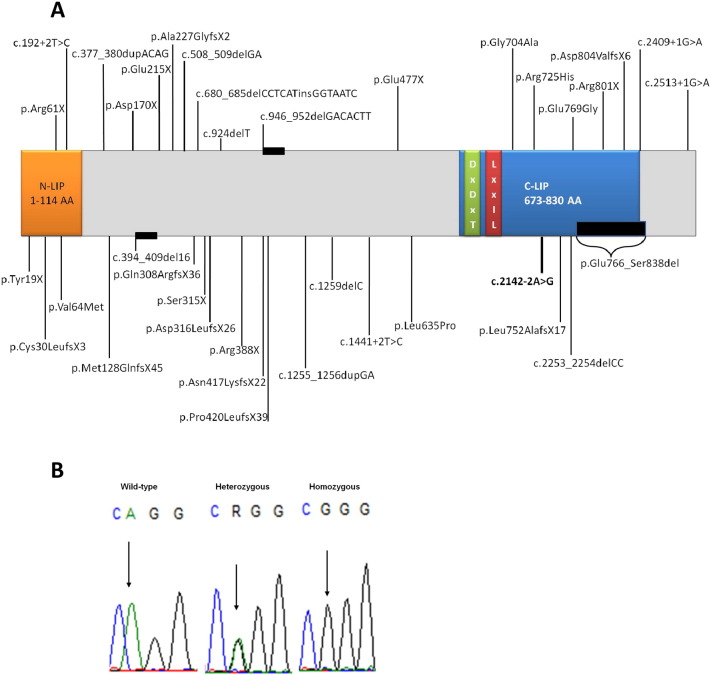
Rhabdomyolysis (RM) is characterized by acute and often severe skeletal muscle damage resulting in myoglobinuria and, in severe cases, acute renal failure [1]. In adults is typically due to trauma, intoxication or infection, whereas in children is frequently associated with inherited muscle disorders [2]. LPIN1 mutations were identified as a cause of severe recurrent RM, which usually begin in childhood, and infections are the most frequent trigger [[3], [4]]. LPIN1 spans 19 exons and encodes lipin-1, an 890 amino acid protein predominantly expressed in skeletal muscle and adipose tissue, which accounts for phosphatidic acid phosphatase activity [[2], [5]]. To date, 36 LPIN1 mutations have been described related with RM (Fig. 1a).
Fig. 1.
— A) Schematic representation of described mutations in LPIN1 gene. The new splicing mutation found in this study is shown in bold. B) LPIN1 splicing mutation (c.2142-2A > G) in heterozygous and homozygous state, compared to the wild-type sequence.
We report a 35-year-old female patient presenting myalgia, muscle weakness, general fatigue and sleep apnea. Her first child born from an apparently non-consanguineous marriage (father already dead), presented normal growth and psychomotor development until the age of 2 years, when developed recurrent RM events precipitated by infections, without symptoms and normal plasma creatine kinase between episodes. The child died at 4-year-old due to a crisis of RM during gastroenteritis. A novel LPIN1 splicing mutation (c.2142-2 A > G) was identified in heterozygous state, in the index case, however, her child was homozygous (Fig. 1b). This novel mutation is probably pathogenic, predicted by bioinformatics tools, due to the break of acceptor site which affect the splicing mechanisms [[6], [7]].
LPIN1 mutations appear as the second most common cause of early-onset RM, after primary fatty acid oxidation defects as a whole [8]. Heterozygous LPIN1 mutations may also produce symptoms of cramps and exercise-induced myalgia or mild muscular symptoms [2], as occurred in our family.
This study allowed the identification of the first LPIN1 mutation in Portuguese patients and corroborates the importance of a molecular testing to confirm LPIN1 patients (children and adults) with recurrent RM.
References
- 1.Scalco R.S., Gardine A.R., Pitceathly R.D. Rhabdomyolysis: a genetic perspective. Orphanet J. Rare Dis. 2015;10:51. doi: 10.1186/s13023-015-0264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meijer I.A., Sasarman F., Maftei C. LPIN1 deficiency with severe recurrent rhabdomyolysis and persistent elevation of creatine kinase levels due to chromosome 2 maternal isodisomy. Mol. Genet. Metab. Rep. 2015;5:85–88. doi: 10.1016/j.ymgmr.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michot C., Hubert L., Romero N.B. Study of LPIN1, LPIN2 and LPIN3 in rhabdomyolysis and exercise-induced myalgia. J. Inherit. Metab. Dis. 2012;38:621–628. doi: 10.1007/s10545-012-9461-6. [DOI] [PubMed] [Google Scholar]
- 4.Pichler K., Scholl-Buergi S., Birnbacher R. A novel therapeutic aproach for LPIN1 mutation -associated rhabdomyolysis – the Austrian experience. Muscle Nerve. 2015;52:437–439. doi: 10.1002/mus.24749. [DOI] [PubMed] [Google Scholar]
- 5.Jaradat S.A., Amayreh W., Al-Qa'qa' K. Molecular analysis of LPIN1 in Jordanian patients with rhabdomyolysis. Meta Gene. 2016;7:90–94. doi: 10.1016/j.mgene.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.http://www.umd.be/HSF3/
- 7.http://www.mutationtaster.org
- 8.Michot C., Hubert L., Brivet M. LPIN1 gene mutations: a major cause of severe rhabdomyolysis in early childhood. Hum. Mutat. 2010;31:1564–1573. doi: 10.1002/humu.21282. [DOI] [PubMed] [Google Scholar]