Abstract
Background
Postoperative complications have been shown to worsen prognoses of various cancer types.
Methods
We retrospectively analyzed 265 patients with stage II-III gastric cancer who underwent curative gastrectomies between 1991 and 2010 at Tottori University Hospital to determine the effect of postoperative intra-abdominal complication (IAC) on prognosis.
Results
Of the 265 patients, 38 (14.3%) developed postoperative IACs of grade ≥ 2, of whom significantly more patients were male. Patients in the IAC group were significantly older than patients in the non-complication (NC) group. The NC group had significantly better survival than did the IAC group (P < 0.0001). Within the IAC group, 5-year survival rates did not significantly differ between patients with infectious complication subgroup (24.6%) and the non-infectious subgroup (46.2%). Grade of complication was not related to prognosis. Lengths of time before starting adjuvant chemotherapy (AC) after surgery were significantly longer for the IAC group (55.3 ± 34.7 days) than for the NC group: (26.6 ± 11.9 days; P = 0.0023). Prognosis of patients who took AC within 6 weeks after surgery tended to be better than that of patients who took AC > 6 weeks after surgery (P = 0.071). In multivariate analysis, IAC was an independent predictor of prognosis, as were age, invasion depth, and lymph node metastasis.
Conclusion
Postoperative IACs were related to poorer survival for patients with stage II–III gastric cancer.
Keywords: gastrectomy, gastric cancer, postoperative complication, prognosis
Although the prognosis of patients with gastric carcinoma has improved with increased availability of diagnostic techniques and better intraoperative and postoperative care, death from gastric cancer (GC) still ranks second among all cancer deaths worldwide.1
Gastrectomy with D2 lymph node dissection has been the standard treatment for advanced GC in Japan,2 and has led to lower morbidity and mortality rates. However, as D2 lymph node dissection and esophago-jejunostomy after total gastrectomy are technically demanding, some patients suffered from postoperative complications,3 which can worsen their short-term outcome (such as lengthening their hospital stay, or requiring longer fasting). Furthermore, some complications, such as anastomotic leakage and pancreatic fistula, may become serious or even life-threatening.
Reportedly, postoperative complications also negatively affect long-term outcomes for a wide range of cancers,4–7 including GC.8, 9 However, reports regarding GC have included only infectious complications and excluded non-infectious complication in their analyses. Adjuvant chemotherapy (AC) is an indispensable component of treatment, aimed at preventing recurrence in patients with resectable advanced GC. The Japanese Gastric Cancer Treatment Guideline recommends adjuvant therapy by S-1 for 1 year after curative surgery,2 based on the Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC), which showed a survival benefit from AC after D2 gastrectomy compared with surgery alone for patients with stage II–III GC.10 They also recommend starting AC within 6 weeks after operation. Late initiation of AC has been significantly associated to poor prognosis in patients with stage II–III GC.11 Therefore, both non-infectious and infectious complications are likely to worsen their prognoses, owing to late initiation of AC. However, few reports have yet shown a correlation between non-infectious complications and prognosis in GC patients. This retrospective study was designed to determine the effect of postoperative intra-abdominal complications (IACs), including both infectious and non-infectious complications on long-term outcomes in patients with stage II–III GC.
MATERIALS AND METHODS
Patients
This study included consecutive 265 patients with stage II–III gastric adenocarcinoma who underwent curative gastrectomies (R0 resections) between 1991 and 2010 at the Tottori University Hospital. Institutional review board approval was obtained (1607A052), and the informed consent requirement was waived for this study. All patients underwent distal partial gastrectomy, proximal partial gastrectomy or total gastrectomy with regional lymph node dissection. At the time of analysis, the median follow-up for the 129 remaining survivors was 91 months. Of the 136 deaths, 86 were related to recurrence of GC, two to another malignancy and 48 to another disease or accident.
With regard to AC, 72 patients underwent chemotherapy, while the remainder did not. Uracil-tegafur (UFT, Taiho, Tokyo, Japan) was used in 16 patients and tegafur-gimeracil-oteracil (S-1, Taiho) was used in 56 patients. These patients received 200–400 mg of UFT, 2–3 × daily orally, or 80 mg/m2/day oral S-1. These regimens were administered for 6 months to a year postoperatively. Because S-1 became available for the treatment of gastric cancer in 1999, UFT was mainly used before 1999 and S-1 was used after 1999.
Clinicopathological findings were generally determined according to the 14th edition of the Japanese Classification of Gastric Carcinoma.12 With regard to the definition of postoperative IACs, the Clavien–Dindo (CD) system was used to classify each patient’s postoperative IAC.13, 14 If an individual patient had multiple complications, the highest grade was used in the analysis. Grade 1 complications were not evaluated to exclude the possibility of a description bias in the patient records.
Statistical analysis
For statistical analyses, chi-square and Fisher’s exact probability tests were used to compare distribution of individual variables between patient groups. Differences between the two groups were evaluated using the Mann–Whitney U test. Disease-specific survival (DSS) was calculated by the Kaplan–Meier method and compared using the log-rank test. Patients who died of causes other than GC were considered lost to follow-up at the time of death for DSS. We used multivariate analysis of factors considered prognostic of DSS, with a Cox’s proportional hazards model and a stepwise procedure. P < 0.05 was considered significant. Statistical analyses were performed with SPSS for Windows version 19.0 (SPSS, Chicago, IL).
RESULTS
Table 1 shows details of complications in patients included in the current study. Thirty-eight patients (14.3%) developed postoperative IACs of grade 2 or higher according to CD classification. Of these patients, 5.3% developed grade III IACs and 1.5% had grade IV or V IACs.
Table 1.
Details of postoperative intra-abdominal complications
| Grade of CD classifications | ||||||||
| Type of complication | II | IIIa | IIIb | IVa | IVb | V | Total | |
| Infectious complication | ||||||||
| Pancreatic fistula | 3 | 2 | 1 | 1 | 0 | 1 | 8 | |
| Anastomotic leakage | 8 | 6 | 0 | 0 | 0 | 1 | 15 | |
| Wound infection | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Non-infectious complication | ||||||||
| Stenosis | 4 | 4 | 0 | 0 | 0 | 0 | 8 | |
| Ileus | 3 | 0 | 0 | 0 | 0 | 1 | 4 | |
| Afferent loop syndrome | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Total | 20 | 12 | 2 | 1 | 0 | 3 | 38 | |
CD, Clavien–Dindo.
Table 2 shows the differences in clinicopathological characteristics between the IAC group (n = 38) and the NC group (n = 227). The IAC had a significantly higher percentage of male patients, and had a significantly older median age, than did the NC group. The IAC and NC groups did not significantly differ in tumor size, depth of invasion, lymph node metastasis, pathological stage of disease, tumor location, type of gastrectomy, and splenectomy.
Table 2.
Clinicopathologic characteristics of patients with stage II–III gastric cancer by the presence of intra-abdominal complications
| Complication group (n = 38) | Non-complication group (n = 227) | P value | ||
| Gender | 0.014 | |||
| Male | 30 | 132 | ||
| Female | 8 | 95 | ||
| Age (years) | 71.2 + 11.5 | 65.9 + 12 | 0.011 | |
| Tumor size (cm) | 7.6 + 4.2 | 6.5 + 3.5 | 0.18 | |
| Tumor location | 0.52 | |||
| Upper | 14 | 64 | ||
| Middle | 11 | 82 | ||
| Lower | 13 | 81 | ||
| Tumor depth | 0.7 | |||
| T1 | 3 | 10 | ||
| T2 | 6 | 43 | ||
| T3 | 12 | 61 | ||
| T4 | 17 | 113 | ||
| Histology | 0.74 | |||
| Differentiated | 16 | 89 | ||
| Undifferentiated | 22 | 138 | ||
| Lymph node metastasis | 0.2 | |||
| N0 | 5 | 47 | ||
| N1 | 9 | 79 | ||
| N2 | 10 | 40 | ||
| N3 | 14 | 61 | ||
| Pathological stage | 0.67 | |||
| II | 15 | 98 | ||
| III | 23 | 129 | ||
| Gastrectomy | 0.25 | |||
| Distal | 13 | 109 | ||
| Proximal | 1 | 8 | ||
| Total | 24 | 110 | ||
| Splenectomy | 0.36 | |||
| Performed | 10 | 45 | ||
| Not performed | 28 | 182 | ||
| Adjuvant chemotherapy | 0.89 | |||
| Performed | 10 | 62 | ||
| Not performed | 28 | 165 | ||
N0, no regional lymph node metastases; N1, metastasis in 1–2 regional lymph nodes; N2, metastasis in 3–6 regional lymph nodes; N3, metastasis in 7 or more regional lymph nodes; T1, tumor has invaded lamina propria or submucosa; T2, tumor has invaded the muscularis propria; T3, tumor has invaded the subserosa; T4, tumor invasion is contiguous to or exposed beyond the serosa or tumor invades adjacent structures.
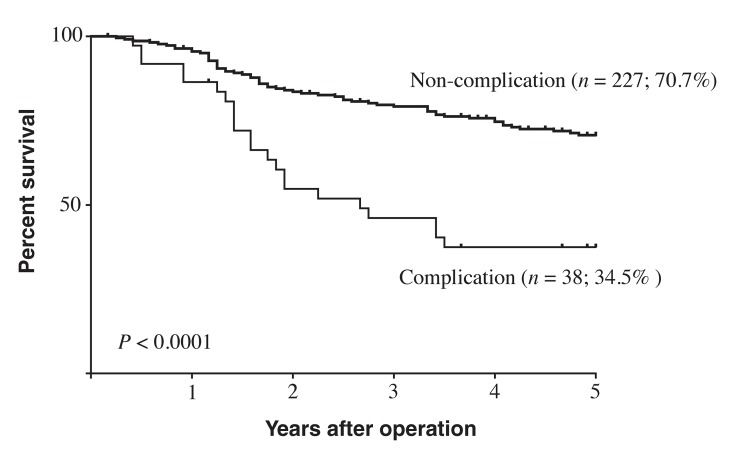
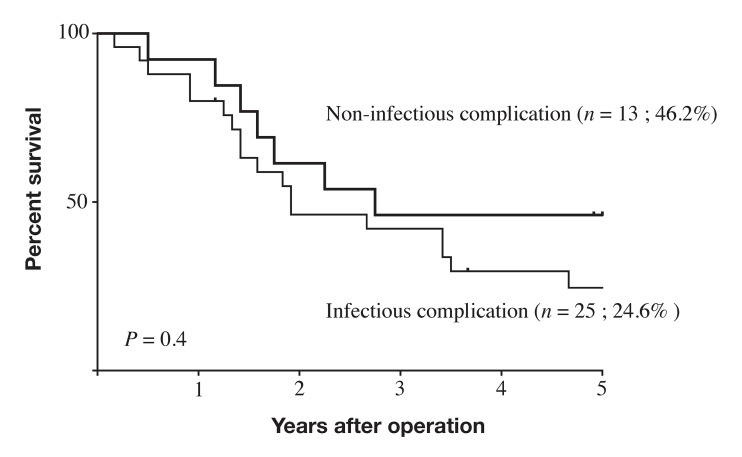
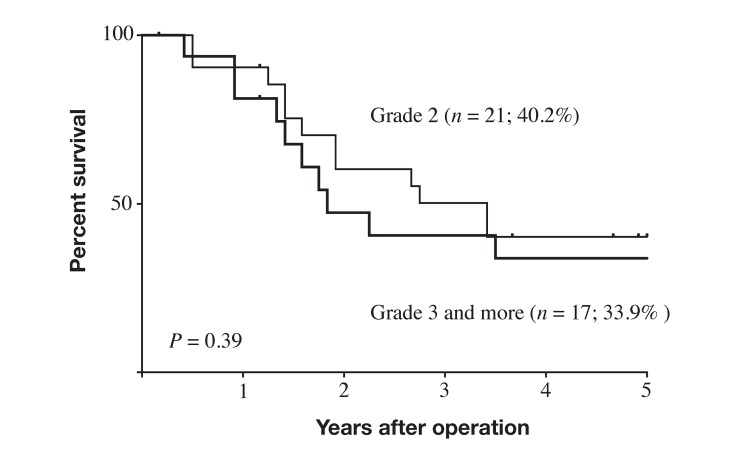
Five-year survival rates differed significantly between the IAC group (34.5%) and the NC group (70.7%; P < 0.0001; Fig. 1). Within the IAC group, 5-year survival rates did not significantly differ between the subgroup with infectious IACs (24.6%) and those with non-infectious IACs (46.2%; P = 0.4; Fig. 2). Five-year survival also did not significantly differ between the subgroup with grade 2 IACs (40.2%) and those with grade 3–5 IACs (33.9%; P = 0.39; Fig. 3).
Fig. 1.
Survival curves for patients with stage II–III gastric cancer, based on presence of postoperative intra-abdominal complications. Patients without intra-abdominal complication had significantly better prognosis than did patients with intra-abdominal complications (P < 0.0001).
Fig. 2.
Survival curves for patients with stage II–III gastric cancer and postoperative intra-abdominal complications. Outcomes did not significantly differ between patient subgroups with infectious and non-infectious intra-abdominal complication (P = 0.4).
Fig. 3.
Survival curves for patients with stage II–III gastric cancer with postoperative intra-abdominal complication by grade of complication (CD classification). Outcomes did not significantly differ between patients with grade 2 intra-abdominal complications and those with grade ≥ 3 complications (P = 0.39). CD, Clavien–Dindo.
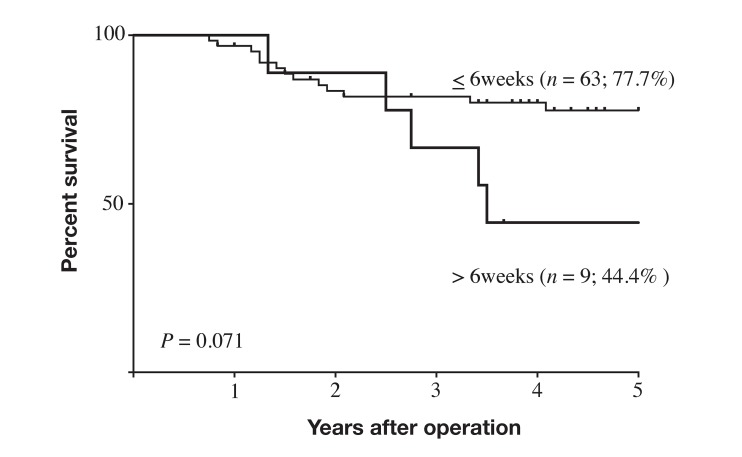
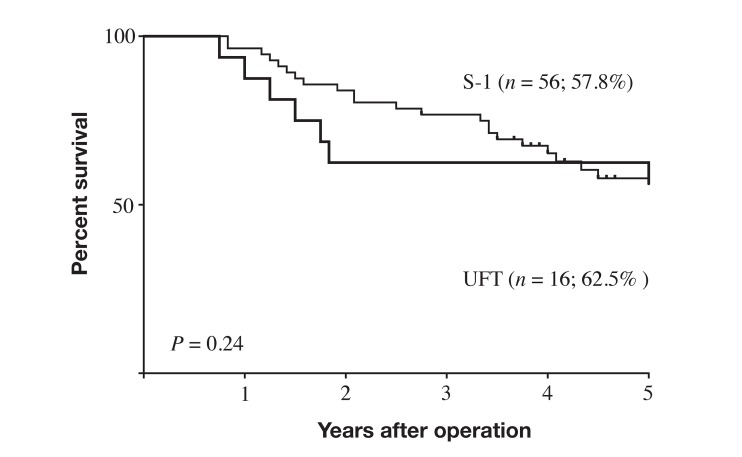
Intervals before starting AC after surgery differed significantly between the IAC group (55. 3 ± 34.7 days; n = 10) and the NC group (26.6 ± 11.9 days; n = 62; P = 0.0023). Because the Japanese Gastric Cancer Treatment Guideline recommends starting AC with S-1 within 6 weeks post-surgery in patients with stage II–III GC, we divided patients who took AC into the non-delayed group (patients who started AC within 6 weeks of surgery), and the delayed group (who started AC later than 6 weeks after surgery). In the NC group, only 4 patients (6.5%) were in the delayed group, while 5 patients (50%) were in the delayed group in the IAC group and the difference was statistically significant (P = 0.0001). The prognosis of patients in non-delayed group tended to be better than that in delayed group, but not significantly so (P = 0.071; Fig. 4). Two anti-cancer drugs, UFT and S-1, were used for AC in the current study. Patients who took UFT and those who took S-1 showed no significant difference in prognosis (P = 0.24, Fig. 5).
Fig. 4.
Survival curves for patients with stage II–III gastric cancer with postoperative intra-abdominal complication based on length of time before starting AC. Prognosis of patients who started AC within 6 weeks after surgery tended to be better (but not significantly so) than that of patients who started AC more than 6 weeks after surgery (P = 0.071). AC, adjuvant chemotherapy.
Fig. 5.
Survival curves for patients with stage II–III gastric cancer and postoperative intra-abdominal complication by drugs used for adjuvant chemotherapy. Prognosis did not significantly differ between patients who took UFT and those who took S-1 (P = 0.24). S-1, tegafur-gimeracil-oteracil; UFT, tegafur-uracil.
We finally applied Cox proportional hazard model and a stepwise procedure on age, sex, tumor size, histology, tumor location, depth of invasion, lymph node metastasis, lymphatic vessel invasion, blood vessel invasion, type of gastrectomy, splenectomy, AC, and IAC, to identify independent prognostic factors. Multivariate analysis indicated that IAC was an independent prognostic indicator, as were age, depth of invasion, and lymph node metastasis (Table 3).
Table 3.
Multivariate analysis using Cox’s proportional hazards model and a stepwise procedure to identify
| Covariates | P value | Hazard ratio | 95% CI |
| Age* | 0.019 | 1.024 | 1.004–1.045 |
| Depth of invasion (T1–T4)† | < 0.0001 | 1.942 | 1.437–2.625 |
| Lymph node metastasis (N0–N3)‡ | < 0.0001 | 1.545 | 1.265–1.887 |
| Intra-abdominal complication | 0.0001 | 2.633 | 1.610–4.305 |
*Continuous variable. †T1: tumor has invaded lamina propria or submucosa; T2: tumor has invaded the muscularis propria; T3: tumor has invaded the subserosa; T4: tumor invasion is contiguous to or exposed beyond the serosa or tumor invades adjacent structures. ‡N0: no regional lymph node metastases; N1: metastasis in 1–2 regional lymph nodes; N2: metastasis in 3–6 regional lymph nodes; N3: metastasis in 7 or more regional lymph nodes. CI, confidence interval.
DISCUSSION
Gastric surgery has had lower morbidity and mortality rates in recent years because of advances in treatment techniques, surgical devices, and perioperative management.15–17 In fact, a recent clinical trial indicates that the rate of overall surgery-related complications among patients who underwent gastrectomy with D2 lymphadenectomy—the current standard operation for advanced GC—is 20.9%, including 2.3 % for anastomotic leakages, 5.3% for pancreatic fistulae, 5.3% for abdominal abscesses, and 4.6% for pneumonia, and with an 0.8% hospital death rate.3
Postoperative complications inversely affect patients’ short-term outcomes, and some complications, such as anastomotic leakage and pancreatic fistula, can become life-threatening. Therefore, every effort should be made to avoid their development. They have also been shown to affect long-term outcomes of patients with various cancer types.4–7 We have clearly demonstrated that postoperative IAC is significantly associated with poorer survival of patients with stage II–III GC in this current study. Other reports have also associated infectious complications to poor prognosis in GC patients.8, 9 Tokunaga et al. compared the prognosis of patients with stage II–III GC with and without infectious IACs and found that postoperative infectious IACs adversely affected overall and relapse-free survival of these patients9; Tokunaga et al. considered pancreas-related complications, anastomotic leakages, and intra-abdominal abscesses as infectious IACs, but excluded ileus and wound infections, whereas all type of IACs were included in the current study. Notably, we found that both infectious and non-infectious IACs were related to poor prognosis. The reason why IACs are related to poor prognosis remains unclear. With regard to infectious complications, immune suppression might be a factor in poor survival. We have previously reported that number of total lymphocytes significantly decreased after gastrectomy for GC although some inflammation markers, such as numbers of white blood cells and neutrophils and serum CRP levels, significantly increased.18 The lymphocyte count reached a minimum on postoperative day (POD) 1 and then increased to preoperative level on day 30. Prolonged inflammation could have induced prolonged suppression of cell-mediated immunity in patients with infectious IACs, which would put those patients at high risk of recurrence.
Non-infectious IACs are also associated with poor survival. In this regard, delayed initiation of AC might worsen survival of patients with non-infectious IAC. In the current study, time before starting AC after surgery differed significantly in the IAC group (55.3 ± 34.7 days) and the NC group (26.6 ± 11.9 days). As the aim of AC is to eradicate micrometastatic tumor cells, delaying AC allows such cells to grow after operation. Some clinicians often believe that chemotherapy may therefore have little or no adjuvant benefit after a 3-month delay19, 20 Time before initiating AC has been associated with survival in breast and colorectal cancers,20, 21 and recently, in prognosis for advanced GC patients who started S-1 AC within or after 6 weeks post-surgery.11 As postoperative complications are a common reason for delaying AC, the poorer prognosis in our IAC group is likely due to delayed AC.
The present retrospective study has limitations. First, clinicopathological factors differed between the IAC and NC groups. As pathological stage is considered to be the strongest prognostic clinicopathological factor for GC after curative gastrectomy,22, 23 we conducted multivariate analysis and found IACs to be an independent prognostic factor. Second, the degree of immune suppression was not assessed in this study, but should be examined in a future trial to verify our hypothesis that patients with intra-abdominal infectious complications have severe immune suppression that leads to high recurrence rates and poor overall and relapse-free survival rates. Third, the two anti-cancer drugs that were used for AC in the current study, UFT and S-1, were mainly used before 2003 and after 2004, respectively. Those two drugs may have differently affected the patients’ prognoses. In this regard, patients who took UFT and those who took S-1 did no significantly differ in prognosis, which indicates that the differences of the two drugs’ effects on outcomes was small for patients included in the current study.
In conclusion, our study clearly shows that postoperative IACs—both infectious and non-infectious—are related to poor survival of patients with stage II–III gastric cancer. Therefore, every effort should be made to avoid the development of these complications.
The authors declare no conflict of interest.
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. DOI: 10.1007/s10120-016-0622-4 Epub 2016 Jun 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-62. [DOI] [PubMed] [Google Scholar]
- 4. Murthy BL, Thomson CS, Dodwell D, Shenoy H, Mikeljevic JS, Forman D, et al. Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer. 2007;97:1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kulu Y, Tarantio I, Warschkow R, Kny S, Schneider M, Schmied BM, et al. Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol. 2015;22:2059-67. [DOI] [PubMed] [Google Scholar]
- 6. Aoyama T, Murakawa M, Katayama Y, Yamaoku K, Kanazawa A, Higuchi A, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res. 2015;35:2401-9. [PubMed] [Google Scholar]
- 7. Saeki H, Tsutsumi S, Tajiri H, Yukaya T, Tsutsumi R, Nishimura S, et al. Prognostic Significance of Postoperative Complications After Curative Resection for Patients With Esophageal Squamous Cell Carcinoma. Ann Surg. Epub 2016 Mar 7 [DOI] [PubMed] [Google Scholar]
- 8. Hayashi T, Yoshikawa T, Aoyama T, Hasegawa S, Yamada T, Tsuchida K, et al. Impact of infectious complications on gastric cancer recurrence. Gastric Cancer. 2015;18:368-74. [DOI] [PubMed] [Google Scholar]
- 9. Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575-83. [DOI] [PubMed] [Google Scholar]
- 10. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-20. [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto M, Sakaguchi Y, Kinjo N, Yamaguchi S, Egashira A, Minami K, et al. S-1 Adjuvant Chemotherapy Earlier After Surgery Clinically Correlates with Prognostic Factors for Advanced Gastric Cancer. Ann Surg Oncol. 2016;23:546-51. [DOI] [PubMed] [Google Scholar]
- 12. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-12. [DOI] [PubMed] [Google Scholar]
- 13. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-96. [DOI] [PubMed] [Google Scholar]
- 14. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Degiuli M, Sasako M, Ponti A. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg. 2010;97:643-9. [DOI] [PubMed] [Google Scholar]
- 16. Katai H, Sasako M, Fukuda H, Nakamura K, Hiki N, Saka M, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer. 2010;13:238-44. [DOI] [PubMed] [Google Scholar]
- 17. McCulloch P. The role of surgery in patients with advanced gastric cancer. Best Pract Res Clin Gastroenterol. 2006;20:767-87. [DOI] [PubMed] [Google Scholar]
- 18. Takaya S, Saito H, Ikeguchi M. Upregulation of Immune Checkpoint Molecules, PD-1 and LAG-3, on CD4+ and CD8+ T Cells after Gastric Cancer Surgery. Yonago Acta Med. 2015;58:39-44. [PMC free article] [PubMed] [Google Scholar]
- 19. Hershman D, Hall MJ, Wang X, Jacobson JS, McBride R, Grann VR, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581-8. [DOI] [PubMed] [Google Scholar]
- 20. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. Jama. 2011;305:2335-42. [DOI] [PubMed] [Google Scholar]
- 21. Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014;32:735-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2006;9:51-66. [DOI] [PubMed] [Google Scholar]
- 23. Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2011;14:301-16. [DOI] [PMC free article] [PubMed] [Google Scholar]