We read the recent findings by Winston et al. [1] with great interest. The quantification of extracellular vesicles (including exosomes) has presented several challenges despite the availability of a number biophysical techniques, including nanoparticle tracking analysis (NanoSight, Ltd.) and tunable resistive pulse sensing (Izon Science, Ltd.) [2]. These challenges are mainly due to the small size and polydispersity of exosomes and the lack of a standardized methodology for measuring exosomes which allows results from different days, users, and instruments to be compared [3].
We recently attempted to characterize exosomes in a small group of Parkinson's disease (PD) patients (detailed methodology is described elsewhere [2], [3]). The patients enrolled in our study were administered the Montreal Cognitive Assessment (MoCA) before obtaining the biofluid samples. The MoCA has recently been validated by our group as superior to the mini mental state examination in assessing cognitive impairment in PD patients [4]. Suggested cutoff scores were PD-normal cognition (PD-N) ≥ 26, PD-mild cognitive impairment (PD-MCI) = 21–25, and PD-dementia (PD-D) ≤ 20.
We recruited 11 PD patients, regardless of cognitive function. The patients, all of whom were male, had a mean age of 66 ± 11.5 years, mean disease duration of 70 ± 30 months, and mean MoCA scores of 25.6 ± 2.6 points. Paired plasma samples, 28 days apart, were obtained from all patients except two (total samples = 20).
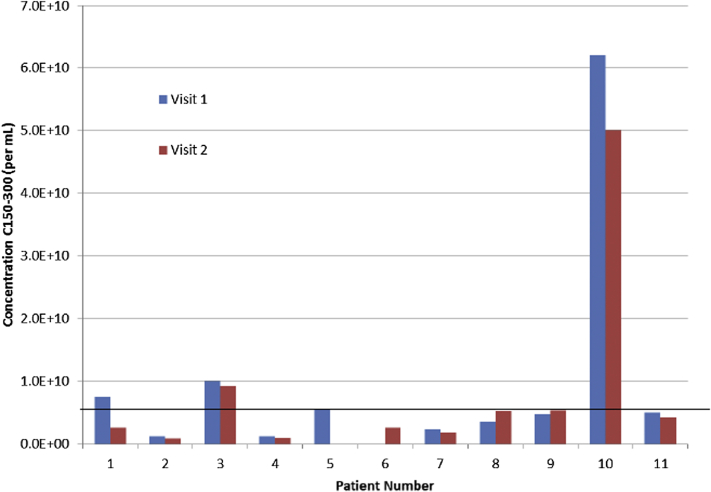
Analysis of exosome concentration in plasma revealed an unexpected pattern. Although a wide variation among our patients (mean, 19.4 ± 21.7 × 109 exosomes/mL; size range, 150–300 nm) was observed, three patients in particular (see Fig. 1) exhibited plasma exosome concentrations that were 10- to 20-fold higher than the rest of the sample (mean, 43.5 vs. 7.4 × 109 exosomes/mL, respectively). This discrepancy was repeatable in plasma samples collected 28 days later (mean, 44.5 vs. 5.4 × 109 exosomes/mL). Incidentally, these three patients had lower MoCA scores compared with the other patients (mean, 22.7 vs. 26.9, respectively).
Fig. 1.
The relative concentrations of exosomes detected in 11 participants are shown, with samples from participants 1, 3, and 10 exhibiting the highest concentrations.
The correlation we describe here is intriguing but insufficiently powered to draw definitive answers. In a recent study [5], 37 PD patients were found to have significantly higher concentrations of cerebrospinal fluid (CSF) exosomes compared with patients with dementia with Lewy bodies, progressive supranuclear palsy, and polyneuropathy. The study, however, did not stratify PD patients by cognitive function, and only CSF samples were analyzed. Cognitive correlates using plasma, much more easily accessible than CSF, may be more appealing in clinical applications.
Ridder et al [6] have recently reported increased transfer of genetic material, via exosomes, between murine hematopoietic cells and Purkinje neurons in states of neuroinflammation. This could afford a potential explanation for the observed higher exosome concentration in PD-MCI, reflecting a more severe and/or underlying neuroinflammatory process compared with PD-N. To provide more definitive conclusions, however, larger studies that examine CSF and plasma concentrations of exosomes in PD-N, PD-MCI, and PD-D patients are much needed.
Acknowledgments
The exosome analysis was conducted by Izon Science, Christchurch, New Zealand.
References
- 1.Winston C.N., Goetzl E.J., Akers J.C., Carter B.S., Rockenstein E.M., Galasko D. Prediction of conversion from mild cognitive impairment to dementia with neuronally derived blood exosome protein profile. Alzheimers Dement (Amst) 2016;3:63–72. doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel R., Willmott G., Kozak D., Roberts G.S., Anderson W., Groenewegen L. Quantitative Sizing of Nano/Microparticles with a Tunable Elastomeric Pore Sensor. Anal Chem. 2011;83:3499–3506. doi: 10.1021/ac200195n. [DOI] [PubMed] [Google Scholar]
- 3.Roberts G.S., Yu S., Zeng Q., Chan L.C., Anderson W., Colby A.H. Tunable pores for measuring concentrations of synthetic and biological nanoparticle dispersions. Biosens Bioelectron. 2012;31:17–25. doi: 10.1016/j.bios.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Dalrymple-Alford J.C., MacAskill M.R., Nakas C.T., Livingston L., Graham C., Crucian G.P. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 5.Stuendl A., Kunadt M., Kruse N., Bartels C., Moebius W., Danzer K.M. Induction of alpha-synuclein aggregate formation by CSF exosomes from patients with Parkinson's disease and dementia with Lewy bodies. Brain. 2016;139(Pt 2):481–494. doi: 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridder K., Keller S., Dams M., Rupp A.K., Schlaudraff J., Del Turco D. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12:e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]