W., Tuft S. J., Chen V., Meek K. M. A morphological study of rabbit corneas after laser keratectomy. Eye (Lond) 1992;6(Pt 6):637–642. doi: 10.1038/eye.1992.137. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Salonen E. M., Tervo T., Törmä E., Tarkkanen A., Vaheri A. Plasmin in tear fluid of patients with corneal ulcers: basis for new therapy. Acta Ophthalmol (Copenh) 1987 Feb;65(1):3–12. doi: 10.1111/j.1755-3768.1987.tb08482.x. [DOI] [PubMed] [Google Scholar]
- Schimmelpfennig B., Beuerman R. A technique for controlled sensory denervation of the rabbit cornea. Graefes Arch Clin Exp Ophthalmol. 1982;218(6):287–293. doi: 10.1007/BF02150440. [DOI] [PubMed] [Google Scholar]
- Shah M., Foreman D. M., Ferguson M. W. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992 Jan 25;339(8787):213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- SundarRaj N., Geiss M. J., 3rd, Fantes F., Hanna K., Anderson S. C., Thompson K. P., Thoft R. A., Waring G. O., 3rd Healing of excimer laser ablated monkey corneas. An immunohistochemical evaluation. Arch Ophthalmol. 1990 Nov;108(11):1604–1610. doi: 10.1001/archopht.1990.01070130106039. [DOI] [PubMed] [Google Scholar]
- Talamo J. H., Gollamudi S., Green W. R., De La Cruz Z., Filatov V., Stark W. J. Modulation of corneal wound healing after excimer laser keratomileusis using topical mitomycin C and steroids. Arch Ophthalmol. 1991 Aug;109(8):1141–1146. doi: 10.1001/archopht.1991.01080080101040. [DOI] [PubMed] [Google Scholar]
- Tengroth B., Epstein D., Fagerholm P., Hamberg-Nyström H., Fitzsimmons T. D. Excimer laser photorefractive keratectomy for myopia. Clinical results in sighted eyes. Ophthalmology. 1993 May;100(5):739–745. doi: 10.1016/s0161-6420(93)31581-2. [DOI] [PubMed] [Google Scholar]
- Tervo K., Tervo T., van Setten G. B., Tarkkanen A., Virtanen I. Demonstration of tenascin-like immunoreactivity in rabbit corneal wounds. Acta Ophthalmol (Copenh) 1989 Jun;67(3):347–350. doi: 10.1111/j.1755-3768.1989.tb01886.x. [DOI] [PubMed] [Google Scholar]
- Tervo K., van Setten G. B., Beuerman R. W., Virtanen I., Tarkkanen A., Tervo T. Expression of tenascin and cellular fibronectin in the rabbit cornea after anterior keratectomy. Immunohistochemical study of wound healing dynamics. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):2912–2918. [PubMed] [Google Scholar]
- Tervo T., Mustonen R., Tarkkanen A. Management of dry eye may reduce haze after excimer laser photorefractive keratectomy. Refract Corneal Surg. 1993 Jul-Aug;9(4):306–306. [PubMed] [Google Scholar]
- Tervo T., Sulonen J., Valtones S., Vannas A., Virtanen I. Distribution of fibronectin in human and rabbit corneas. Exp Eye Res. 1986 Apr;42(4):399–406. doi: 10.1016/0014-4835(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Tervo T., van Setten G. B., Pällysaho T., Tarkkanen A., Tervo K. Wound healing of the ocular surface. Ann Med. 1992 Feb;24(1):19–27. doi: 10.3109/07853899209164141. [DOI] [PubMed] [Google Scholar]
- Trokel S. L., Srinivasan R., Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983 Dec;96(6):710–715. doi: 10.1016/s0002-9394(14)71911-7. [DOI] [PubMed] [Google Scholar]
- Tucker R. P., Hammarback J. A., Jenrath D. A., Mackie E. J., Xu Y. Tenascin expression in the mouse: in situ localization and induction in vitro by bFGF. J Cell Sci. 1993 Jan;104(Pt 1):69–76. doi: 10.1242/jcs.104.1.69. [DOI] [PubMed] [Google Scholar]
- Tuft S. J., Gartry D. S., Rawe I. M., Meek K. M. Photorefractive keratectomy: implications of corneal wound healing. Br J Ophthalmol. 1993 Apr;77(4):243–247. doi: 10.1136/bjo.77.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Laitinen L., Närvänen O., Cutolo M., Thornell L. E., Zardi L., Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci. 1987 Nov;88(Pt 4):419–430. doi: 10.1242/jcs.88.4.419. [DOI] [PubMed] [Google Scholar]
- Weinstock S. J., Machat J. J. Excimer laser keratectomy for the correction of myopia. CLAO J. 1993 Apr;19(2):133–136. [PubMed] [Google Scholar]
- Wilson S. E., Lloyd S. A., He Y. G. EGF, basic FGF, and TGF beta-1 messenger RNA production in rabbit corneal epithelial cells. Invest Ophthalmol Vis Sci. 1992 May;33(6):1987–1995. [PubMed] [Google Scholar]
- Wu W. C., Stark W. J., Green W. R. Corneal wound healing after 193-nm excimer laser keratectomy. Arch Ophthalmol. 1991 Oct;109(10):1426–1432. doi: 10.1001/archopht.1991.01080100106053. [DOI] [PubMed] [Google Scholar]
- van Setten G. B., Koch J. W., Tervo K., Lang G. K., Tervo T., Naumann G. O., Kolkmeier J., Virtanen I., Tarkkanen A. Expression of tenascin and fibronectin in the rabbit cornea after excimer laser surgery. Graefes Arch Clin Exp Ophthalmol. 1992;230(2):178–183. doi: 10.1007/BF00164660. [DOI] [PubMed] [Google Scholar]
- van Setten G. B., Viinikka L., Tervo T., Pesonen K., Tarkkanen A., Perheentupa J. Epidermal growth factor is a constant component of normal human tear fluid. Graefes Arch Clin Exp Ophthalmol. 1989;227(2):184–187. doi: 10.1007/BF02169794. [DOI] [PubMed] [Google Scholar]