Abstract
Ketotifen has recently been reported to inhibit the growth of both asexual and sexual malaria parasites. A parasite transporter, PfgABCG2, has been implicated in its mechanism of action. Human dihydrofolate reductase (hDHFR) is the most commonly used selectable marker to create transgenic Plasmodium falciparum cell lines. Growth assays using transgenic P. falciparum parasites with different selectable markers revealed that the presence of hDHFR rather than the absence of PfgABCG2 is responsible for a shift in the parasite's sensitivity to ketotifen. Employing a range of in vitro assays and liquid chromatography-mass spectrometry we show that ketotifen influences hDHFR activity, but it is not metabolised by the enzyme. Our data also highlights potential pitfalls when functionally characterising transgenic parasites.
Keywords: hDHFR, Ketotifen fumarate, Plasmodium falciparum, Malaria, Selectable marker, Transfection, Folate
Graphical abstract
Highlights
-
•
The use of hDHFR as selectable marker alters sensitivity of P. falciparum to ketotifen.
-
•
Anti-malarial mechanism of ketotifen is unrelated to expression of parasite gABCG2.
-
•
hDHFR interacts with ketotifen.
-
•
Folate concentration used in P. falciparum culture limits parasite growth in vitro.
1. Introduction
Ketotifen, a tricyclic antihistamine, suppresses the proliferation of rodent malaria parasites in vivo (Milner et al., 2012) and human malaria parasites (P. falciparum) in vitro (Eastman et al., 2013). Both ketotifen and its metabolite norketotifen kill schizonts and liver-stage P. berghei parasites (Milner et al., 2012). Ketotifen and other antihistamines have also been shown to reverse chloroquine resistance in P. falciparum (Basco et al., 1991) and in P. yoelii (Singh and Puri, 2000). The potential of ketotifen as an antimalarial is therefore of significant interest.
Dihydrofolate reductase (DHFR) converts dihydrofolate (DHF) into tetrahydrofolate (THF) in the folate pathway. This pathway is essential for DNA synthesis and amino acid metabolism in the parasite (Hyde, 2005) and DHFR inhibitors such as pyrimethamine have been widely used for the treatment of malaria. Another antifolate, WR99210, inhibits P. falciparum growth by inhibiting P. falciparum DHFR (Kinyanjui et al., 1999) and is used as a selectable marker for the transfection of P. falciparum. Human dihydrofolate reductase (hDHFR) is insensitive to WR99210 (De Koning-Ward et al., 2000, Fidock and Wellems, 1997) and parasites transfected with a plasmid containing the gene encoding hDHFR are resistant to WR99210 and survive WR99210-selection pressure.
Eastman et al. reported that disruption of the gene encoding the ABC-transporter PfgABCG2 reduces the sensitivity of asexual blood-stage 3D7 parasites to a range of tricyclic compounds, including ketotifen (Eastman et al., 2013). From this, the authors concluded that PfgABCG2 plays a role in the parasite response to these compounds. In this study, we investigated the sensitivity to ketotifen of an independently-generated 3D7 parasite line lacking PfgABCG2 (Tran et al., 2014), comparing it with that of a number of other parasite lines.
2. Material and methods
2.1. Parasites
Transfections were performed on chloroquine sensitive 3D7 wild-type parasites as previously described with some modifications (Fidock and Wellems, 1997; Rug and Maier, 2013). Six different lines were used (Table S1): (I) wild-type parasites; (II) parasites containing an episomal human dihydrofolate reductase (hDHFR) selection cassette (hDHFR (e)) (Tran et al., 2014) ((e) referring to an episomal locus, (i) to integration into the genome); (III) PfgABCG2 knock-out parasites generated by genomic integration of the hDHFR selection cassette into the gene encoding PfgABCG2 (ΔPfgABCG2-hDHFR (i)) (Tran et al., 2014); (IV) ΔPfgABCG2 parasites complemented with an episomal copy of gABCG2 (ΔPfgABCG2-hDHFR (i)/PfgABCG2-BSD (e)) (Tran et al., 2014); (V) PFD1170c knock-out parasites (ΔPFD1170c-hDHFR (i)) (Nguyen et al., manuscript in preparation), generated by genomic integration of the hDHFR selection cassette into the gene encoding PFD1170c (an exported protein unrelated to PfgABCG2; see Supplementary Fig. S1 for the integration strategy); and (VI) PF14_0124-RFP-BSD (e) parasites, containing an episomal plasmid pRREP-4/PF14_0124 (see Supplementary Fig. S2 for a schematic representation of the episomal plasmid) expressing both Aspergillus terreus blasticidin-S deaminase (BSD) and P. falciparum actin II (encoded by PF14_0124) fused to red fluorescent protein (BSD (e)). BSD confers resistance to blasticidin-S (Yamaguchi et al., 1965, Mamoun et al., 1999) and the gene encoding BSD thereby serves as a selectable marker.
The parasites were cultured using standard methods (Trager and Jensen, 1976) with slight modifications (Maier and Rug, 2013). Parasites and erythrocytes were grown in RPMI 1640-Hepes medium with Glutamax (ThermoFisher Scientific #72400120) supplemented with 10 mM glucose (Sigma), 480 μM hypoxanthine (Sigma), 20 μg/ml gentamicin (ThermoFisher Scientific), 0.25% (w/v) Albumax II (ThermoFisher Scientific), and 5% heat inactivated human serum. The use of human erythrocytes was approved by the ANU Human Ethics committee 2011/266. Ring-stage parasites were synchronized by sorbitol treatment (Lambros and Vanderberg, 1979).
2.2. In vitro proliferation assay
Synchronous ring-stage cultures (100 μL, 0.2% parasitemia, 2% haematocrit) were incubated with ketotifen fumarate (Sigma) at a range of concentrations for 72 h at 37 °C, after which parasitised erythrocytes were stained with 1 μM SYTO16 (Invitrogen) at 37 °C for 30 min, then counted using a flow cytometer (BD LSR II, BD Biosciences) on the FITC channel (488/525 nm). Each parasite cell line was assayed in triplicate and 50,000 events (total RBCs) were counted for each sample and processed using FlowJo v887 software. The drug concentrations were log-transformed, the parasite number was normalised relative to the percentage of no-drug control and sigmoidal curve-fitted. The drug responses were graphed using GraphPad Prism 5.0 and the 50% inhibitory concentrations (IC50) were calculated and compared using best-fit values and t-test.
2.3. Biochemical assays
The possible metabolism of ketotifen by hDHFR was investigated using liquid chromatography-mass spectrometry, as described by Chooi et al. (2015).
The effect of ketotifen on the conversion of DHF to THF by recombinant hDHFR was investigated using an in vitro assay (Bailey and Ayling, 2009, Loveridge et al., 2009). Reactions were carried out at 27 °C in a flat bottom 96-well plate containing 0.1 M K3PO4, 0.1 M NaCl, pH 7.0; 0.1 mM NADPH2 (Sigma), 50 mM 2-mercaptoethanol, 100 nM purified recombinant hDHFR (Creative Biomart) and a range of concentrations of ketotifen fumarate (Sigma). The reduction of NADPH2 to NADP+ was measured at OD340.
3. Results and discussion
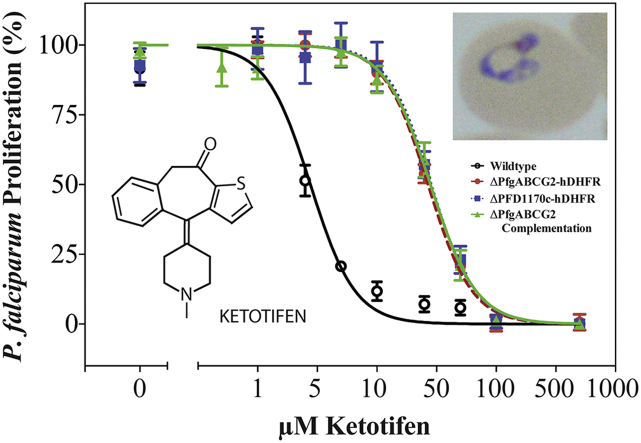
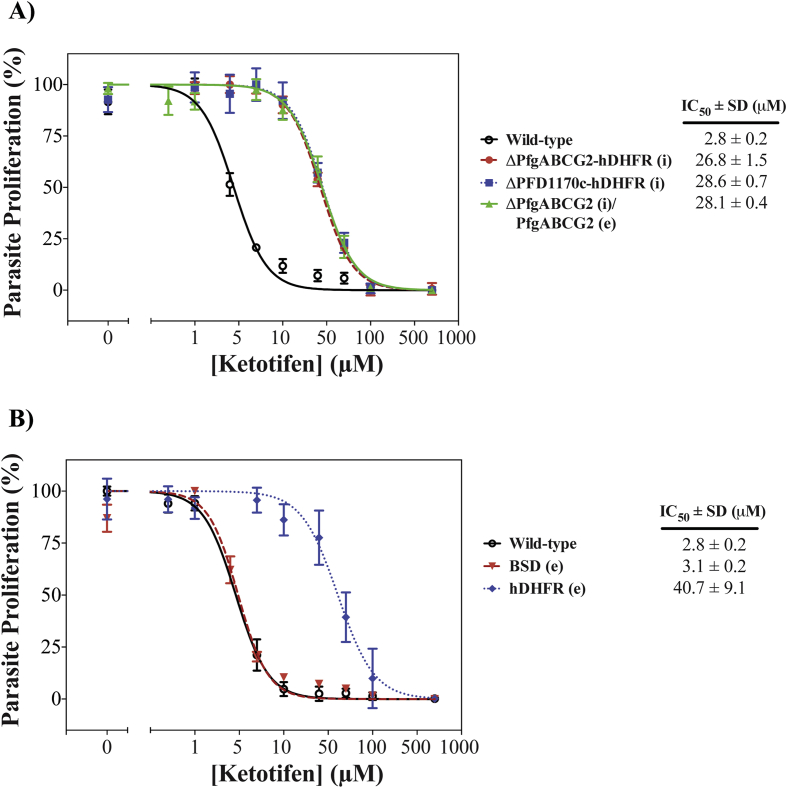
In order to compare the ketotifen-sensitivity of parasites with or without PfgABCG2 we performed an in vitro proliferation assay (Fig. 1A). As has been reported previously (Eastman et al., 2013), parasites in which the PfgABCG2 gene was disrupted showed a significant reduction in ketotifen-sensitivity, relative to parental 3D7 parasites. The IC50 (i.e. the concentration at which parasite proliferation was reduced by 50%) for inhibition of the proliferation of ΔPfgABCG2-hDHFR (i) parasites by ketotifen was ten-fold higher than that for the parental 3D7 line (p < 0.001, unpaired Student's t-test). However, a similar ten-fold increase in the IC50 for inhibition of proliferation by ketotifen was seen also for a cell-line in which an entirely unrelated protein (PFD1170c) was knocked out using the same selectable marker (hDHFR) (ΔPFD1170c-hDHFR (i)). Furthermore, the sensitivity of the ΔPfgABCG2-hDHFR (i) parasites to ketotifen was not restored by transfection with a functional episomal copy of the PfgABCG2 gene under the influence of the endogenous promoter (ΔPfgABCG2-hDHFR (i)/PfgABCG2-BSD (e)). These parasites retain the hDHFR selection cassette in the disrupted endogenous PfgABCG2 locus. These findings are consistent with the expression of the selectable marker (hDHFR), rather than disruption of either of the two unrelated genes, being responsible for the observed altered ketotifen sensitivity.
Fig. 1.
Expression of the selectable marker hDHFR antagonises the inhibition of P. falciparum parasite proliferation by ketotifen. The proliferation of asexual blood-stage parasites in the presence of a range of ketotifen concentrations was assessed over 72 h. Selectable marker cassettes integrated into the genome are indicated by (i), selectable marker cassettes located on episomal plasmids are indicated by (e). A) Sensitivity of parasites to ketotifen is independent of the presence of PfgABCG2. Wild-type: 3D7 parental line; ΔPfgABCG2-hDHFR (i): PfgABCG2 knock-out parasites expressing the hDHFR selectable marker. ΔPFD1170c-hDHFR (i): PFD1170c knock-out parasites expressing the hDHFR selectable marker. ΔPfgABCG2-hDHFR (i)/PfgABCG2-BSD (e): PfgABCG2 knock-out parasites expressing the hDHFR selectable marker and PfgABCG2 expressed from an episomal plasmid, under the control of the PfgABCG2 promoter, using blasticidin-S deaminase (BSD) as the selectable marker. B) Sensitivity to ketotifen is modulated by the presence of hDHFR. BSD (e): parasites expressing actin II (tagged by red fluorescent protein) and the blasticidin-S deaminase (BSD) selectable marker from an episomal plasmid. hDHFR (e): parasites expressing the hDHFR selectable marker from an episomal plasmid. Mean and standard deviation (SD) values of biological triplicates are shown.
Next, we investigated the effect of hDHFR and a different selectable marker (blasticidin-S deaminase (BSD)) on the parasite's response to ketotifen (Fig. 1B and S2). When parasites were transfected with an episomal plasmid containing the hDHFR selection cassette (hDHFR (e)), we detected a >10-fold decrease in ketotifen sensitivity, similar to what was observed for the other cell lines containing hDHFR (p < 0.001, unpaired Student's t-test). However, when an episomal plasmid containing BSD was transfected (BSD (e)), the parasites maintained the same sensitivity to ketotifen as the parental wild-type cells (p = 0.1481, unpaired Student's t-test). These data, too, are consistent with expression of hDHFR causing decreased ketotifen-sensitivity.
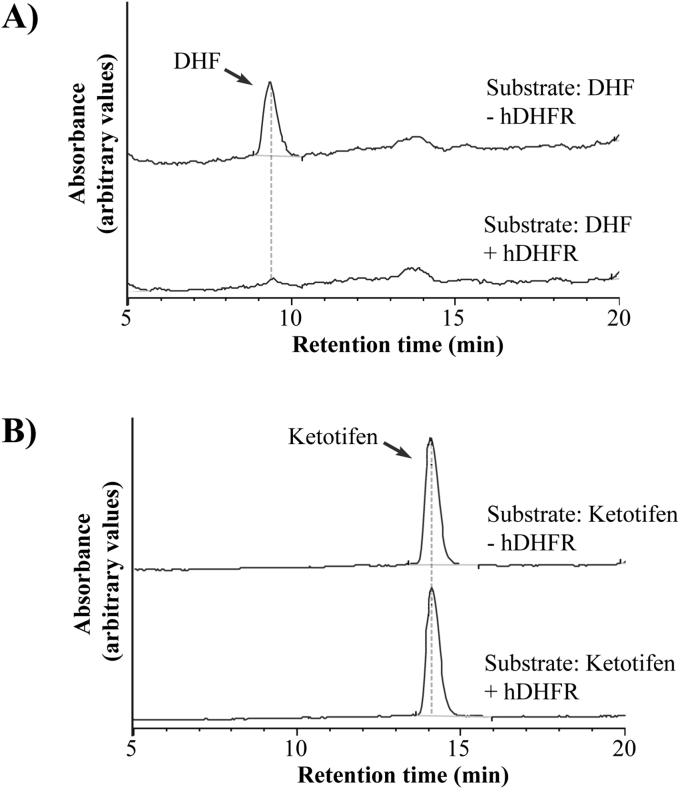
One potential explanation for the effect of hDHFR on the ability of ketotifen to inhibit parasite growth is that hDHFR metabolises ketotifen, thereby reducing its concentration in the culture medium. To explore this possibility we compared the natural metabolism of DHF by hDHFR to the effect of hDHFR on ketotifen, using liquid chromatography-mass spectrometry (Fig. 2), as described by Chooi et al. (2015). In the course of a 10 min incubation, the recombinant hDHFR metabolized DHF, as expected (Fig. 2A). Under the same conditions, however, ketotifen remained unaltered, consistent with it not being metabolized by hDHFR (Fig. 2B).
Fig. 2.
Recombinant hDHFR does not modify ketotifen in an in vitro enzyme assay. DHF or ketotifen fumarate (100 μM) was incubated in the presence or absence of recombinant hDHFR (100 nM) for 10 min at 27 °C. The LC/MS trace shown is representative of those obtained in three independent experiments. A) In the presence of recombinant hDHFR, the substrate 7,8-dihydrofolate (DHF), was broken down. B) Ketotifen is not broken down in the presence of recombinant hDHFR.
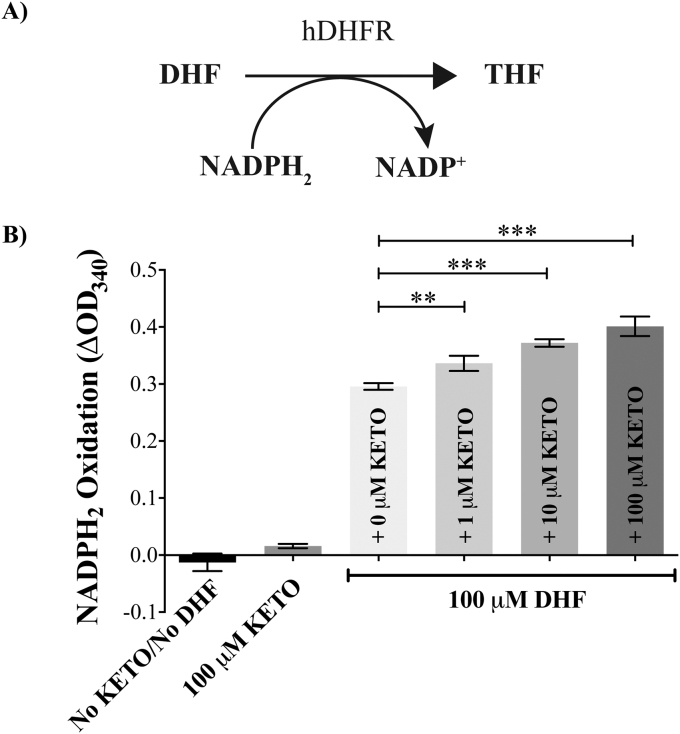
Since hDHFR did not metabolise ketotifen, we used an alternative approach to investigate the possibility of an interaction between the two molecules. For this we used recombinant hDHFR in conjunction with an in vitro assay for the hDHFR-mediated conversion of DHF to THF (Fig. 3A) (Bailey and Ayling, 2009). The oxidation of NADPH2 to NADP+ was measured at OD340. As the ketotifen concentration was increased from 0 to 100 μM, hDHFR activity increased (p > 0.001) (Fig. 3B), consistent with there being an interaction between ketotifen and hDHFR.
Fig. 3.
Ketotifen increases the activity of hDHFR. A) Schematic outline of the conversion of 7,8-dihydrofolate (DHF) to 5,6,7,8-tetrahydrofolate (THF) through the enzymatic action of human dihydrofolate-reductase (hDHFR). The reaction involves the oxidation of NADPH2, which can be measured at OD340. B) Ketotifen (KETO) caused a significant concentration-dependent increase in the conversion of DHF to THF (as measured by oxidation of NADPH2 over 10 min) by recombinant hDHFR. The NADPH2 oxidation is indicative of hDHFR activity. The bars indicate mean values at equilibrium of biological triplicates and are shown ± standard deviation. *** (p < 0.001) and ** (p < 0.01) indicate a statistically significant difference in hDHFR activity in the presence of ketotifen, relative to the activity measured in the absence of ketotifen (Student's unpaired t-test). Time courses for the conversion of DHF to THF are shown in Supplementary Fig. S3.
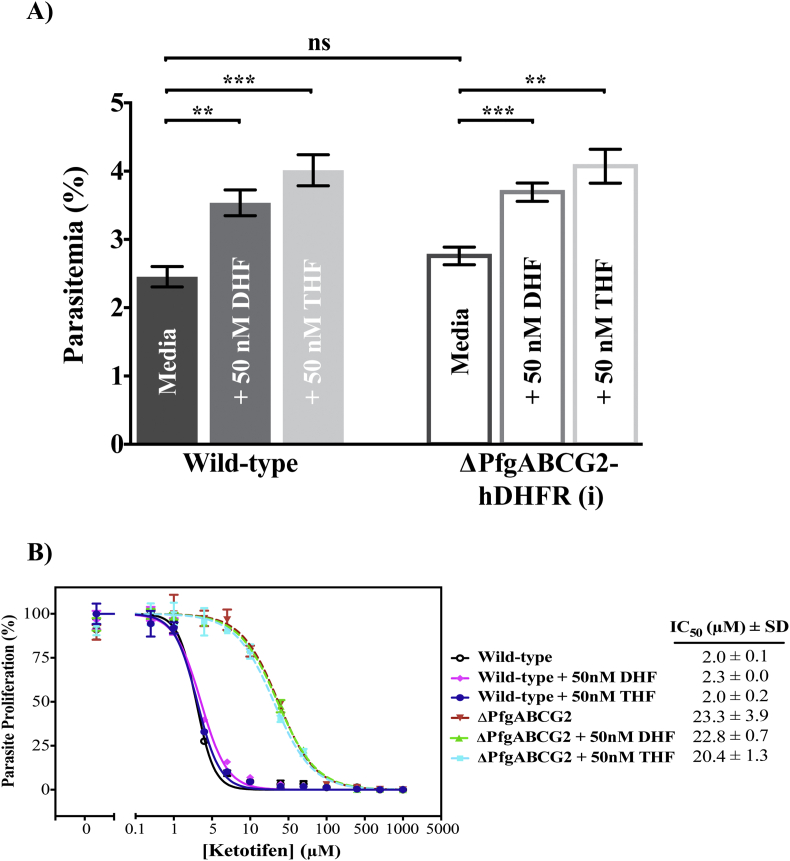
The possibility that hDHFR counters the growth-inhibitory effect of ketotifen by increasing the intracellular concentration of THF was explored by testing the effect of exogenously-supplied DHF and THF on parasite growth. The addition of 50 nM DHF or THF to the culture medium significantly increased the proliferation of P. falciparum parasites (p = 0.0015, 0.0006 respectively, unpaired Student's t-test) (Fig. 4A), indicating (i) that the supplemented DHF/THF (or metabolites thereof) are taken up by the parasites and (ii) that the folate concentration is growth-limiting under our experimental conditions. However, the addition of exogenous DHF or THF was without significant effect (p = 0.6632, 0.8020; unpaired Student's t-test) on the growth-inhibitory effect of ketotifen (Fig. 4B). These data argue against the hypothesis that the protective effect of hDHFR is a consequence of increased THF levels within the parasite.
Fig. 4.
Addition of DHF or THF to the culture medium increases the proliferation of P. falciparum, but does not alter the sensitivity to ketotifen. A) Growth of wild-type and ΔPfgABCG2-hDHFR (i) P. falciparum parasites over 72 h, measured in the presence of increasing concentrations of DHF or THF and in the absence of ketotifen. Mean and standard deviation (SD) values of biological triplicates are shown. ***, p < 0.001; **, p < 0.01; ns, not significant (Student's unpaired t-test). B) Proliferation of asexual blood-stage parasites measured over 72 h in the presence of a range of ketotifen concentrations, and in the presence or absence of 50 nM DHF or THF. “ΔPfgABCG2″ refers to ΔPfgABCG2-hDHFR (i) parasites. Mean and standard deviation (SD) values of biological triplicates are shown.
To test the possibility that hDHFR antagonizes the antiplasmodial activity of ketotifen by acting as a ‘sink’ and thereby reducing its effective concentration, we measured the antiplasmodial activity of ketotifen in the presence and absence of extracellular hDHFR (100 nM). The addition of hDHFR to the medium did not influence the antiplasmodial activity of ketotifen (Fig. S4), indicating that, under these conditions at least, hDHFR does not act as a substantial sink for ketotifen.
In summary, the observation of decreased ketotifen sensitivity of parasites in which the gene encoding the transporter PfgABCG2 is disrupted (Eastman et al., 2013) is due not to the absence of the protein but, rather, to the presence of the selection marker, hDHFR. We have some evidence for an interaction between hDHFR and ketotifen including a moderate increase in hDHFR activity in the presence of ketotifen; however the nature and significance of this interaction is unknown. Our observations serve as a reminder of the potential pitfalls associated with interpreting functional assays when selectable markers are used.
Conflicts of interest
The authors have no conflicts of interest concerning the work reported in this paper.
Acknowledgements
The authors are grateful to Uyen Nguyen (Australian National University) for providing the 3D7 ΔPFD1170c-hDHFR (i) parasites, to Yit-Heng Chooi (Australian National University) for expert technical assistance with the LC/MS analysis, and to the Australian Red Cross for supplying human red blood cells and serum.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2016.09.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Bailey S.W., Ayling J.E. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc. Natl. Acad. Sci. 2009;106:15424–15429. doi: 10.1073/pnas.0902072106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco L.K., Ringwald P., Le Bras J. Chloroquine-potentiating action of antihistaminics in Plasmodium falciparum in vitro. Ann. Trop. Med. Parasitol. 1991;85:223–228. doi: 10.1080/00034983.1991.11812549. [DOI] [PubMed] [Google Scholar]
- Chooi Y.H., Krill C., Barrow R.A., Chen S., Trengove R., Oliver R.P., Solomon P.S. An In planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Appl. Environ. Microbiol. 2015;81:177–186. doi: 10.1128/AEM.02745-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koning-Ward T.F., Fidock D.A., Thathy V., Menard R., Van Spaendonk R.M.L., Waters A.P., Janse C.J. The selectable marker human dihydrofolate reductase enables sequential genetic manipulation of the Plasmodium berghei genome. Mol. Biochem. Parasitol. 2000;106:199–212. doi: 10.1016/s0166-6851(99)00189-9. [DOI] [PubMed] [Google Scholar]
- Eastman R.T., Pattaradilokrat S., Raj D.K., Dixit S., Deng B., Miura K., Yuan J., Tanaka T.Q., Johnson R.L., Jiang H., Huang R., Williamson K.C., Lambert L.E., Long C., Austin C.P., Wu Y., Su X.Z. A class of tricyclic compounds blocking malaria parasite oocyst development and transmission. Antimicrob. Agents Chemother. 2013;57:425–435. doi: 10.1128/AAC.00920-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock D.A., Wellems T.E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J.E. Exploring the folate pathway in Plasmodium falciparum. Acta Trop. 2005;94:191–206. doi: 10.1016/j.actatropica.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinyanjui S.M., Mberu E.K., Winstanley P.A., Jacobus D.P., Watkins W.M. The antimalarial triazine WR99210 and the prodrug PS-15: folate reversal of in vitro activity against Plasmodium falciparum and a non-antifolate mode of action of the prodrug. Am. J. Trop. Med. Hyg. 1999;60:943–947. doi: 10.4269/ajtmh.1999.60.943. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- Loveridge E.J., Rodriguez R.J., Swanwick R.S., Allemann R.K. Effect of dimerization on the stability and catalytic activity of dihydrofolate reductase from the hyperthermophile Thermotoga maritima. Biochemistry. 2009;48:5922–5933. doi: 10.1021/bi900411a. [DOI] [PubMed] [Google Scholar]
- Maier A.G., Rug M. In vitro culturing Plasmodium falciparum erythrocytic stages. In: Ménard R., editor. Malaria : Methods and Protocols. second ed. Humana Press; 2013. pp. 3–15. [DOI] [PubMed] [Google Scholar]
- Mamoun C.B., Gluzman I.Y., Goyard S., Beverley S.M., Goldberg D.E. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8716–8720. doi: 10.1073/pnas.96.15.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner E., Sousa J., Pybus B., Auschwitz J., Caridha D., Gardner S., Grauer K., Harris E., Hickman M., Kozar M.P., Lee P., Leed S., Li Q., Melendez V., Moon J., Ngundam F., O'Neil M., Parriott S., Potter B., Sciotti R., Tangteung A., Dow G.S. Ketotifen is an antimalarial prodrug of norketotifen with blood schizonticidal and liver-stage efficacy. Eur. J. Drug Metabolism Pharmacokinet. 2012;37:17–22. doi: 10.1007/s13318-012-0080-2. [DOI] [PubMed] [Google Scholar]
- Rug M., Maier A.G. Transfection of Plasmodium falciparum. Methods Mol. Biol. 2013;923:75–98. doi: 10.1007/978-1-62703-026-7_6. [DOI] [PubMed] [Google Scholar]
- Singh N., Puri S.K. Interaction between chloroquine and diverse pharmacological agents in chloroquine resistant Plasmodium yoelii nigeriensis. Acta Trop. 2000;77:185–193. doi: 10.1016/s0001-706x(00)00133-9. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J.B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Tran P.N., Brown S.H.J., Mitchell T.W., Matuschewski K., McMillan P.J., Kirk K., Dixon M.W.A., Maier A.G. A female gametocyte-specific ABC transporter plays a role in lipid metabolism in the malaria parasite. Nat. Commun. 2014;5:4773. doi: 10.1038/ncomms5773. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Yamamoto C., Tanaka N. Inhibition of protein synthesis by blasticidin S: I. studies with cell-free systems from bacterial and mammalian cells. J. Biochem. 1965;57:667–677. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.