Abstract
Although major steps have been recently made in understanding the role of the distinct subsets of dendritic cells (DC)/antigen-presenting cells (APC), further studies are required to unravel their precise role, including in-depth immunophenotypic characterisation of these cells. Here, we used eight-colour flow cytometry to investigate the reactivity of a panel of 72 monoclonal antibodies (including those clustered in seven new Cluster of Differentiation, CD) on different subsets of APC in peripheral blood (PB) samples from five healthy adults. These experiments were performed in the context of the Tenth International Workshop on Human Leukocyte Differentiation Antigens (HLDA10). Plasmacytoid DC was the only cell population that expressed CD85g and CD195, whereas they lacked all of the other molecules investigated. In contrast, myeloid DC mostly expressed inhibitory C-type lectin receptors (CLRs) and other inhibitory-associated molecules, whereas monocytes expressed both inhibitory and activating CLRs, together with other phagocytosis-associated receptors. Within monocytes, progressively lower levels of expression were generally observed from classical monocytes (cMo) to SLAN− and SLAN+ non-classical monocytes (ncMo) for most of the molecules expressed, except for the CD368 endocytic receptor. This molecule was found to be positive only in cMo, and the CD369 and CD371 modulating/signalling receptors. In addition, the CD101 inhibitory molecule was found to be expressed at higher levels in SLAN+ vs SLAN− ncMo. In summary, the pattern of expression of the different signalling molecules and receptors analysed in this work varies among the distinct subsets of PB APCs, with similar profiles for molecules within each functional group. These findings suggest unique pattern-recognition and signalling capabilities for distinct subpopulations of APCs, and therefore, diverse functional roles.
‘Mononuclear phagocytes' (that is, dendritic cells (DCs), monocytes and macrophages) are functionally related immune cells that have an essential role as antigen-presenting cells (APCs).1 Among them, DCs are widely recognised as the most potent APCs capable of efficiently activating naive T cells. In turn, the main role of macrophages is to remove apoptotic/damaged cells and foreign components, such as microorganisms and their pathogenic factors, to ensure tissue integrity.1 Despite these general roles, it is well known that both DCs and monocytes/macrophages are heterogeneous cell populations.2 In fact, both consist of different functionally specialised subsets of cells with diverse effector and immunomodulatory functions that give rise to a complex cellular network capable of integrating multiple environmental signals leading to either immunity or tolerance. Regarding this, two clearly different subsets of human circulating DCs have been identified: plasmacytoid DCs (pDCs), a unique subset of DC that secrete large amounts of type I interferon in response to viruses,3 and myeloid DCs (mDCs). mDCs can be further subdivided into a major subset of CD1c+(BDCA1+) mDCs, which are highly effective at the uptake of antigens, migration and antigen presentation to CD4+ T cells, and a minor subset of CD141+(BDCA3+) mDCs that are specialised in antigen cross-presentation.1 Similarly, circulating peripheral blood (PB) human monocytes are also heterogeneous, consisting of three distinct populations based on their differential expression of the CD14 lipopolysaccharide co-receptor and the CD16 Fcγ receptor. These populations include: (a) CD14++/CD16− or classical monocytes (cMo) that represent 90–95% of all PB monocytes in healthy subjects; (b) CD14+/lo/CD16lo or intermediate monocytes (iMo); and (c) CD14lo/−/CD16+non-classical monocytes (ncMo). The latter two subsets represent ~5–10% of all circulating monocytes in healthy adults.4, 5
All PB subsets of DCs and monocytes are functionally related cells, which share antigen-presentation functions, among other roles. Despite all of the above, the precise role of each subset and the exact relationship among them still remains to be fully understood. For example, the number of ncMo has consistently been found to be increased in response to microbial and/or inflammatory stimuli, and under these conditions they display phenotypic and functional features intermediate between those of cMo and DCs.6 Thus, further studies are still needed, including a more detailed analysis of the phenotypic profiles of PB APCs, to better understand their precise roles and functional interactions.
Since the first Human Leukocyte Differentiation Antigen (HLDA) Workshop and Conference that took place in 1982, efforts have been made to identify and characterise a large number of molecules present in hematopoietic cells. Indeed, the increase of knowledge regarding the phenotypic profiles of different subsets of Leukocytes has permitted a more rational understanding of their functions. Here, we analyse the reactivity of different subpopulations of human circulating PB DCs and monocytes for a large panel of new monoclonal antibodies. This work forms part of the Tenth Human Leukocyte Differentiation Antigen Workshop—DC section (HLDA10-DC task), which aims to contribute to a better understanding on their precise functional roles.
Results
Expression of C-type lectin receptors (CLRs) on different subsets of normal human PB APCs
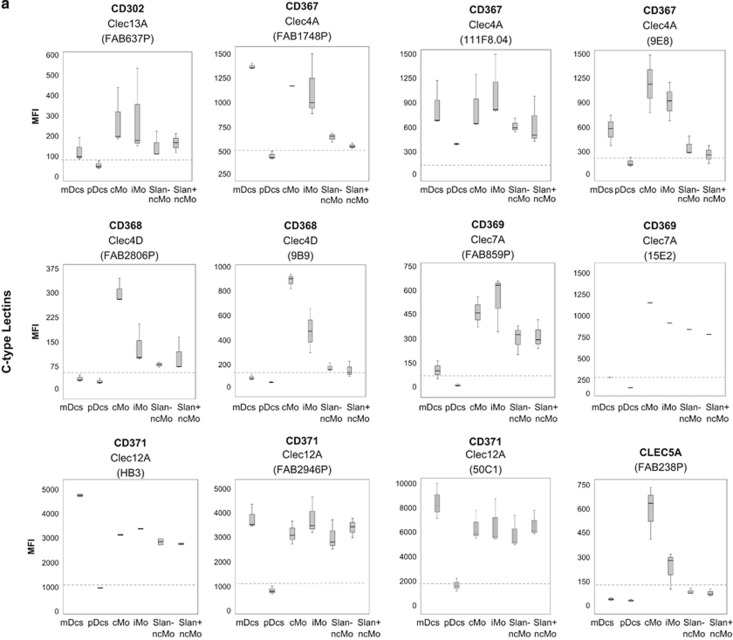
Overall, CLRs showed variable patterns of expression on the different subsets of PB APC investigated. However, this was not the case for pDCs, in which none of the CLRs analysed were found to be clearly positive on the cell surface membrane. Actually, for pDCs, only low levels of expression of CD367 (CLEC4A/DCIR) and CD371 (CLEC12A/MICL/CCL-1) were observed on the PB by using the 111F8.04 and 50C1 monoclonal antibodies (mAb), respectively (Figure 1a). Interestingly, all mDCs from the five samples analysed were found to be CD367+ and CD371++, the two molecules known to have inhibitory roles in the immune response.7 In addition, mDCs partially expressed the CD302 (CLEC13A/DCL-1) endocytic receptor (65% of CD302+ mDCs from one out of the three samples analysed) at low levels, corresponding mostly to activating CLRs (Figure 1a and Table 1), where the rest of the CLRs studied lacked reactivity (Figure 1a and Table 1). Conversely, monocytes expressed the majority of the CLRs analysed, including CD302, and both inhibitory (for example, CD367 and CD371) and activating (for example, CD368, CD369 and CLEC5A) CLRs, although they were negative for CD370, CLEC2D, CLEC8A and CLEC14A (Figure 1a and Table 1). Interestingly, the different subsets of PB monocytes showed distinct patterns of expression for the CLRs: (a) some molecules (CD302, CD367 and CD369) were expressed on all subsets of monocytes, but with progressively lower levels from cMo to SLAN− and SLAN+ ncMo; (b) other CLRs (for example, CD371) were expressed at high and similar levels in all subsets of monocytes; (c) CD368 and CLEC5A expression was restricted to some monocyte subsets, being clearly positive on cMo, while weakly expressed on iMo and negative in both SLAN+ and SLAN− ncMo.
Figure 1.
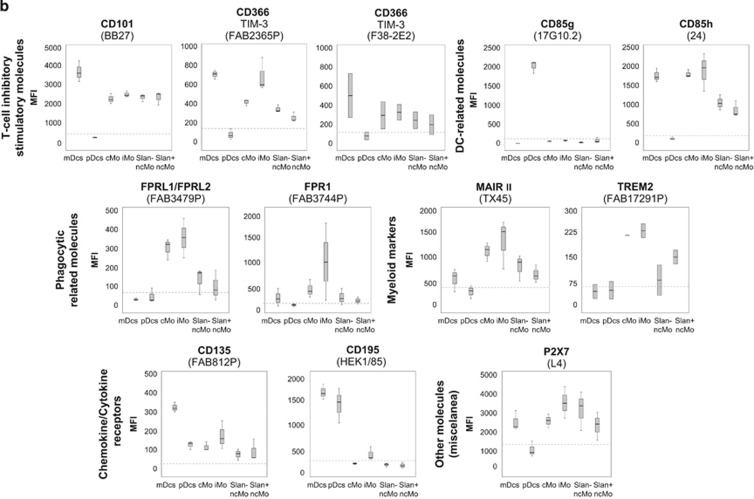
Immunophenotypical characterisation of the different subsets of circulating DCs and monocytes in normal PB. Panel a shows the expression of C-type lectins, and Panel b shows the expression of T-cell inhibitory/stimulatory molecules, DC-related markers, phagocytic-related molecules, other myeloid markers, chemokine/cytokine receptors and other cell surface molecules, in the different APC subpopulations of mDCs, pDCs, cMo, iMo, SLAN− ncMo and SLAN+ ncMo present in normal adult PB in basal conditions. Within each group of molecules, only those markers found to be positive in at least one cell subset in most samples stained are represented. They are classified according to the ‘CD' code and/or their alternative name, as well as the different monoclonal antibodies used (in brackets). Results are expressed as MFI (mean fluorescence intensity) for each whole cell population (arbitrary relative mean units, scaled from 0 to 262 144). Notched boxes represent 25th and 75th percentile values; the line in the middle and vertical lines correspond to the median value and the minimum-maximum values (without the extreme values and outliers), respectively. The dotted lines correspond to the cutoff value for positivity in each plot. The graph for CD369 (clone 15E2) includes data from one single case. Data represented in graphs for CD371 (clone HB3), CD366 (clone F38–2E2) and TREM2 correspond to two cases, not to five cases, owing to an insufficient amount of the corresponding antibody available. Abbreviations: mDCs: myeloid dendritic cells; pDCs: plasmacytoid dendritic cells; cMo: classical monocytes; iMo: intermediate monocytes; ncMo: non-classical monocytes; MFI: mean fluorescence intensity (arbitrary mean units scaled from 0 to 262 144).
Table 1. Biological and technical features of the HLDA10-DC monoclonal antibody reagents tested on normal human PB DC and monocyte subsets.
| Specificity | Target molecule | Clone | Antibody (workshop codes) | Reactivity |
|---|---|---|---|---|
| C-type lectins | CD302 | FAB637P | 10–54 | mDCd+, cMo+, iMo−/+, ncMod+ |
| CD367 | FAB1748P | 10–13 | mDC+++, cMo++, iMo+, ncMod+ | |
| CD367 | 111F8.04 | 10–71 | mDC++, pDCd+, cMo++, iMo++, ncMo+ | |
| CD367 | 9E8 | 10–72 | mDC+, cMo++, iMo++, ncMod+ | |
| CD368 | FAB2806P | 10–21 | cMo++ | |
| CD368 | 9B9 | 10–78 | cMo++, iMo+ | |
| CD369 | GE2 | 10–01 | − | |
| CD369 | FAB1859P | 10–35 | cMo+, iMo+, ncMod+ | |
| CD369 | 15 E 2 | 10–79 | cMo++, iMo++, ncMo+ | |
| CD370 | 8F9 | 10–02 | − | |
| CD370 | 9A11 | 10–09 | − | |
| CD370 | FAB6049P | 10–45 | − | |
| CD370 | 8F9 | 10–65 | iMo+ | |
| CD371 | HB3 | 10–17 | mDC+++, cMo++, iMo++, ncMo+ | |
| CD371 | FAB2946P | 10–51 | mDC++, cMo+, iMo++, ncMo+ | |
| CD371 | 50C1 | 10–73 | mDC+++, pDCd+, cMo++, iMo++, ncMo++ | |
| Clec2D | FAB3480P | 10–06 | − | |
| Clec5A | FAB238P | 10–28 | cMo+ | |
| Clec5C | FAB1900P | 10–31 | − | |
| Clec8A | FAB1798P | 10–40 | − | |
| Clec14A | FAB7436P | 10–57 | − | |
| T-cell co stimulatory inhibitory molecules | CD101 | BB27 | 10–34 | mDC+++, cMo++, iMo++, ncMo++ |
| CD245 | DY12 | 10–43 | − | |
| CD245 | DY35 | 10–48 | − | |
| CD273 | ANC8D12 | 10–61 | − | |
| CD365 | FAB1750P | 10–14 | − | |
| CD365 | 1D12 | 10–67 | − | |
| CD366 | FAB2365P | 10–24 | mDC++, cMo+, iMo++, ncMod+ | |
| CD366 | F38–2E2 | 10–75 | mDC−/+, cMod+, iMod+, ncMod+ | |
| ULBP-3 | FAB1517P | 10–52 | mDC+, iMo+ | |
| B7-H4 | MIH43 | 10–64 | − | |
| TSLP-R | 1B4 | 10–68 | − | |
| TIM4 | 9F4 | 10–81 | − | |
| DC-related markers | CD1a | 010e | 10–03 | − |
| CD1a | 0619 | 10–10 | − | |
| CD1b | O249 | 10–18 | − | |
| CD1c | L161 | 10–26 | − | |
| CD85g | 17G10.2 | 10–66 | pDC+++ | |
| CD85h | 24 | 10–74 | mDC++, cMo++, iMo++, ncMo+ | |
| CD209 | 118A8.05 | 10–83 | − | |
| Phagocytic-related molecules | FPRL1/FPRL2 | FAB3479P | 10–36 | cMo+, iMo++ |
| FPR1 | FAB3744P | 10–47 | mDCd+, cMod+, iMo−/+, SLAN−ncMod+ | |
| Calreticulin | FMU-CRT-2 | 10–23 | − | |
| Calreticulin | FMU-CRT-8 | 10–29 | − | |
| Calreticulin | FMU-CRT-17 | 10–42 | − | |
| Myeloid markers | MAIR II | TX45 | 10–80 | mDCd+, cMo++, iMo++, ncMod+ |
| TREM2 | FAB17291P | 10–07 | cMo++, iMo++, SLAN+ncMod+ | |
| FDF03 | 36H2 | 10–84 | - | |
| Chemokine and cytokine receptors | CD135 | FAB812P | 10–15 | mDC++ |
| CD195 | HEK/1/85 | 10–76 | mDC+++, pDC++, iMod+ | |
| CD213a2/ IL13Ra2 | FMU-IL-13RA2–7 | 10–30 | − | |
| CD213a2/ IL13Ra2 | FMU-IL-13RA2–8 | 10–37 | − | |
| CD213a2/ IL13Ra2 | FMU-IL-13RA2–14 | 10–41 | − | |
| Other molecules (miscelanea) | Tie-2 | FAB3131P | 10–56 | − |
| P2X7 | L4 | 10–70 | mDC+, cMo+, iMo++, SLAN− ncMo++, SLAN+ ncMo+ | |
| LPAP | CL3 | 10–04 | − | |
| LPAP | CL4 | 10–11 | − | |
| LPAP | CL7 | 10–19 | − | |
| FAT1 cadherin | FMU-FAT-6 | 10–08 | − | |
| Other molecules (miscelanea) | FAT1 cadherin | FMU-FAT1–7 | 10–16 | − |
| unknown | BGA69 | 10–38 | − | |
| Axl | FAB154P | 10–50 | − | |
| IL-1RAcP | AY19 | 10–53 | − | |
| Vimentin | SC5 | 10–55 | − | |
| unknown | MDR64 | 10–59 | − | |
| unknown | CMRF-44 | 10–82 | − | |
| GARP | ANC8C9 | 10–62 | − | |
| GARP | ANC10G10 | 10–63 | − | |
| unknown | CMRF-56 | 10–69 | − | |
| DORA | 104A10.01 | 10–77 | − | |
| Tetanus toxoid | CMRF-81 | 10–85 | − |
Abbreviations: cMo, classical monocytes; iMo, intermediate monocytes; mDCs, myeloid dendritic cells; ncMo, non-classical monocytes; pDCs, plasmacytoid dendritic cells;.
Reactivity for each cell population is displayed as superscripts; symbols used for the assessment of the expression are based on median values of fluorescence intensity:−(negative); −/+ (variable reactivity, from negative to positive); d+ (dim positive);+(positive); ++ (strong positive); +++ (very strong positive). Peripheral blood cell subsets other than APCs were used as internal controls, to establish the cutoff for positivity per marker/monoclonal antibody reactivity.
Expression of other phagocytosis-associated proteins, myeloid molecules and DC-related markers on different subsets of normal human PB APCs
The expression of the phagocytosis-associated proteins and myeloid molecules studied, other than the CLRs, was also restricted to monocytes and, to a lesser extent, to mDCs, whereas pDCs did not express any of these. Accordingly, mDCs were found to dimly express FPR1 and MAIR II and monocytes expressed FPRL1/FPRL2 and TREM2 (in addition to FPR1 and MAIR II), with progressively lower levels of expression from cMo/iMo to SLAN− and SLAN+ ncMo for all the above molecules, except TREM2 (Figure 1b and Table 1).
The CD85h immunoglobulin-like transcript-activating factor was expressed at high levels on mDCs, cMo and iMo, whereas the intensity of expression was markedly lower on ncMo, particularly in the SLAN+ subset, and negative on pDCs (Figure 1b). In contrast, CD85g, a molecule known to mediate signals that negatively modulate IFNα production, was selectively expressed by pDCs (Figure 1b).
The other molecules from these groups of markers were systematically absent on all of the APC subsets analysed (Table 1).
Expression of T-cell inhibitory/stimulatory molecules on different subsets of normal human PB APCs
Only a minor number of the T-cell-related molecules investigated were expressed by both mDCs and monocytes. These molecules were absent on pDCs: CD101, CD366 (TIM-3/Hepatitis A virus cellular receptor 2) and, to a lesser extent, ULBP-3 (Table 1). The two former molecules (CD101 and CD366) are known to exhibit modulatory/co-regulatory functions related to tolerance induction, and were found to be expressed at higher levels on mDCs vs monocytes. Within the monocyte population, expression of CD101 was similar among cMo, iMo and ncMo, whereas CD366 expression progressively decreased from cMo/iMo to SLAN− and SLAN+ ncMo (Figure 1b and Table 1).
Expression of chemokine and cytokine receptors on PB subsets of normal human APCs
CD135 (the receptor for Flt3L) and CD195 (CCR5) were both expressed by mDC, and the latter marker was also present on pDCs. In contrast, the different populations of monocytes were negative or weakly positive for CD135 and CD195 (Figure 1b). Likewise, none of the APC subsets analysed expressed the interleukin 13 receptor alpha2 (CD213a2) (Table 1).
Other molecules
None of the molecules included within the group ‘miscellanea' were expressed by the APC subsets studied (Table 1), with the exception of P2X7 (Figure 1b). Accordingly, this latter molecule was expressed at high levels in mDCs and at lower levels in the different monocyte subsets, but was absent in pDCs.
Discussion
Here we report on the result of an immunophenotypic analysis of a large panel of mAb directed against DC-related molecules, submitted to the HLDA10-DC workshop. Notably, the pattern of reactivity of the antibodies received by our lab was blindly tested on normal human PB subsets of DCs and monocytes, using non-cultured whole blood specimens from five healthy adults. Overall, our results show strikingly different patterns of expression of the different molecules analysed on the different subsets of PB APC. Common expression profiles were detected, although this depended on the functional group of the molecules studied and the specific subset of APC analysed.
CLRs comprise>1000 receptors, which are typically found on phagocytes. These receptors are essential for antigen capturing,8, 9 and the subsequent activation of intracellular signalling cascades, triggering numerous cellular and immunological responses for maintaining the homoeostasis of the immune system and controlling immune responses during infection. In addition, CLRs also have an important role in diseases and conditions such as autoimmunity, allergy and cancer.10 CLRs are associated with carbohydrate binding (that is, mannose, N-acetylglucosamine, L-fucose, glucose, galactose and N-acetyl-galactosamine, among other pathogen-related carbohydrates) and act as highly effective pattern-recognition receptors. This leads to the activation and/or modulation of immune functions upon encountering ligands from ‘non-self' (pathogen-associated molecular patterns), ‘damaged self' (damage-associated molecular patterns) or ‘altered self' (tumour-associated molecular patterns).11 CLRs are usually classified into two groups, based on their dominant signalling potential (activating receptors and inhibitory receptors), depending on whether they mediate cell activation or suppression of cellular activation, respectively.11 pDC did not express any of the CLRs studied, except for partial and low expression of the CD367 and CD371 inhibitory molecules. In contrast, mDCs expressed CD367 and high levels of CD371, together with partial and low expression of CD302. Interestingly, CD367 (CLEC4A/DCIR) and CD371 (CLC12A/MICL/CCL-1) contain ITIMs (immunoreceptor tyrosine-based inhibitory motifs) in their cytoplasmic tails and both act as inhibitory receptors, havig an important role in the interaction between innate and adaptive immunity during tolerogenic immune responses.11, 12 In addition, CD367 also drives antigen cross-presentation in DCs13, 14 and CD371 has been recently shown to sense dead cells, and therefore, regulates inflammation in response to cell death.12 These two inhibitory markers were also expressed by the different subsets of normal PB monocytes, but with a distinct immunophenotypic profile: mDCs and both cMo and iMo showed relatively high reactivity for CD367 expression, whereas ncMo expressed this marker at markedly lower levels (particularly on the subset of SLAN+ ncMo). Interestingly, SLAN+ ncMo have been found to induce potent proinflammatory Th1 and probably Th17 immune responses,15 which could be consistent with the low expression of the CD367 tolerogenic marker. Alternatively, the lower expression of CLEC4A/DCIR observed on ncMo may reflect a more mature phenotype for this cell subset as CD367 expression has been shown to decrease on DCs by signals that have been induced to mature (for example, CD40 ligand, lipopolysaccharide and tumor necrosis factor α). In turn, CD371 was highly expressed by mDCs and, to a lesser extent, by all monocyte subsets, potentially reflecting a shared role for both cMo and ncMo as sensors of necrotic/dead cells and/or as regulators of inflammatory responses.
In contrast to the inhibitory CLRs, expression of activating CLRs such as CD368, CD369, involved in cellular activation through the Syk kinase,16 and CLEC5A (a receptor associated with proinflammatory effects17) was restricted to monocytes, although higher levels of expression for the three molecules were observed on cMo and iMo vs ncMo. This suggests that these activating receptors might be associated with a specific monocyte-dependent immunological response. It is now known that CD368 (CLEC4D/Dectin-3) acts as a receptor for mycobacteria,18 CD369 (CLEC7A/Dectin-1) functions as a receptor specific for beta glucans from fungal cell walls19 and CLEC5A is a Dengue virus receptor,20 although little is known about the ligands for this receptor. However, independently of their pathogen partner, these three receptors are Syk-coupled CLRs and, when they are stimulated, all lead to hem-ITAM-mediated signalling cascades inside the cell, triggering phagocytosis, production of reactive oxygen species, and proinflammatory chemokines/cytokines.19, 21, 22, 23 The fact that ncMo had lower expression levels of activating Syk-coupled CLRs versus both cMo and iMo, could suggest that the former monocyte subset has less-phagocytic activity, together with a lower capacity for producing both reactive oxygen species and proinflammatory chemokines/cytokine. The results obtained regarding the pattern of expression of the CLEC13A/CD302 endocytic receptor, involved in phagocytosis, would further support this hypothesis.24
Expression of other phagocytic-related molecules (FPR1 and FPRL1/FPRL2), myeloid molecules (MAIR II and TREM2) and the CD85h DC-related marker here analysed, were restricted to monocytes and, to a lesser extent, also to mDCs. Although absent on pDCs, ncMo systematically had lower levels of expression for these proteins as compared to both cMo and iMo. Therefore, ncMo seem to represent a final stage of maturation when antigen presentation or phagocytosis events no longer take place, at least via CLRs, Ig-like V-type receptors (that is, TREM2) or G protein-coupled receptors (that is, FPR1). Overall these results could indicate that these cells may be more prone to undergo apoptosis and die. As expected, CD85g was selectively expressed by pDCs, as it mediates signals that negatively modulate IFNα production, CD85g expression potentially representing a homoeostatic regulatory mechanism on immature circulating PB pDC.25
The T-cell immunoglobulin mucin receptors (TIM) investigated in this study (TIM-1, initially cloned as hepatitis A virus cellular receptor 1, and TIM-3 or hepatitis A cellular receptor 2) were allocated as belonging to the CD365 and CD366 new CD codes. Both molecules are expressed on activated/effector T cells.26, 27 However, although the former molecule is able to associate with the TCR complex and mediate T-cell activation signals,28 the latter has been shown to inhibit Th1-mediated autoimmune and alloimmune responses and to promote immunological tolerance.26 In addition to their classical roles, TIM receptors have been recently suggested to mediate phagocytosis of apoptotic cells by APCs.29 In addition, none of the populations of APC analysed were found to express CD365, whereas both mDCs and monocytes (but not pDCs) expressed CD366, together with two other T-cell inhibitory molecules tested (CD101 and ULBP-3). The expression on mDCs was significantly higher than on the monocytes. Therefore, as for the above referred molecules, mDCs seemed to display a more pronounced inhibitory receptor molecule-associated profile than the monocytes.
As expected, the CD135 chemokine/cytokine receptor was highly expressed on mDCs, where it has a central role in cell proliferation and differentiation.30 In turn, CD195 was expressed both on mDCs and pDCs. Monocytes showed a lower expression for both markers, suggesting they both might play a preferential role on mDCs and pDCs immune-dependent responses. Finally, P2X7, a purinergic receptor for extracellular ATP that triggers downstream events such as activation of membrane metalloproteases and intracellular caspase activation leading to apoptosis,31 was highly expressed on iMo and SLAN- ncMo, further supporting the notion that ncMo could be an end-stage maturation subset of monocytes that could easily be triggered to undergo apoptosis. Interestingly, we observed slight differences within ncMo, according to the expression of SLAN (O-linked sugar modification of P-selectin glycoprotein ligand-1). Previous studies showed that antigens properly processed and presented by SLAN+ APCs were successfully taken up by CD4+ T cells.32 Moreover, SLAN− and SLAN+ ncMo have recently been studied as separate cell subpopulations based on their different gene expression profiles and immunophenotypes.33 SLAN+ cells were found to show a higher level of expression of the ubiquitin C transcript, which is associated with cell signalling, transcription, apoptosis, among other cellular functions. In addition, the immunophenotype of SLAN+ ncMo was characterised by a higher expression of tumor necrosis factor-α, CX3CR1, and lower CCR2, contrasting with the SLAN− ncMo expression profile for these molecules.33 These differences would suggest that SLAN+ and SLAN− ncMo would have different functional roles, also consistent with the phenotypic differences observed in our study.
In summary, we show that the patterns of expression of the different signalling molecules and receptors evaluated (and recognised by the APC-related mAb submitted to the HLDA10-DC workshop) vary substantially among the different subsets of DC and monocytes analysed. Such differences potentially reflect distinct pattern-recognition and signalling as well as different antigen uptake and phagocytic activities for the different subsets of APCs evaluated. Further investigations regarding the functional correlation of the immunophenotype reported here are required to better understand the precise and unique roles of the different subsets of PB.
Methods
Subjects and samples
A total of five healthy adult volunteers (three males and two females, with a median age of 42 years, ranging from 25 to 78 years) were included in this study. PB samples from the donors were collected into tubes containing K3-EDTA. The study was approved by the Ethics Committee of the University Hospital of Salamanca and performed following the Declaration of Helsinki. Each participant gave his/her informed consent prior to entering the study.
Flow cytometry immunophenotypic studies
A total of 72 new mAb were submitted to our laboratory (Cancer Research Center –CIC/IBMCC– and Service of Cytometry, Nucleus Platform for Research Support, University of Salamanca, Salamanca, Spain), being one of the contributors to the HLDA10-DC. These included mAb clustered in seven new CD that recognise cell surface molecules expressed by PB APC subsets. The remaining mAb tested, either recognised already established CDs or they required further validation. Details on the specificity, target molecules, clones, formats and workshop codes of the HLDA10-DC mAb tested in our laboratory are shown in Table 1.
Immunophenotypic studies were performed on fresh, whole PB samples, using eight-colour flow cytometry; antigen expression was analysed at the cell surface membrane level by staining ~5 × 105 cells in 100 μl per test. Seven common markers (backbone) were constantly present in all sample aliquots for identifying the different subsets of PB APC for which reactivity; for each mAb being studied, was tested. These included: anti-HLADR-Pacific Blue (PacB)-(Clone L243); CD45-Pacific Orange (PacO)-(Clone HI30); CD33-peridinin chlorophyll protein-cyanin 5.5 (PerCPCy5.5)-(Clone P67.6); CD123-allophycocyanin (APCy)-(Clone AC145); CD16-phycoerythrin-cyanine 7 (PECy7)-(Clone 3G8); CD14-APC-Hilite 7 (APCyH7)-(Clone MϕP9) and anti-SLAN-(Clone DD1), conjugated with either phycoerythrin (PE) or fluorescein isothiocyanate, depending on the conjugate format of the mAb reagent available for testing. Accordingly, anti-SLAN-fluorescein isothiocyanate was used for testing PE-conjugated primary mAb, whereas anti-SLAN-PE was used with primary mAb conjugated with Alexa Fluor 488, as well as with unconjugated antibodies (biotinylated, purified or unpurified reagents). The secondary antibody used against the primary mAb was fluorescein isothiocyanate-conjugated (see below). Backbone reagents were all purchased from Becton Dickinson Biosciences (BD; San Jose, CA, USA), except for the anti-HLADR-PacB (Biolegend, San Diego, CA, USA), CD45-PacO (Invitrogen, Carlsbad, CA, USA) and anti-SLAN reagents (Milteny Biotech, Cologne, Germany).
In all samples, a direct immunofluorescence stain-lyse-and-then-wash procedure was used for the 8-colour combination tubes including PE or Alexa Fluor 488-conjugated primary mAb, following well-established techniques, which have been described elsewhere.34 In the case of the eight-colour mAb combinations with unconjugated primary mAb, the following technique was strictly followed in order to avoid unspecific staining: first, indirect labelling was performed (using a fluorescein isothiocyanate-conjugated rabbit anti-mouse Ig F(ab)2 antibody fragments; Dako, Glostrup, Denmark), followed by two washing steps in phosphate buffered saline (PBS; pH=7.6) to remove the residual soluble secondary antibody; then, labelling with the directly conjugated mAb was carried out.35 In two additional normal PB samples, a cocktail of PECy7-conjugated anti-CD3 (Clone SK7; BD), plus anti-CD19 (Clone J3-119; Beckman Coulter, Brea, CA, USA), plus anti-CD56 (Clone N901 (NKH-1); Beckman Coulte) monoclonal antibodies, was added together with the backbone markers, to confirm that the strategy of identification of the different APC subsets in the remaining tubes was robust enough to unequivocally identify DC subsets without the presence of T-, B- and NK-cell-related markers (Supplementary Figure 1).
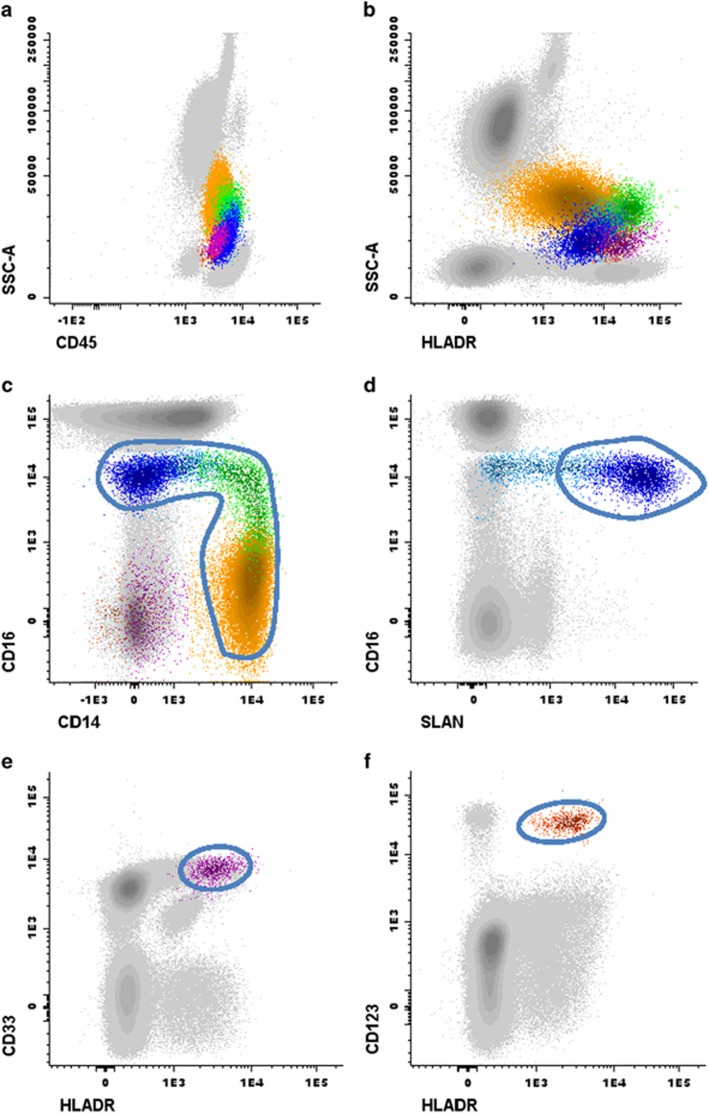
Immediately after the completion of the sample preparation, the samples were acquired in a FACSCanto II flow cytometer (BD), using the FACSDiva software programme (BD). For data analysis, the INFINICYT software programme (Cytognos SL, Salamanca, Spain) was used. The different DC and monocyte cell subsets, for which reactivity against the HLDA10-DC mAb was assessed, were identified following the gating strategy illustrated in Figure 2. The minimum number of clustered events considered to constitute an APC cell population to be accurately characterised was 50.36 In addition, T cells were also identified and used as internal controls (data not shown).
Figure 2.
Gating strategy for the identification of the different subsets of circulating monocytes and dendritic cells (DCs) in a representative peripheral blood (PB) sample from a healthy donor. Coloured events in Panels a and b correspond to all the antigen-presenting cell (APC) subsets under study (that is, total PB monocytes and DCs), firstly gated from leukocytes (grey dots) according to their typical expression pattern of sideward scatter (SSCint) together with CD45 (Panel a) and HLADR (Panel b). Next, monocytes were identified within total APCs, based on their expression of CD33+/++ (not shown), together with their expression profile for CD14 and CD16 (coloured dots included in the gate in Panel c). Their differential expression of CD14 and CD16 further allowed the identification of three major subpopulations of monocytes (Panel c): CD14++/CD16− classical monocytes (cMo), CD14+/lo/CD16lo intermediate monocytes (iMo) and CD14lo/−/CD16+non-classical monocytes (ncMo). Within this latter monocytic subset, both SLAN− and SLAN+ ncMo were identified, as shown in Panel d: the region including SLAN+ ncMo (blue dots included in the gate) was set based on internal negative controls for SLAN (grey dots in Panel d). Myeloid DCs (mDCs, Panel e) were identified based on their high expression of CD33 and HLADR in the absence of CD14 and CD16, to exclude monocytes, while plasmacytoid DCs (pDCs, Panel f) were identified by the strong CD123 expression and positivity for HLADR, also in the absence of CD14 and CD16. Colour codes: yellow dots correspond to cMo, iMo are painted in green, whereas blue dots correspond to ncMo; dark purple and orange dots represent mDC and pDC, respectively.
Statistical methods
For each parameter under study, median, range and the 25th and 75th percentiles were calculated using the SPSS.21 software programme (IBM SPSS Statistics, IBM, Armonk, NY, USA).
Acknowledgments
This work has been partially supported by the following grants: RTICC RD12/0036/0048-FEDER and PI13/01412-FEDERfrom the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Madrid, Spain.
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Clinical and Translational Immunology website (http://www.nature.com/cti)
Supplementary Material
References
- Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology 2013; 140: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida J, Bueno C, Alguero MC, Sanchez ML, de Santiago M, Escribano L et al. Comparative analysis of the morphological, cytochemical, immunophenotypical, and functional characteristics of normal human peripheral blood lineage(−)/CD16(+)/HLA-DR(+)/CD14(−/lo) cells, CD14(+) monocytes, and CD16(−) dendritic cells. Clin Immunol 2001; 100: 325–338. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol 2015; 15: 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116: e74–e80. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5: 953–964. [DOI] [PubMed] [Google Scholar]
- Hofer TP, Zawada AM, Frankenberger M, Skokann K, Satzl AA, Gesierich W et al. Characterization of subsets of the CD16-positive monocytes: impact of granulomatous inflammation and M-CSF-receptor mutation. Blood 2015; 126: 2601–2610. [DOI] [PubMed] [Google Scholar]
- Yan H, Kamiya T, Suabjakyong P, Tsuji NM. Targeting C-Type lectin receptors for cancer immunity. Front Immunol 2015; 6: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa N. Dendritic cell immunoreceptors: C-type lectin receptors for pattern-recognition and signaling on antigen-presenting cells. J Dermatol Sci 2007; 45: 77–86. [DOI] [PubMed] [Google Scholar]
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J 2005; 272: 6179–6217. [DOI] [PubMed] [Google Scholar]
- Kerscher B, Willment JA, Brown GD. The Dectin-2 family of C-type lectin-like receptors: an update. Int Immunol 2013; 25: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol 2015; 32: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K, Castineiras-Vilarino M, Hockendorf U, Hannesschlager N, Lemeer S, Kupka D et al. Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity 2014; 40: 389–399. [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Benitez-Ribas D, Tacken PJ, Punt CJ, Figdor CG, de Vries IJ et al. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood 2008; 111: 4245–4253. [DOI] [PubMed] [Google Scholar]
- Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJ, Figdor CG et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol 2009; 85: 518–525. [DOI] [PubMed] [Google Scholar]
- Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res 2012; 53: 41–57. [DOI] [PubMed] [Google Scholar]
- Graham LM, Gupta V, Schafer G, Reid DM, Kimberg M, Dennehy KM et al. The C-type lectin receptor CLECSF8 (CLEC4D) is expressed by myeloid cells and triggers cellular activation through Syk kinase. J Biol Chem 2012; 287: 25964–25974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Dominguez E, Samaniego R, Flores-Sevilla JL, Campos-Campos SF, Gomez-Campos G, Salas A et al. CD163L1 and CLEC5A discriminate subsets of human resident and inflammatory macrophages in vivo. J Leukoc Biol 2015; 98: 453–466. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, Marakalala MJ, Hoving JC, van Laarhoven A, Drummond RA, Kerscher B et al. The C-type lectin receptor CLECSF8/CLEC4D is a key component of anti-mycobacterial immunity. Cell Host Microbe 2015; 17: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 2003; 197: 1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ et al. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 2013; 121: 95–106. [DOI] [PubMed] [Google Scholar]
- Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 2006; 6: 33–43. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 2005; 106: 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun WK, Lu X, Li X, Sun QY, Su X, Song Y et al. Dectin-1 is inducible and plays a crucial role in Aspergillus-induced innate immune responses in human bronchial epithelial cells. Eur J Clin Microbiol Infect Dis 2012; 31: 2755–2764. [DOI] [PubMed] [Google Scholar]
- Kato M, Khan S, d'Aniello E, McDonald KJ, Hart DN. The novel endocytic and phagocytic C-Type lectin receptor DCL-1/CD302 on macrophages is colocalized with F-actin, suggesting a role in cell adhesion and migration. J Immunol 2007; 179: 6052–6063. [DOI] [PubMed] [Google Scholar]
- Tavano B, Galao RP, Graham DR, Neil SJ, Aquino VN, Fuchs D et al. Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1.24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood Leukocytes. J Immunol 2013; 190: 2622–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JV, Colgan JD. Regulation of T cell responses by the receptor molecule Tim-3. Immunol Res 2014; 59: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennert PD. Novel roles for TIM-1 in immunity and infection. Immunol Lett 2011; 141: 28–35. [DOI] [PubMed] [Google Scholar]
- Binne LL, Scott ML, Rennert PD. Human TIM-1 associates with the TCR complex and up-regulates T cell activation signals. J Immunol 2007; 178: 4342–4350. [DOI] [PubMed] [Google Scholar]
- Brooks CR, Yeung MY, Brooks YS, Chen H, Ichimura T, Henderson JM et al. KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J 2015; 34: 2441–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandasabapathy N, Breton G, Hurley A, Caskey M, Trumpfheller C, Sarma P et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant 2015; 50: 924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu BJ, Zhang WY, Bendall LJ, Chessell IP, Buell GN, Wiley JS. Expression of P2X(7) purinoceptors on human lymphocytes and monocytes: evidence for nonfunctional P2X(7) receptors. Am J Physiol Cell Physiol 2000; 279: C1189–C1197. [DOI] [PubMed] [Google Scholar]
- Bippes CC, Feldmann A, Stamova S, Cartellieri M, Schwarzer A, Wehner R et al. A novel modular antigen delivery system for immuno targeting of human 6-sulfo LacNAc-positive blood dendritic cells (SLANDCs). PLoS One 2011; 6: e16315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer TP, Zawada AM, Frankenberger M, Skokann K, Satzl AA, Gesierich W et al. SLAN-defined subsets of CD16-positive monocytes: impact of granulomatous inflammation and M-CSF receptor mutation. Blood 2015; 126: 2601–2610. [DOI] [PubMed] [Google Scholar]
- Kalina T, Flores-Montero J, van der Velden VH, Martin-Ayuso M, Bottcher S, Ritgen M et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia 2012; 26: 1986–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin L, Almeida J, Hernandez-Campo PM, Sanchez ML, Lecrevisse Q, Orfao A. Immunophenotypical, morphologic, and functional characterization of maturation-associated plasmacytoid dendritic cell subsets in normal adult human bone marrow. Transfusion 2009; 49: 1692–1708. [DOI] [PubMed] [Google Scholar]
- Rawstron AC, Fazi C, Agathangelidis A, Villamor N, Letestu R, Nomdedeu J et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: an European Research Initiative on CLL study. Leukemia 2016; 30: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.