Abstract
Background
Vitamin E (VE, α-tocopherol) is a fat-soluble vitamin and is well known as an antioxidant. A deficiency in VE induces oxidative stress in the brain and causes motor and memory dysfunction. The consumption of a VE-rich diet has been given much attention in recent years, in regards to anti-aging and the prevention of age-related neuronal disorders.
Methods
A VE-deficient mouse model was prepared by feeding the animals a diet lacking VE. In addition, to evaluate the effect of VE-containing rice bran (RB) on VE deficiency, a diet including RB was also provided. VE levels in the brain tissue, as well as in the RB, were measured using an HPLC system. Behavioral tests, including rotarod, wheel running activity, Y-maze, and elevated plus maze were performed. To clarify the effect of VE deficiency and RB, we investigated the induction of heme oxygenase-1 (HO-1). Histological studies were performed using HE staining and immunohistochemical studies were performed using antibodies against glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1).
Results
VE in the mouse brain under a VE-deficient diet was decreased, and recovered α-tocopherol levels were observed in the brain of mice fed an RB diet. Motor behavioral scores were decreased in VE-deficient conditions, while the supplementation of RB improved motor function. HO-1, a marker of oxidative stress, was upregulated in the mouse brain under VE deficiency, however, RB supplementation inhibited the increase of HO-1. Histological analyses showed neuronal degeneration of Purkinje cells and decreased GFAP-immunoreactivity of Bergmann glia in the cerebellum. In addition, activated astrocytes and microglia were observed in mice fed the VE-deficient diet. Mice fed the RB diet showed improvement in these histological abnormalities.
Conclusion
A VE-deficient diet induced motor dysfunction in mice due to the degeneration of Purkinje cells in the cerebellum. Oral supplementation of RB increases VE in the brain and improved the motor dysfunction caused by VE deficiency. Thus, RB or unpolished rice may be a promising VE supplement.
Keywords: antioxidant, Bergmann glia, oxidative stress, rice bran, vitamin E
Vitamin E (VE) is a fat-soluble vitamin and is well known as a cell membrane antioxidant that protects cells and organs against oxidative stress. α-Tocopherol (αTOC) is the most studied form of VE, however, there are 8 identified vitamin E derivatives: α-, β-, γ- and δ-tocopherol (αTOC, βTOC, γTOC and δTOC, respectively) and α-, β-, γ- and δ-tocotrienol (αT3, βT3, γT3 and δT3, respectively).1, 2 These classes, TOCs and T3s, can be differentiated by an unsaturated side chain with three double bonds in the farnesyl isoprenoid tail of TOCs and an isoprenoid tail with 3 double bonds in T3s.1, 2 Furthermore, differences between α-, β-, γ- and δ- are characterized by the number and location of methyl groups on the chromanol ring. Numerous reports have suggested that all types of VE scavenge reactive oxygen species and protect cells and organs against oxidative stress.3–5 Furthermore, it has been reported that VE derivatives also stimulate intracellular signal transduction and protect cells in antioxidant independent manner.2, 6
Epidemiological and clinical studies suggest that VE is important for the prevention of oxidative stress-related neurodegenerative disorders such as Parkinson’s disease,7–9 Alzheimer’s disease10 and amyotrophic lateral sclerosis.11 Moreover, it has been reported that αTOC deficiency induced through the lack of αTOC transfer protein (αTTP) induces cerebellar ataxia in humans and mice.12-14 These findings suggest that VE is essential for the maintenance of brain integrity.
αTTP is an abundant cytosolic liver protein with high binding affinity to αTOC, but not to other VE derivatives. αTTP can transport αTOC between separate membranes.15 αTTP is present in the brain and is concentrated in Purkinje cell layers of the cerebellum.16 Moreover, a detail examination of the rat brain revealed that αTTP is expressed in Bergmann glia located between Purkinje cells, which are important in maintaining the functional stability of cerebellar Purkinje cells.16 These facts suggest that the cerebellum is more vulnerable to αTOC deficiency compared with other brain regions.
Rice brain (RB), a byproduct of the rice milling process, is known to be a rich source of antioxidants including VE. Although the concentrations of TOCs and T3s in RB vary according to the rice species and milling techniques, RBs have abundant TOCs and T3s.17, 18 It has been reported that dietary RB is useful for intestinal regulation, decreasing low density lipoprotein, and lowering blood pressure.19–21 In the present study, we investigated the effects of dietary RB on neuronal abnormalities such as cerebellar ataxia induced by VE deficiency.
MATERIALS AND METHODS
Animals
C57BL/6 mice were obtained from CLEA Japan (Tokyo, Japan). The mice were housed under standardized conditions of light (06:00–18:00), temperature (25 °C), and humidity (approximately 50%), and were allowed free access to food and water. The mice used in this study were treated in accordance with the Guideline for Animal Experimentation of Tottori University (No. 14-Y-03).
VE-deficient feed (VEdef) (Funabashi Farm, Chiba, Japan), normal feed and RB were prepared. In order to investigate an effect of RB, mice were fed 5 types of feed. The diets were as follows: i) VEdef alone (n = 4); ii) a mixture of VEdef and RB at a concentration 2% RB (n = 4); iii) a mixture of VEdef and RB at a concentration 5% RB (n = 4); iv) normal diet alone (n = 6) and v) a mixture of normal diet and RB at a concentration 5% RB (n = 6). Hereafter, these diets are referred to as VEdef, 2%RB, 5%RB, NR and NR5%RB, respectively.
Mice were supplied with the above-mentioned diets from 8 to 20 weeks of age. Behavior analyses were performed at 0, 0.5, 1, 2, 3 months, and, after 3 months, the brain was sampled.
Behavioral test
Rotarod test
The motor coordination of mice was investigated using an accelerating rotarod apparatus (MK-630B treadmill; Muromachi, Tokyo, Japan). The mice were placed on the rod for four successive trials. The two highest scores of the four trials were used to calculate an average score, which indicates the latency to fall. During each trial, the rotating rod accelerates gradually from 0 to 40 rpm over a period of 5 min, and, thereafter, is maintained at the 40 rpm speed.
Wheel running activity (WRA)
WRA was investigated using the MK-750PC (Muromachi). Locomotor and running activity was converted to revolutions per day. The mice were allowed free exercise, rest, food and water for 24 hours.
Y-maze
The Y-maze apparatus was composed of three arms with walls of 10 cm wide, 13 cm high, and 35 cm long, allowing the mice to see distal spatial features. The insides of the arms were identical to each other, and provided no interior cues.
Continuous spontaneous alternation testing was performed by placing the mice in the Y-maze for 8 min with all three arms. The number and sequence of arms entered were recorded manually.
One alternation was counted when mice visited three different arms consecutively. Immediate reentries were discounted. The percentage of alternation was indicated as spatial working memory. The spontaneous alternative rate (%) was calculated as the number of alternations/(the number of total arm entries –2) × 100.
Elevated plus maze test
The elevated plus maze consisted of two arms without walls (6 cm wide × 30 cm length) and two enclosed by walls (6 cm wide × 30 cm length × 20 cm height), with a center area of 6 × 6 cm. This apparatus was elevated to height of 50 cm from the ground. The mice were placed at the center area, and allowed to explore the maze for 5 min. The time spent in the open arms and the number of open arm entries were counted and estimated as parameter of anti-anxiolytic behavior. All measuring were recorded manually.
Quantification of vitamin E
Mice were decapitated under deep anesthesia (pentobarbital, 50 mg/kg, i.p.) and brain tissues were collected. A portion of the brain tissue (approximately 200 mg) was crushed by a polytron homogenizer in 1.5 mL phosphate buffered saline (PBS). To prevent oxidation of the samples, this procedure was performed on ice and under a nitrogen gas stream.
The homogenates were used for the measurement of VE and quantification of protein concentration. Mixture of the brain homogenate and 2.0 mL ethanol was transferred to a glass tube with a screw cap, then, after added 5.0 mL n-hexane, it was shaken vigorously for 20 min.
The tube was centrifuged at 750 × g for 5 min, and the hexane layer was collected in another glass tube. After 5.0 mL of n-hexane was added again, re-extraction (shaking, centrifuging and collection of hexane layer) was performed. The collected hexane layer was evaporated under nitrogen gas stream. The fat-soluble extract that remained was dissolved in 0.5 mL ethanol, passed through a 0.22 μm filter to remove impurities, and collected in a storage glass tube.
Similarly, 1.0 g of each feed (5 types of feed and the RB used for the experiment) was treated with the same procedures as extraction of the brain.
αTOC, γTOC, δTOC, αT3, γT3 and δT3 in the fat-soluble fraction extracted from brain or feed were quantified by a high performance liquid chromatography (HPLC) system. VE levels in the brain were measured using an HPLC system equipped with an absorbance detector (295 nm) and a phenylhexyl column (COSMOSIL πNAP; Nacalai Tesque, Kyoto, Japan). Methanol 90%/H2O 10% delivered at a flow rate of 1 mL/min was used as eluent. αT3, γT3 and δT3 purified from palm for standards in HPLC were donated by Eisai Food & Chemical (Tokyo, Japan). αTOC, γTOC and δTOC were obtained from Sigma (St. Louis, MO).
Immunoblot
Brain tissues were lysed in SDS sample buffer (50 mM Tris–HCl, pH 6.8, 2% SDS, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, and 2 mM ethylenediaminetetraacetic acid). Aliquots (20 μg) were separated on the basis of molecular size on 10% polyacrylamide gels, transferred onto polyvinylidene difluoride membranes (Hybond-P; GE Healthcare, Buckinghamshire, UK), and hybridized with HO-1 or β-actin antibody in PBS with 0.1%Tween 20 at room temperature for 1 h. Primary antibodies against hemeoxigenase-1 (HO-1) and β-actin were obtained from Santa Cruz Biotechnology (Dallas, TX) and Cell Signaling Technology (Danvers, MA), respectively. The immunoreactive signal was detected using horseradish peroxidase-linked anti-rabbit or mouse IgG and ECL detection reagents (GE Healthcare). The protein content of each sample was measured using the bicinchoninic acid (BCA) protein assay system (Thermo Fisher Scientific Inc, Rockford, IL). The density of immunoblot signal (expressed as the ratio of HO-1/β-actin) was semi-quantified using Image J software. Semi-quantitative data were assessed by ANOVA using the Stat View software, and the criterion for statistical significance was P < 0.05.
Histological study
Immunohistochemistry was performed on paraffin-embedded sections of mouse brain tissues. A portion of brain tissue was fixed by immersion in 4% paraformaldehyde (Wako, Osaka, Japan), and embedded in paraffin. Paraffin sections (4 μm) were deparaffinized by placing slides into three changes of xylene, followed by rehydration in a graded ethanol series. Sections were treated with methanol with 3% H2O2 at room temperature for 30 min to reduce endogenous peroxidase activity.
For glial fibrillary acidic protein (GFAP) and ionized calcium binding adaptor molecule 1 (Iba1) staining, the sections were rinsed in water and subjected to antigen retrieval in 10 mM citrate (pH 6.0) at 121 °C for 10 min in an autoclave. GFAP was detected by incubating the sections in GFAP antibody at a 1:1000 dilution in PBS at 4 °C overnight. Iba1 antibody was used at a 1:1000 dilution. The labeled antigens were visualized using the Histo Fine kit (Nichirei, Tokyo, Japan) for GFAP and Iba1, followed by 3,3-diamiobenzidine (DAB) reaction. Finally, the sections were counterstained with hematoxylin and observed by microscopy (BZ-9000; Keyence, Osaka, Japan). Primary antibody comprised goat antibody to GFAP and Iba1 were obtained from Abcam (Cambridge, UK).
RESULTS
Vitamin E quantification of the brain and feeds
In order to investigate the effect of VE in the RB on VE deficiency in the brain, quantification of VE homologs in the brain tissue and feeds used in this study were performed by HPLC.
First, all VE homologs in the RB used in this study were measured, except for βTOC and βT3 (Table 1). Although αTOC, αT3, γTOC, γT3 and δTOC were detected, detectable levels of δT3 were not observed. The RB used in this experiment included predominately αTOC, γTOC and δTOC. The concentrations of T3s were lower than those of TOCs. As a next step, we measured the concentration of αTOC in each feed used in this experiment. The concentration of αTOC in VEdef, 2%RB, 5%RB, NR and NR5%RB were 0.66 ± 0.83 nmol/g, 15.55 ± 25.14 nmol/g, 31.51 ± 59.05 nmol/g, 477.93 ± 82.74 nmol/g and 546.16 ± 33.24 nmol/g, respectively.
Table 1.
Concentration of tocopherols and tocotrienols in rice bran (nmol/g)
| αTOC | γTOC | δTOC | αT3 | γT3 | δT3 |
| 60.86 ± 6.06 | 182.97 ± 16.36 | 125.05 ± 19.86 | 28.02 ± 2.02 | 3.94 ± 2.45 | N.D. |
αT3, α-tocotrienol; αTOC, α-tocopherol; δT3, δ-tocotrienol; δTOC, δ-tocopherol; γT3, γ-tocotrienol; γTOC, γ-tocopherol; N.D., not detected.
Furthermore, we measured the concentration of VE homologs in the brain tissue at 3 months after the start of special feeds administration. As expected, the amount of αTOC in the VEdef mouse brain was notably lower, while αTOC levels in 5%RB mice were comparable to those in NR mice (Table 2). The concentrations of αTOC in the brains of each feed group is shown in Table 2. Since αTOC levels in the brain from NR5%RB mice was similar to that from NR mice, we omitted NR5%RB mice by the later experiments. Other VE homologs, such as γTOC, δTOC, αT3, γT3 and δT3, were not detected because their concentrations in the brain were lower than that detectable by our HPLC system.
Table 2.
Vitamin E concentration in each feed
| Feed | αTOC (nmol/g) |
| NR | 477.93 ± 82.74 |
| NR5%RB | 546.16 ± 33.24 |
| VEdef | 0.66 ± 00.83 |
| 2%RB | 15.55 ± 25.14 |
| 5%RB | 31.51 ± 59.05 |
2%RB, a mixture of vitamin E deficient feed and RB at a concentration 2% rice bran; 5%RB, a mixture of vitamin E deficient feed and RB at a concentration 5% rice bran; αTOC, α-tocopherol; NR, normal diet alone; NR5%RB, a mixture of normal diet and RB at a concentration 5% rice bran; VE, vitamin E; VEdef, vitamin E-deficient feed.
Behavioral analyses
To evaluate the effects of oral supplementation of RB on the improvement of behavioral disorders caused by dietary loss of VE, we utilized the rotarod test, WRA, Y-maze test, and elevated plus maze test in all groups of mice.
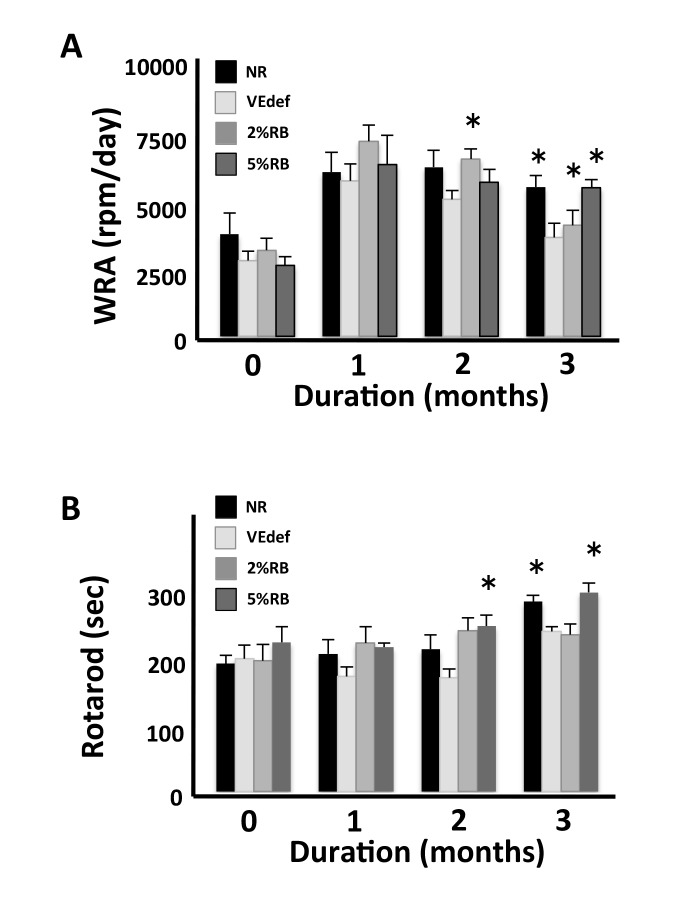
The rotarod test was performed in order to investigate motor coordination or movement memory of mice (Fig. 1A). Although the rotarod score of normal mice is progressed with every test attempt, this progression was not observed in VEdef mice. VEdef mice displayed a significant decrease of duration time on the rotarod compared with NR mice at 3 months, while the 5%RB mice showed a significant increase of the rotarod score compared with that of VEdef mice.
Fig. 1.
Behavioral changes due to administration of VE-deficient diet or RB-containing diet.
5%RB mice displayed increased movement behavior in the rotarod (A) and WRA tests (B) compared with the VEdef mice. Each group of mice NR (n = 6), VEdef (n = 4), 2%RB (n = 4) and 5%RB (n = 4) was subjected to the rotarod and WRA test. (A) Performance of each group of mice in the rotarod test. This score displays the mean of latency to fall. There is a significant difference between the VEdef and 5%RB mice at 3 months. (B) WRA was measured each month after the experiment started. There is a significant difference between VEdef and 5%RB mice at 3-months. *P < 0.05 vs. VEdef at same month (ANOVA). NR, normal diet alone; RB, rice bran; 2%RB, a mixture of vitamin E deficient feed and RB at a concentration 2% rice bran; 5%RB, a mixture of vitamin E deficient feed and RB at a concentration 5% rice bran; VE, vitamin E; VEdef, vitamin E-deficient feed; WRA, wheel running activity.
We measured the spontaneous activity levels of mice managed under the VE-deficient feed with or without δT3 using an activity wheel for mice (Fig. 1B). Although the mice under VE-deficient conditions showed a decrease in activity, the mice managed under VEdef with 5% RB showed a recovery of spontaneous activity.
To investigate anxiety behavior or deficits in short-term memory in these mice, the Y-maze and the elevated plus maze were performed at the same time as the locomotor analysis. At 3 months after the start of the experiment, we observed no difference between the VEdef mice and the other groups in the non-motility tests. Briefly, spontaneous alternative scores in the Y-maze test were 51.8 ± 5.14%, 57.7 ± 7.83% and 59.5 ± 2.78% in the VEdef, 2%RB and 5%RB groups, respectively. Moreover, the average of the time spent on open arms were 18.0 sec, 20.0 sec and 17.72 sec in the VEdef, 2%RB and 5%RB groups, respectively.
Measurement of oxidative stress marker
Because VE has anti-oxidative activity, chronic VEdef may induce oxidative stress. We investigated the expression of HO-1 in the brain by immunoblot analyses as an oxidative stress marker.
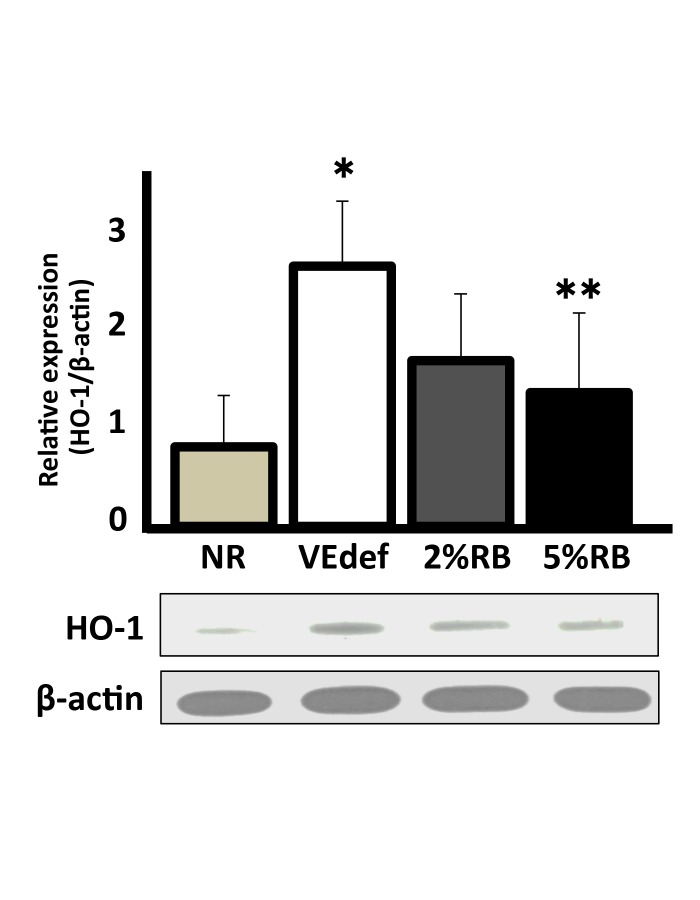
The expression of HO-1 in the brain increased significantly in VEdef mice compared with NR mice, whereas the expression significantly decreased in 5%RB mice compared with VEdef mice (Fig. 2).
Fig. 2.
The expression of heme oxygenase-1 in the mouse brain.
(Above) Quantified data of the relative expression of HO-1 in the brain normalized against β-actin expression. Homogenized whole brains were used for this analysis. A graph indicates relative values against NR (a reference value of 1.0). (Below) A representative example of an immunoblotting experiment. Upper panel shows HO-1 (32kDa) and lower panel shows β-actin (45kDa). HO-1 is upregulated in the brain of VEdef mouse, whereas that in 5%RB is significantly decreased. *P < 0.05 vs. NR, **P < 0.05 vs. VEdef (ANOVA). n = 4–6 (mean ± S.E.). HO-1, heme oxygenase-1; NR, normal diet alone; VEdef, vitamin E-deficient feed.
These results suggest that the influence of VEdef on the brain is reflected in increasing oxidative stress, which may be prevented by ingestion of (at least 5%) RB.
Histological analysis
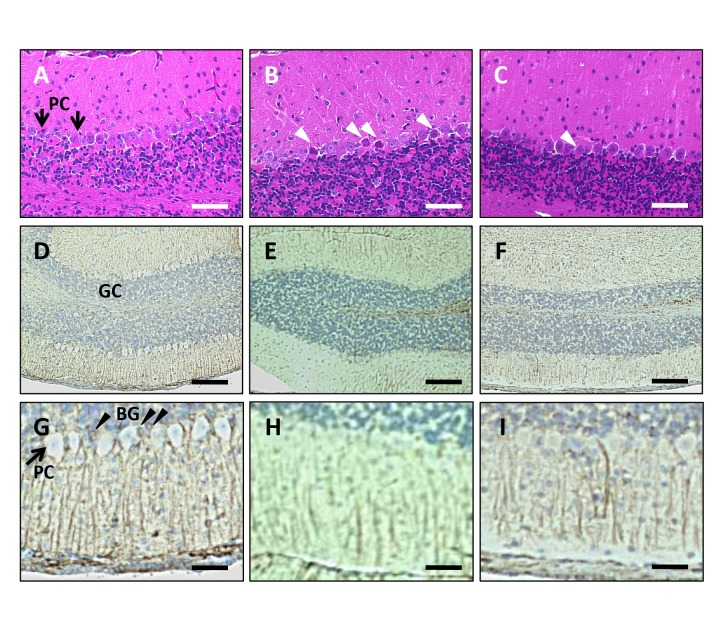
Histological analyses were performed by taking advantage of the brain tissue sampled at the time of HPLC analysis. Degeneration of Purkinje cells was observed in the cerebellum of VEdef mice at 3 months after the start of the experiment (Fig. 3B). On the other hand, in the RB5% mice, degeneration of Purkinje cells was mild (Fig. 3C) compared with the VEdef mouse cerebellum. Furthermore, we investigated the effect of the VEdef and RB diets on Bergmann glia, which help maintain Purkinje cells (Figs. 3D–I). Although GFAP-positive Bergman glia were observed around Purkinje cells in NR mice (Figs. 3D and G), GFAP immunoreactivity of the Bergmann glia decreased in the VEdef mouse cerebellum (Figs. 3E and H). On the other hand, GFAP immunoreactivity was recovered in the cerebellum from 5%RB mice (Figs. 3F and I).
Fig. 3.
Histological analyses of the cerebellum.
(A–C) HE staining of the cerebellum. Bar = 100 μm. (A) The cerebellum from NR mice. Arrows indicate Purkinje cells (PC). (B) VEdef mouse cerebellum shows neuronal degeneration of Purkinje cells (white arrow heads). (C) Addition of 5%RB inhibits the neuronal degeneration of Purkinje cells. Neurodegeneration (white arrow head) is reduced compared to that in VEdef mice. (D–I) Immunohistochemistry using anti-GFAP antibody. (D–F) Low magnification. Bar = 200 μm. (G–I) High magnification. Bar = 50 μm. GC: Granule cell layer. PC: Purkinje cells. BG: Bergmann glia. (D and G) GFAP positive Bergmann glia (black arrow heads) are observed around Purkinje cells. (E and H) GFAP immunoreactivity in Bergmann glia is reduced in the cerebellum of VEdef mice compared to NR or RB5% mice. (F and I) The GFAP immunoreactivity of Bergmann glia is recovered by 5%RB supplementation. GFAP, glial fibrillary acidic protein; HE, hematoxylin-eosin; NR, normal diet alone; RB, rice bran; 5%RB, a mixture of vitamin E deficient feed and RB at a concentration 5% rice bran; VE, vitamin E; VEdef, vitamin E-deficient feed.
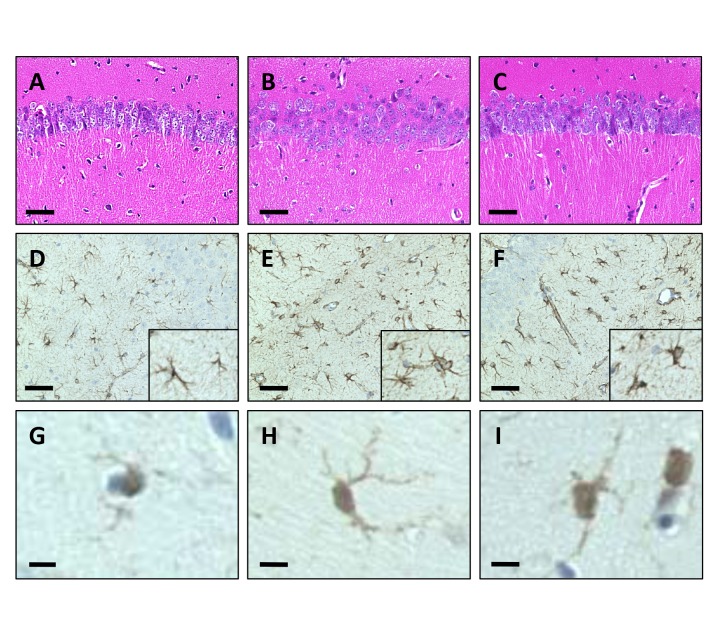
Next, we investigated the effect of VEdef and RB supplement on neuronal loss in the hippoccampus. In our experimental conditions, a significant loss of pyramidal neurons in the hippocampus was not observed (Figs. 4A–C). Furthermore, to confirm the activity of astrocytes and microglia in the hippocampus, we performed immunostaining with anti-GFAP (Figs. 4D–F), as well as anti-Iba1 antibody (Figs. 4G–I). In the hippocampus of each group, although there was a consistent level of staining intensity of glial cells, the immunoreactivity in VEdef mice was more intense than that of the other feed group (Figs. 4E and H). The activation of astrocyte and microglia, as indicated by complicating spinous processes and swollen cell bodies was observed in the VEdef mouse hippocampus (Figs. 4E and H). In the hippocampus of the brain from 5%RB mice, the degree of activation of astrocytes and microglia was less than that observed in VEdef mice (Figs. 4F–I).
Fig. 4.
Histological analyses of the hippocampus.
(A–C) HE staining of CA1 area in the hippocampus. Significant cell loss of the pyramidal neurons was not observed even in VEdef mice. Bar = 100 μm. (D–F) Immunohistochemistry for astrocytes using anti-GFAP antibody. Bar = 100 μm. (D) GFAP positive astrocytes are observed in the hippocampus. (E) Numbers of reactive astrocyte with complicating spinous processes and swelling of the cell body are observed in VEdef mouse hippocampus. (F) Fewer reactive astrocytes are observed in the hippocampus of 5%RB mice than VEdef mice. (G–I) Immunohistochemistry for microglia using the anti-Iba1 antibody. Bar = 10 μm. (G) Iba1 immunoreactive microglia. (H) Iba1 immunoreactive microglia shows a swollen cell body with complicated spinous processes. (I) Microglia with decreased spinous processes compared with that in the VEdef mouse hippocampus are observed in the 5%RB mouse brain. GFAP, glial fibrillary acidic protein; HE, hematoxylin-eosin; Iba1, ionized calcium biding adaptor molecule 1; 5%RB, a mixture of vitamin E deficient feed and RB at a concentration 5% rice bran.
DISCUSSION
Traditionally, VE has been described as having antioxidant activity.3–5 The αTOC-mediated free radical quenching reaction is extremely fast (~108/M/sec), and the water soluble antioxidant ascorbic acid (vitamin C) is thought to recycle the oxidized αTOC radical generated during a redox reaction back to reduced αTOC.5, 22 Therefore, VE is a powerful antioxidant in vivo.
VE derivatives are found in the cell membrane of various foods. However, T3s are found only in a few vegetable fats such as palm oil and RB oil.23, 24 RB is byproduct of the rice milling process and has abundant nutrients including both TOCs and T3s. Therefore, RB is a promising oral supplement to provide VE.
A mutation of the αTTP gene has been reported as autosomal recessive familial VE deficiency in human.25, 26 In this family, patients show phonotypes that include cerebellar ataxia similar to spinocerebellar ataxia, and high dose supplementation of VE early in the disease process may, to some extent, reverse ataxia and mental deterioration. Since αTTP is expressed in Bergmann glia, which help maintain cerebellar Purkinje cells,16 a loss of function of αTTP may cause cerebellar degeneration. This suggests that the cerebellum may be vulnerable against VE deficiency, even with the normal αTTP gene product activity. αTTP-deficient mice also show a phenotype similar to human αTTP deficiency.12
In this study, we investigated the effect of oral supplementation with RB as a source of VE on motor and cognitive function in mice fed a VE-deficient diet. In our experimental conditions, VE deficiency induced decreased activity of motor function, but not cognitive function and anxiety. The rotarod score, an index of motor function including balance and exercise learning, showed a low latency in the VEdef group, and also showed recovered latency in the RB5% group (Fig. 1A). Furthermore, we observed Purkinje cell degeneration and reduced GFAP immunoreactivity of Bergmann glia in the cerebellum, suggesting that cerebellar dysfunction by VE deficiency caused motor dysfunction and that RB supplement rescued this cerebellar dysfunction (Fig. 3).
The WRA test showed mildly lowered scores in VEdef mice and a recovery effect of RB against VE deficiency (Fig. 1B). In order to investigate the cause of this lowered activity, we examined the loss of dopaminergic neurons in the substantia nigra, which has been shown to decrease activity.27 In our experimental conditions, significant neuronal loss was not observed in the substantia nigra (data not shown). Therefore, we concluded the reduced WRA score in VEdef mice is due to cerebellar dysfunction.
Since VE deficiency induces non-motor dysfunction and neuronal loss in the brain,28 we examined the effect of VE deficiency and oral supplementation of RB on cognitive function and anxiety. Although reactive astrocytes and activated microglia were observed in the hippocampus, a loss of pyramidal neurons was not observed in the VEdef mouse brain (Fig. 4). This result is consistent with the result of the cognitive and anxiety examination using the Y-maze system and the elevated plus maze test. Since our experimental procedures was designed for only 3 months, longer-term experiments using the VEdef diet may show similar results as those described by Fukui, et al.28
It has been reported that TOCs and T3s have cytoprotective function due to not only antioxidative activity, but also antioxidant-independent activities including intracellular signal transduction.6, 27 However, judging from the induction and/or inhibition of HO-1, the protective effect of RB may due to an antioxidative function. Several antioxidative ingredients, as well as VE, may be included in RB. Therefore, the effect by other ingredients should also be considered, and further study is needed.
The RB used in this study was rich in TOCs rather than in T3s (Table 1). Therefore, oral supplementation of RB increased αTOC in the brain, decreased oxidative stress, and improved motor function and histological abnormalities induced by VE deficiency. These results suggest that RB is useful as a supplement food for VE.
Acknowledgments
Ackonwlegments: This work was supported by JSPS KAKENHI 24591262 (KN), 15K09315 (KN), 23580168 (TM), 15K00816 (TM), Eisai Food & Chemical Co., Ltd. (KN) and The Tojuro Iijima Foundation for Food Science and Technology (KN).
The authors declare no conflict of interest.
REFERENCES
- 1. Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671-701. [DOI] [PubMed] [Google Scholar]
- 2. Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;27:2088-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Packer L. Interactions among antioxidants in health and disease: vitamin E and its redox cycle. Proc Soc Exp Biol Med. 1992;200:271-6. [DOI] [PubMed] [Google Scholar]
- 4. Niki E, Yamamoto Y, Takahashi M, Komuro E, Miyama Y. Inhibition of oxidation of biomembranes by tocopherol. Ann N Y Acad Sci. 1989;570:23-31. [DOI] [PubMed] [Google Scholar]
- 5. Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: A critical review. Free Radic Biol Med. 2008;44:739-64. [DOI] [PubMed] [Google Scholar]
- 6. Nakaso K, Tajima N, Horikoshi Y, Nakasone M, Hanaki T, Kamizaki K, et al. The estrogen receptor-β-PI3K/Akt pathway mediates the cytoprotective effects of tocotrienol in a cellular Parkinson’s disease model. Biochem Biophys Acta Mol Bas Dis. 2014;1842:1303-12. [DOI] [PubMed] [Google Scholar]
- 7. de Rijk MC, Breteler MM, den Breeijen JH, Launer LJ, Grobbee DE, van der Meche FG, et al. Dietary antioxidants and Parkinson disease. The Rotterdam study. Arch Neurol. 1997;54:762-5. [DOI] [PubMed] [Google Scholar]
- 8. Zhang SM, Hernan MA, Chen H, Spiegelman D, Willett WC, Ascherio A. Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology. 2002;59:1161-9. [DOI] [PubMed] [Google Scholar]
- 9. Fariss MW, Zhang JG. Vitamin E therapy in Parkinson’s disease. Toxicology. 2003;189:129-46. [DOI] [PubMed] [Google Scholar]
- 10. Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, α-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s disease cooperative study. N Engl J Med. 1997;336:1216-22. [DOI] [PubMed] [Google Scholar]
- 11. Ascherio A, Weisskopf MG, O’Reilly EJ, Jacobs EJ, McCullough ML, Calle EE, et al. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann Neurol. 2005;57:104-10. [DOI] [PubMed] [Google Scholar]
- 12. Yokota T, Igarashi K, Uchihara T, Jisage K, Tomita H, Inaba A, et al. Delayed-onset ataxia in mice lacking α-tocopherol transfer protein: Model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci USA. 2001;98:15185-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida Y. The role of α-tocopherol in motor hypofunction with aging in α-tocopherol transfer protein knockout mice as assessed by oxidative stress biomarkers. J Nutr Biochem. 2010;21:66-76. [DOI] [PubMed] [Google Scholar]
- 14. Ulatowski L, Parker R, Warrier G, Sultana R, Butterfield DA, Manor D, et al. Vitamin E is essential for Purkinje neuron integrity. Neuroscience. 2014;260:120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mowri H, Nakagawa Y, Inoue K, Nojima S. Enhancement of the transfer of α-tocopherol between liposomes and mitochondria by rat-liver protein(s). Eur J Biochem. 1981;117:537-42. [DOI] [PubMed] [Google Scholar]
- 16. Hosomi A, Goto K, Kondo H, Iwatsubo T, Yokota T, Ogawa M, et al. Localization of α-tocopherol transfer protein in rat brain. Neurosci Lett. 1998;256:159-62. [DOI] [PubMed] [Google Scholar]
- 17. Kawakami Y, Tsuzuki T, Nakagawa K, Miyazawa T. Distribution of tocotrienols in rats fed a rice bran tocotrienol concentrate. Biosci Biotechnol Biochem. 2007;71:464-71. [DOI] [PubMed] [Google Scholar]
- 18. Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyans, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr. 2014;2:75-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugano M, Tsuji E. Rice bran oil and cholesterol metabolism. J Nutr. 1997;127:5215-45. [DOI] [PubMed] [Google Scholar]
- 20. Gerhardt AL, Gallo NB. Full-fat rice bran and oat bran similarly reduce hypercholesterolemia in humans. J Nutr. 1998;128:865-9. [DOI] [PubMed] [Google Scholar]
- 21. Ardiansyah , Shirakawa H, Koseki T, Ohinata K, Hashizume K, Komai M. Rice bran fractions improve blood pressure, lipid profile, and glucose metabolism in stroke-prone spontaneously hypertensive rats. J Agric Food Chem. 2006;54:1914-20. [DOI] [PubMed] [Google Scholar]
- 22. Burton GW, Traber MG. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Ann Rev Nutr. 1990;10:357-82. [DOI] [PubMed] [Google Scholar]
- 23. Elson CE. Tropical oils: nutritional and scientific issues. Crit Rev Food Sci Nutr. 1992;31:79-102. [DOI] [PubMed] [Google Scholar]
- 24. Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: The other half of the natural vitamin E family. Mol Aspects Med. 2007;28:692-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gotoda T, Arita M, Arai H, Inoue K, Yokota T, Fukuo Y, et al. Adult-onset spinocerebellar dysfunction caused by a mutation in the gene for the α-tocopherol transfer protein. New Engl J Med. 1995;333:1313-8. [DOI] [PubMed] [Google Scholar]
- 26. Ouahchi K, Arita M, Kayden HJ, Hentanti F, Hamida MB, Sokol R, et al. Ataxia with isolated vitamin E deficiency is caused by mutations in the α-tocopherol transfer protein.. Nat Genet. 1995;9:141-5. [DOI] [PubMed] [Google Scholar]
- 27. Nakaso K, Horikoshi Y, Takahashi T, Hanaki T, Nakasone M, Kitagawa Y, et al. Estrogen receptor-mediated effect of δ-tocotrienol prevents neurotoxicity and motor deficit in the MPTP mouse model of Parkinson’s disease. Neurosci Lett. 2016;610:117-22. [DOI] [PubMed] [Google Scholar]
- 28. Fukui K, Nakamura K, Shirai M, Hirano A, Takatsu H, Urano S, et al. Long-term vitamin E-deficient mice exhibit cognitive dysfunction via elevation of brain oxidation. J Nutr Sci Vitaminol. ・・2015・;61:362-8. [DOI] [PubMed] [Google Scholar]