Abstract
Background
Left ventricle (LV) in patients with aortic stenosis (AS) faces a double hemodynamic load incorporating both valvular stenosis and reduced systemic arterial compliance (SAC). This study aimed to evaluate the impact of global LV afterload on LV hypertrophy (LVH) before and after aortic valve replacement (AVR).
Methods
The study cohort included 453 patients (247 males; mean age, 64 ± 11 years) who underwent AVR. Pre- and post-AVR echocardiographic examinations were retrospectively analyzed including an index of valvuloarterial impedance (ZVA) and LV mass index/LV end-diastolic volume index (LVMI/LVEDVI) as a parameter of LVH.
Results
Pre-AVR LVMI/LVEDVI was 2.7 ± 0.9 g/mL with an aortic valve area (AVA) of 0.6 ± 0.2 cm2. ZVA was 5.9 ± 1.9 mm Hg/mL/m2 and showed a stronger correlation (β = 0.601, p < 0.001) with pre-AVR LVMI/LVEDVI than indexed AVA (β = 0.061, p = 0.19), transvalvular peak velocity (β = 0.211, p < 0.001). During a median follow-up of 3.5 years, patients had a 18.8 ± 10.4% decrease in the LV geometry index with a decrease in SAC from 1.20 ± 0.48 to 1.00 ± 0.38 mL/m2/mm Hg (p < 0.001). Pre-AVR LV ejection fraction (r = 0.284, p < 0.001) and ZVA (r = 0.523, p < 0.001) were independent factors associated with LVH regression in 322 patients with follow-up duration >1 year after AVR.
Conclusion
ZVA is a major determinant of concentric remodeling in AS before AVR and LVH regression after AVR, which should be incorporated in routine evaluation of AS.
Keywords: Aortic stenosis, Left ventricular hypertrophy, Afterload, Echocardiography
Introduction
Recent clinical investigations showing a high prevalence of reduced systemic arterial compliance (SAC) in patients with degenerative aortic stenosis (AS) have established a new concept: the left ventricle (LV) in calcific AS faces a double hemodynamic load incorporating both valvular and arterial pressure overloading.1,2,3,4,5,6) This supports the idea that degenerative AS should be considered as a potential manifestation of a systemic process, rather than as a disease solely limited to the aortic valve.7) The valvuloarterial impedance (ZVA) is a new index to assess this global LV afterload in AS during non-invasive echocardiographic examination and it has been proved to be an independent determinant of LV intrinsic myocardial dysfunction.1),4) Many LV deformational indices, not the conventional LV ejection fraction (EF), showed a good correlation with ZVA8),9) and prognostic implications predicting symptom development and even mortality have been confirmed in several outcome studies.2),3),9),10) Thus, ZVA with certain cut-off values has been proposed as a more useful prognostic index than conventional indices including a transvalvular pressure gradient and valve orifice area. However, ZVA does not separate the relative contributions of AS, the associated hypertension, and low SAC of the global LV afterload, and cannot be used in isolation as a standalone parameter.7),11) Moreover, in patients with low flow and low gradient AS, ZVA fails to provide prognostic information.12) These findings necessitate other approaches to prove the clinical usefulness and physiologic significance of ZVA.
LV hypertrophy (LVH) is a classic compensatory mechanism for LV pressure overloading in AS patients and is a fundamental pathologic change. However, the relationship between ZVA and the development of LVH or LVH regression after aortic valve replacement (AVR) has not been investigated. In this study, we addressed this issue to evaluate the physiologic role of ZVA and SAC measurements in patients with severe AS.
Methods
Subjects
We included patients with severe AS who had undergone uneventful AVR and a comprehensive echocardiographic evaluation before and after AVR [2.4 years after surgery (interquartile range 0.7–4.7)] at our institution from March 2000 to March 2006. Patients who had coexistent moderate to severe aortic regurgitation or mitral valve disease were excluded. Patients with occult coronary artery disease who needed concurrent bypass surgery were included. The study cohort included 453 consecutive patients (247 males; mean age, 63.9 ± 10.9 years) and their echocardiographic and clinical data were used for the analysis. This retrospective study was approved by our Institutional Review Board.
Echocardiography
Comprehensive 2-dimensional and Doppler echocardiographic examinations were performed in all patients. We followed the standards and techniques recommended by the American Society of Echocardiography. LV mass was calculated using the formula recommended by the society13) and LV geometry index was defined by the ratio of LV mass index (LVMI) to LV end-diastolic volume index (LVEDVI).14) Doppler echocardiographic assessment of AS severity included measuring peak velocities and the mean transvalvular pressure gradient with calculation of the aortic valve area (AVA) using the continuity equation.
ZVA, global LV afterload in AS patients, was calculated using the formula: ZVA = [(systolic brachial artery pressure + mean net pressure gradient) / stroke volume index].1) The mean net pressure gradient was calculated with the equation proposed by Baumgartner and Otto7) to obtain a more accurate estimate of LV systolic pressure. LV stroke volume index was divided by aortic pulse pressure to calculate SAC.
Statistical analysis
Continuous data were expressed as mean ± SD. Data acquired before and after AVR were analyzed using the paired t-test. Categorical data were given as a percentage and compared with a chi-square test. A forward stepwise linear regression analysis was performed to identify the variables that are independently associated with LV geometry and its change after AVR. Variables with a p value < 0.1 in univariate analysis were entered in the multivariate analysis. A value of p < 0.05 was considered statistically significant. We used the Hittner, May, and Silver's test to assess comparison of two overlapping correlations based on dependent groups. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) and R program version 3.1.1 (http://www.r-project.org).
Results
Patient characteristics
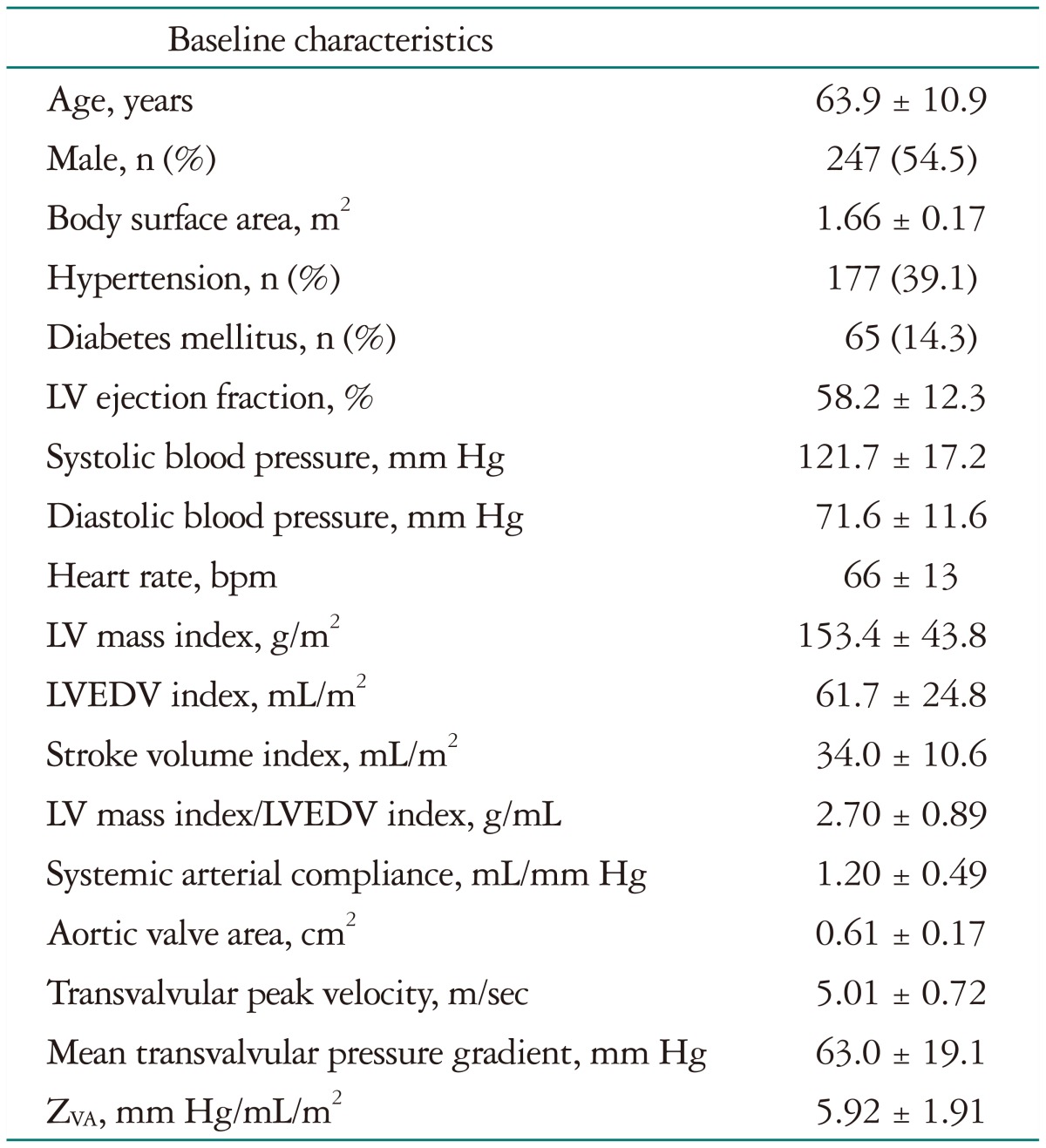
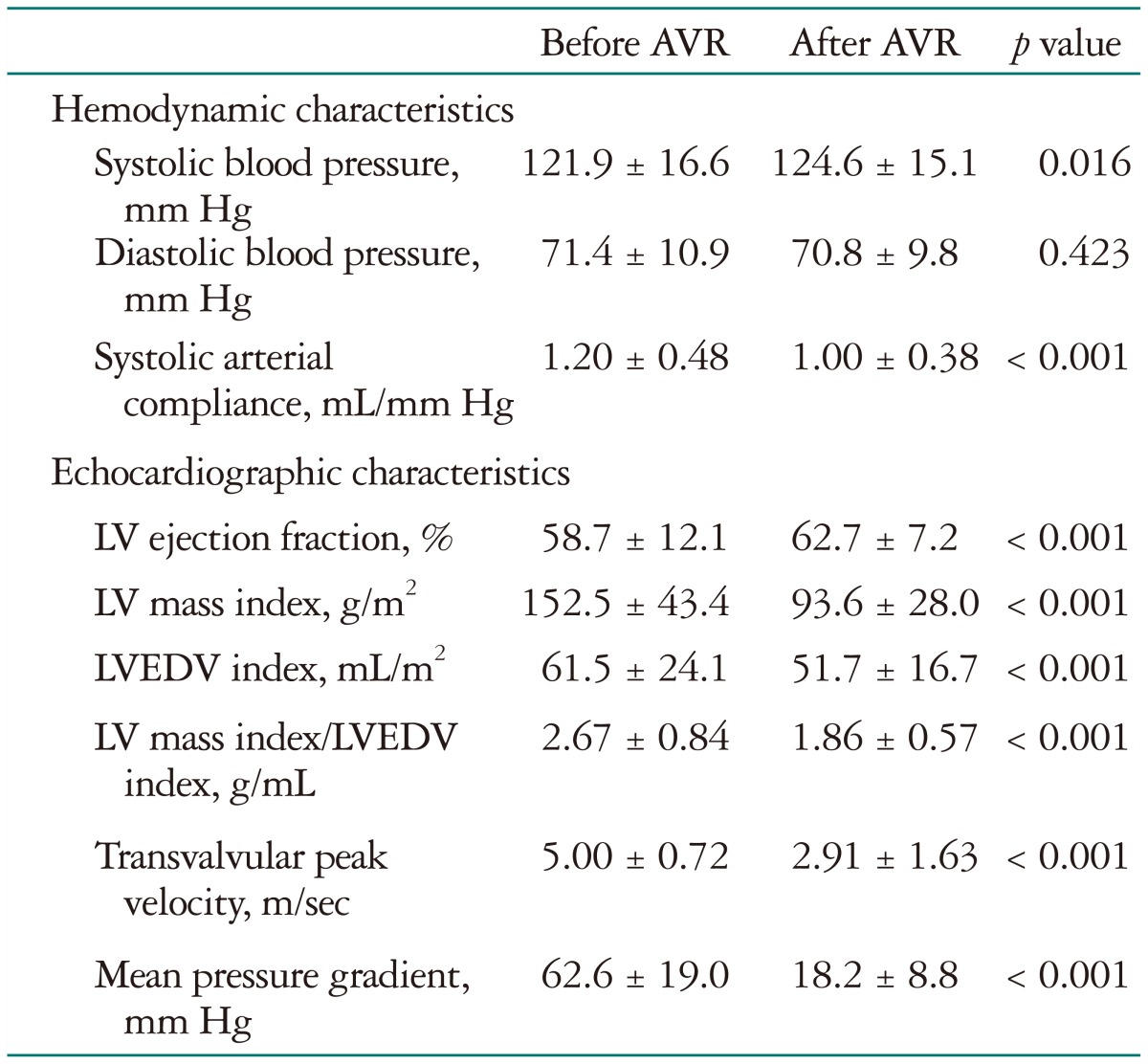
Clinical and hemodynamic characteristics before AVR are summarized in Table 1. Hypertension and diabetes mellitus were present in 177 (39.1%) and in 65 patients (14.3%), respectively. The pre-AVR LVMI was 153 ± 44 g/m2. The dominant surgical procedure was AVR with a mechanical prosthesis. It was performed in 264 patients (58.3%) and concomitant coronary artery bypass graft surgery was performed in 107 patients (23.6%). Table 2 shows the echocardiographic parameters before and after AVR. As expected, LVMI and LVEDVI decreased significantly after AVR with the index of LV geometry being significantly regressed after AVR. Effective AVA increased from 0.6 ± 0.2 to 1.4 ± 0.4 cm2 after AVR (p < 0.001) and SAC decreased from 1.20 ± 0.49 to 1.04 ± 0.40 mL/mm Hg (p < 0.001).
Table 1. Baseline characteristics before AVR.
AVR: aortic valve replacement, LV: left ventricle, LVEDV: LV end-diastolic volume, ZVA: valvuloarterial impedance
Table 2. Hemodynamic and echocardiographic characteristics before and after AVR among patients followed up more than 1 year.
AVR: aortic valve replacement, LV: left ventricle, LVEDV: LV end-diastolic volume
Determinant of LV geometry index before AVR
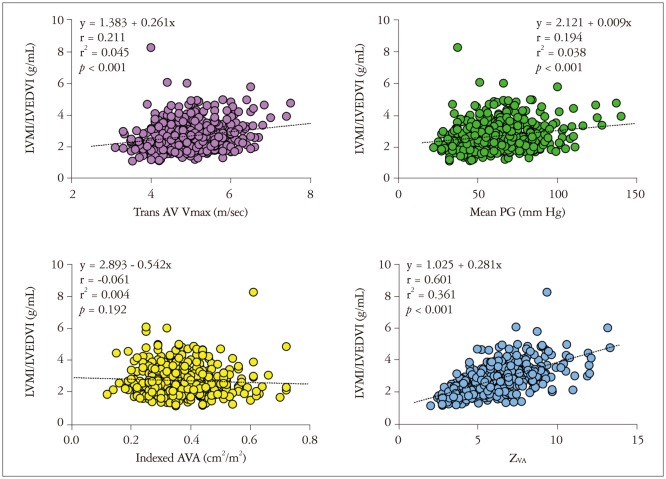
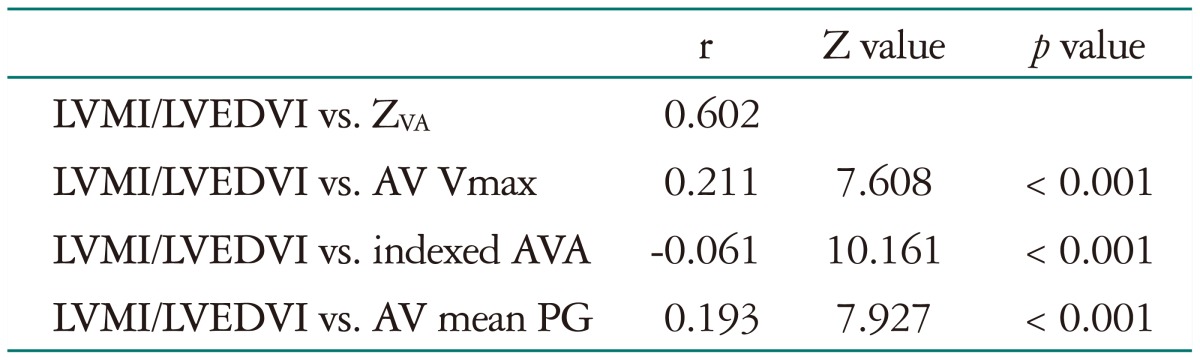
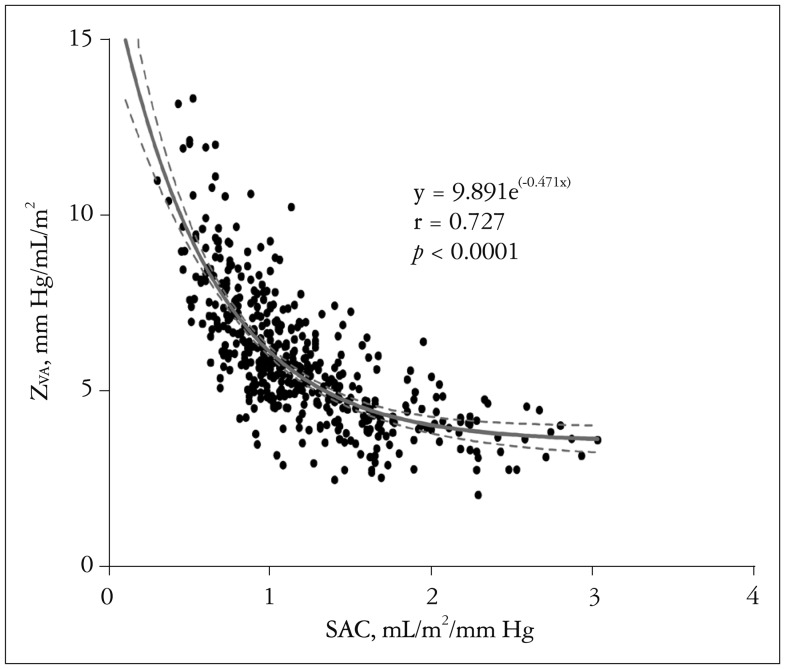
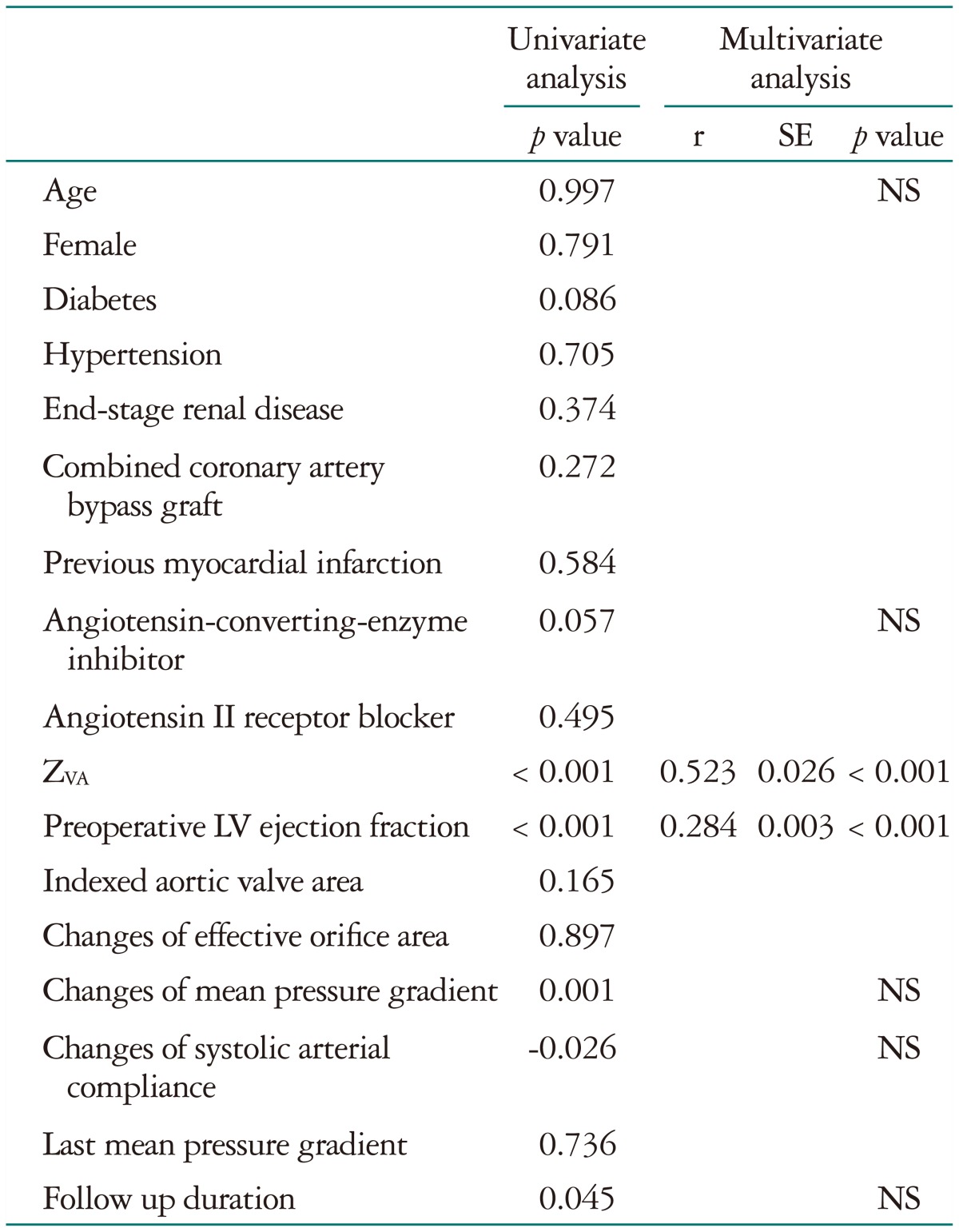
Transvalvular peak velocity (r = 0.211, p < 0.001) and thus the Doppler-derived pressure gradient (r = 0.194, p < 0.001) showed a weak positive correlation with the ratio of LVMI and LVEDVI (Fig. 1), whereas there was no significant correlation between the indexed AVA and the LV geometry index (r = -0.061, p = 0.192). ZVA had a good positive correlation with the LV geometry index (r = 0.601, p < 0.001) (Fig. 1). The correlation between ZVA and the LV geometry index was significantly higher than that between LV geometry and the transvalvular pressure gradient or peak velocity (Table 3). Other variables associated with the LV geometry index included age (r = 0.091, p = 0.053), female gender (r = -0.135, p = 0.004), and LV EF (r = 0.267, p < 0.001). Multivariate analysis showed that female gender (r = -0.071, p = 0.046), LV EF (r = 0.354, p < 0.001), and ZVA (r = 0.664, p < 0.001), were independent factors of the LV geometry index. ZVA showed an excellent correlation with SAC before AVR (Fig. 2). Even after exception for patients with LV dysfunction (LV EF lower than 50%), female (r = -0.101, p = 0.013), LV EF (r = 0.126, p = 0.001), and ZVA (r = 0.69, p < 0.001) were associated with LV geometry index before AVR.
Fig. 1. Correlation between the various hemodynamic variables with LVMI/LVEDVI in patients with severe aortic stenosis. AV: aortic valve, PG: pressure gradient, AVA: aortic valve area, LVMI: left ventricle mass index, LVEDVI: left ventricle end-diastolic volume index, ZVA: valvuloarterial impedance, Vmax: transvalvular peak velocity.
Table 3. Comparison of correlation coefficients.
LVMI: left ventricle mass index, LVEDVI: left ventricular end-diastolic volume index, ZVA: valvuloarterial impedance, AV: aortic valve, AVA: aortic valve area, PG: pressure gradient, Vmax: transvalvular peak velocity
Fig. 2. Correlation between ZVA and SAC. SAC: systemic arterial compliance, ZVA: valvuloarterial impedance.
Regression of LV geometry after AVR
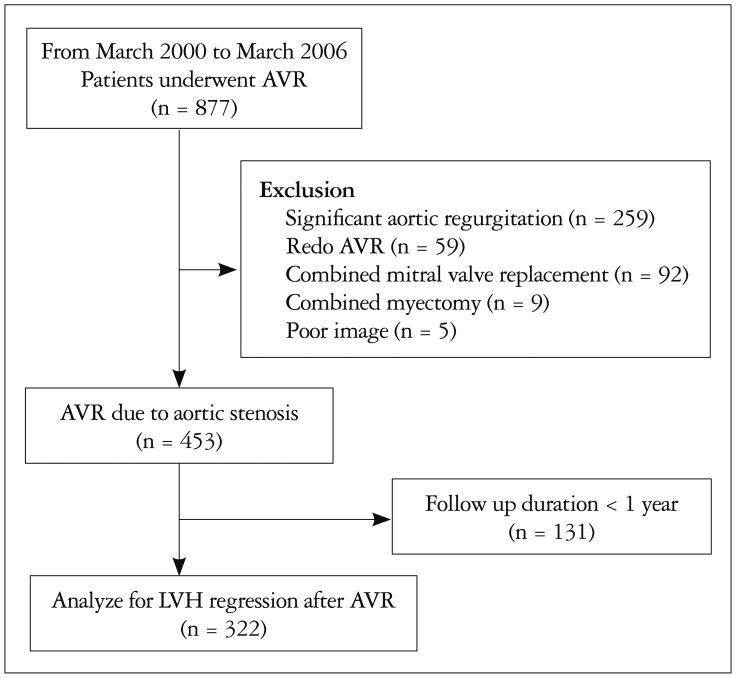
Because the effect of time duration for LVH regression, we excluded patients with follow up interval less than 1 year (Fig. 3). Finally, a total of 322 patients were analyzed for LVH regression. The median echocardiographic follow-up duration was 3.49 years (interquartile range 2.18–5.37) an patients had a 30.6 ± 32.1% decrease in the LV geometry index (from 2.68 ± 0.84 to 1.86 ± 0.57, p < 0.001) and a 25.5 ± 27.4% decrease in the LVMI (from 152 ± 43 to 94 ± 28 g/m2, p < 0.001) with a decrease of SAC from 1.20 ± 0.48 to 1.00 ± 0.38 mL/m2/mm Hg (p < 0.001).
Fig. 3. Study flow. AVR: aortic valve replacement, LVH: left ventricle hypertrophy.
And a degree of reduction in indexed SAC between before AVR and last echocardiography was lower (-0.04 ± 0.38 vs. -0.19 ± 0.33, p < 0.001) in aged 65 or older group (n = 165) compared with younger group (age < 65, n = 157) but changes in LVMI or LV geometric index were not different between groups. Among these patients, the difference of indexed SAC is higher in patients with bicuspid aortic valve (-0.16 ± 0.35 vs. -0.07 ± 0.37, p = 0.03) compared to other etiology of AS but the amount of LVH regression (0.89 ± 0.86 vs. 0.73 ± 0.92, p = 0.099) was not different. In multivariate liner regression analysis, not bicuspid aortic valve but age (r = 0.223, p < 0.001) was an independent associated factor for change in SAC index after AVR.
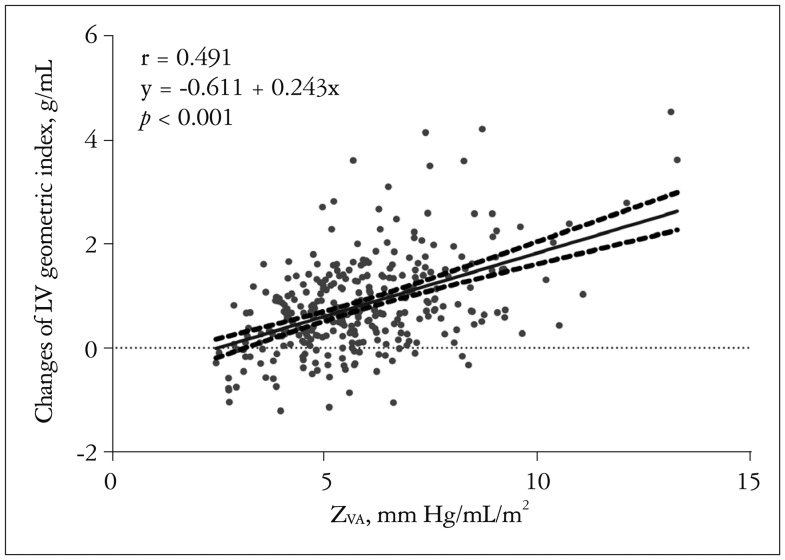
Multivariate analysis showed that the pre-AVR LV EF (r = 0.284, p < 0.001) and ZVA (r = 0.523, p < 0.001) were independent factors associated with the amount of LV mass regression after AVR (Table 4). Patients who showed significant regression of LVH, defined as a difference in the LVH geometry index statistically higher than the median value (0.70 g/mL), had a higher ZVA (6.4 ± 1.8 mm Hg/mL/m2 vs. 5.2 ± 1.6 mm Hg/mL/m2, p < 0.001) without any difference in SAC index after AVR than those without significant LVH regression (-0.08 ± 0.31 mm Hg/mL/m2 vs. -0.15 ± 0.41 mm Hg/mL/m2, p = 0.103). The difference in LVH parameters after AVR showed a positive correlation with ZVA (r = 0.491, p < 0.001) (Fig 4).
Table 4. Factors associated with LV mass regression after AVR.
LV: left ventricle, AVR: aortic valve replacement, ZVA: valvuloarterial impedance
Fig. 4. Correlation between ZVA and changes in LVMI/LVEDVI after aortic valve replacement. ZVA: valvuloarterial impedance, LV: left ventricle, LVMI: LV mass index, LVEDVI: LV end-diastolic volume index.
Discussion
The major finding of this study was that ZVA representing the total load to LV is an independent factor associated with LVH in patients with severe AS requiring AVR and it is also associated with remodeling of LV geometry after successful AVR. Our finding that the association of ZVA with pre- and post-AVR LV geometry is more powerful compared to the conventional indices representing the severity of valvular stenosis per se, such as pressure gradient and AVA, is a strong evidence to support the important physiologic meaning of systemic arterial loading in these AS patients.
LVH in AS before and after AVR: role of systemic arterial loading
The absence of an association between the magnitude of LVH and classic indices of AS severity such as AVA has been reported by many other investigators,15,16,17) indicating the complex nature of LVH. Our observation that ZVA is more strongly correlated with the LV geometry index than does the transvalvular pressure gradient or AVA suggests a significant physiologic impact of total LV afterload on the development of LVH. Thus, although genetic factors such as polymorphism of the angiotensin converting enzyme genotype18,19,20) have been suggested to explain the lack of a correlation between valve stenosis and the hypertrophic response in AS patients, we believe that triggering effects of global LV afterload or systemic arterial loading associated with the pathogenesis of LVH should not be underestimated when the feasibility and advantage of comprehensive physiologic assessment, including both valvular and arterial components, is taken into consideration.
AVR is a definite treatment option to improve survival in symptomatic AS patients, but persistent LVH or incomplete reverse remodeling after successful AVR have been well recognized, and results in diastolic dysfunction and poor long-term outcomes.14),16),21) Persistent LVH, even several years after AVR, may be related to patient prosthesis mismatch, and several technical alterations and valvular substitutes aimed at decreasing the valvular gradient have been proposed. However, the valvular gradient alone cannot explain the lack of LVH regression completely, and several preoperative variables, including LV EF, cardiothoracic ratio, anti-anginal medications, and LV dysfunction, are associated with LVH regression.22) The association between pre-AVR LV function and LV mass regression is conceivable, considering that the transition from hypertrophy to heart failure marks the tipping point at which the LV fails in the face of an increased pressure afterload and is no longer able to maintain forward flow through the valve. This key progression is associated with increased myocyte apoptosis and fibrosis and it is postulated that these two processes are responsible for the transition from hypertrophy to heart failure.23) This is believed to herald the onset of symptoms, adverse events, and a poor prognosis. Irreversible myocardial fibrosis has been confirmed to be an integral part of the hypertrophic process.24) Recent clinical studies using cardiac magnetic resonance imaging with gadolinium enhancement, which allows non-invasive visualization of replacement fibrosis, confirmed that a significant proportion of AS patients showed fibrosis, which is associated with a more advanced hypertrophic response and is irreversible after successful AVR.25,26,27) Our finding that pre-AVR LV EF was an independent factor associated with LVH regression after AVR is compatible with previous observations, and supports the idea that, despite many limitations, LV EF could be a useful marker for intrinsic contractility and maybe provide useful histopathologic information in AS patients.
Arterial hypertension is an independent factor associated with poor LV mass regression after AVR,21),28) but how hypertension affects LVH regression has not been established. Measurement of ZVA and SAC before and after AVR was performed in our study, as we believe that it can provide some insight into mechanism of LVH regression. A previous assumption was that SAC would be generally unchanged by AVR because only the valve, and not the aorta, is replaced at the time of operation.1),29) However, we have confirmed that SAC decreases significantly during follow-up after uneventful AVR. This is conceivable considering the advanced age of the AS patient population. Our finding that the association between ZVA and the amount of LVH regression is a consequence of relief of LV afterload from AVR, emphasizes the importance of the hemodynamic contribution of LV afterload in LVH regression. This suggests that ZVA or SAC could be used as a therapeutic target after AVR. The efficacy of load-independent LV mass regression using angiotensin II receptor blockade on successful LV mass regression has been reported in an animal model and in AS patients.30) Although it is controversial whether the positive effect of this drug is truly load-independent or effective via a reduction of LV afterload, as the data regarding the change in LV afterload or arterial stiffness with this medication are not available, the potential impact of LV afterload manipulation after AVR should be adequately addressed in future investigations.
Limitations
Cardiac magnetic resonance imaging was not performed in our study and may be argued that the echocardiographic assessment of LV mass for comparison of absolute changes within subjects can be problematic due to its dependence on image quality. However, the reliability of echocardiographic measurement has been thoroughly validated in a previous study,31) where an absolute change in between-study assessment of LVMI > 18 g/m2 was shown to have a ≥ 95% likelihood of representing a true change in LV mass. In our study, the mass index changed from 152 ± 43 to 94 ± 28 g/m2, indicating a 25.5 ± 27.4% decrease, which is sufficient to guarantee the reliability of our measurement.
An inappropriately high LV mass before AVR and persistent LVH after AVR25) have been confirmed to be associated with poor long-term outcomes, and the necessity of adding LVH criteria to the current guideline for surgical intervention has been suggested. However, the effect of LV mass regression on survival after AVR remains controversial28) and our study could not address this question due to the short follow-up period. Further clinical investigations with a longer follow-up are necessary to evaluate the real clinical impact of pharmacological modulation of global LV afterload after uneventful AVR.
Clinical implications
The absence of any association between the indices representing valvular pressure overloading and the degree of LVH in AS patients can be easily explained by the addition of arterial pressure overloading. Additionally, ZVA representing total LV afterload seems to be a critical step in understanding the degree of LVH before AVR and the amount of LV mass regression after AVR. Thus, we believe that ZVA should be incorporated in routine evaluation of AS.
References
- 1.Briand M, Dumesnil JG, Kadem L, Tongue AG, Rieu R, Garcia D, Pibarot P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: implications for diagnosis and treatment. J Am Coll Cardiol. 2005;46:291–298. doi: 10.1016/j.jacc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 2.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 3.Hachicha Z, Dumesnil JG, Pibarot P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J Am Coll Cardiol. 2009;54:1003–1011. doi: 10.1016/j.jacc.2009.04.079. [DOI] [PubMed] [Google Scholar]
- 4.Cramariuc D, Cioffi G, Rieck AE, Devereux RB, Staal EM, Ray S, Wachtell K, Gerdts E. Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging. 2009;2:390–399. doi: 10.1016/j.jcmg.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Weisz SH, Magne J, Dulgheru R, Caso P, Piérard LA, Lancellotti P. Carotid artery and aortic stiffness evaluation in aortic stenosis. J Am Soc Echocardiogr. 2014;27:385–392. doi: 10.1016/j.echo.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Rashedi N, Otto CM. Aortic stenosis: changing disease concepts. J Cardiovasc Ultrasound. 2015;23:59–69. doi: 10.4250/jcu.2015.23.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumgartner H, Otto CM. Aortic stenosis severity: do we need a new concept? J Am Coll Cardiol. 2009;54:1012–1013. doi: 10.1016/j.jacc.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Lancellotti P, Donal E, Magne J, O'Connor K, Moonen ML, Cosyns B, Pierard LA. Impact of global left ventricular afterload on left ventricular function in asymptomatic severe aortic stenosis: a two-dimensional speckle-tracking study. Eur J Echocardiogr. 2010;11:537–543. doi: 10.1093/ejechocard/jeq014. [DOI] [PubMed] [Google Scholar]
- 9.Zito C, Salvia J, Cusmà-Piccione M, Antonini-Canterin F, Lentini S, Oreto G, Di Bella G, Montericcio V, Carerj S. Prognostic significance of valvuloarterial impedance and left ventricular longitudinal function in asymptomatic severe aortic stenosis involving three-cuspid valves. Am J Cardiol. 2011;108:1463–1469. doi: 10.1016/j.amjcard.2011.06.070. [DOI] [PubMed] [Google Scholar]
- 10.Giannini C, Petronio AS, De Carlo M, Guarracino F, Benedetti G, Delle Donne MG, Dini FL, Marzilli M, Di Bello V. The incremental value of valvuloarterial impedance in evaluating the results of transcatheter aortic valve implantation in symptomatic aortic stenosis. J Am Soc Echocardiogr. 2012;25:444–453. doi: 10.1016/j.echo.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Samarendra P. Usefulness of valvuloarterial impedance to predict adverse outcomes in patients with asymptomatic aortic stenosis. J Am Coll Cardiol. 2010;55:1164–1165. doi: 10.1016/j.jacc.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Levy F, Luc Monin J, Rusinaru D, Petit-Eisenmann H, Lelguen C, Chauvel C, Adams C, Metz D, Leleu F, Gueret P, Tribouilloy C. Valvuloarterial impedance does not improve risk stratification in low-ejection fraction, low-gradient aortic stenosis: results from a multicentre study. Eur J Echocardiogr. 2011;12:358–363. doi: 10.1093/ejechocard/jer022. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Seo JS, Jang MK, Lee EY, Yun SC, Kim DH, Song JM, Kang DH, Song JK. Evaluation of left ventricular diastolic function after valve replacement in aortic stenosis using exercise Doppler echocardiography. Circ J. 2012;76:2792–2798. doi: 10.1253/circj.cj-12-0446. [DOI] [PubMed] [Google Scholar]
- 15.Kupari M, Turto H, Lommi J. Left ventricular hypertrophy in aortic valve stenosis: preventive or promotive of systolic dysfunction and heart failure? Eur Heart J. 2005;26:1790–1796. doi: 10.1093/eurheartj/ehi290. [DOI] [PubMed] [Google Scholar]
- 16.Beach JM, Mihaljevic T, Rajeswaran J, Marwick T, Edwards ST, Nowicki ER, Thomas J, Svensson LG, Griffin B, Gillinov AM, Blackstone EH. Ventricular hypertrophy and left atrial dilatation persist and are associated with reduced survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg. 2014;147:362–369.e8. doi: 10.1016/j.jtcvs.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Pagé A, Dumesnil JG, Clavel MA, Chan KL, Teo KK, Tam JW, Mathieu P, Després JP, Pibarot P ASTRONOMER Investigators. Metabolic syndrome is associated with more pronounced impairment of left ventricle geometry and function in patients with calcific aortic stenosis: a substudy of the ASTRONOMER (Aortic Stenosis Progression Observation Measuring Effects of Rosuvastatin) J Am Coll Cardiol. 2010;55:1867–1874. doi: 10.1016/j.jacc.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 18.Dellgren G, Eriksson MJ, Blange I, Brodin LA, Rådegran K, Sylvén C. Angiotensin-converting enzyme gene polymorphism influences degree of left ventricular hypertrophy and its regression in patients undergoing operation for aortic stenosis. Am J Cardiol. 1999;84:909–913. doi: 10.1016/s0002-9149(99)00464-6. [DOI] [PubMed] [Google Scholar]
- 19.Orlowska-Baranowska E, Placha G, Gaciong Z, Baranowski R, Zakrzewski D, Michalek P, Hoffman P, Rawczynska-Englert I. Influence of ACE I/D genotypes on left ventricular hypertrophy in aortic stenosis: gender-related differences. J Heart Valve Dis. 2004;13:574–581. [PubMed] [Google Scholar]
- 20.Chang SA, Kim HK, Sohn DW. Impact of afterload on the assessment of severity of aortic stenosis. J Cardiovasc Ultrasound. 2012;20:79–84. doi: 10.4250/jcu.2012.20.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund O, Erlandsen M, Dørup I, Emmertsen K, Flø C, Jensen FT. Predictable changes in left ventricular mass and function during ten years after valve replacement for aortic stenosis. J Heart Valve Dis. 2004;13:357–368. [PubMed] [Google Scholar]
- 22.Lund O, Emmertsen K, Dørup I, Jensen FT, Flø C. Regression of left ventricular hypertrophy during 10 years after valve replacement for aortic stenosis is related to the preoperative risk profile. Eur Heart J. 2003;24:1437–1446. doi: 10.1016/s0195-668x(03)00316-6. [DOI] [PubMed] [Google Scholar]
- 23.Hein S, Arnon E, Kostin S, Schönburg M, Elsässer A, Polyakova V, Bauer EP, Klövekorn WP, Schaper J. Progression from compensated hypertrophy to failure in the pressure-overloaded human heart: structural deterioration and compensatory mechanisms. Circulation. 2003;107:984–991. doi: 10.1161/01.cir.0000051865.66123.b7. [DOI] [PubMed] [Google Scholar]
- 24.Krayenbuehl HP, Hess OM, Monrad ES, Schneider J, Mall G, Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–755. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 25.Weidemann F, Herrmann S, Störk S, Niemann M, Frantz S, Lange V, Beer M, Gattenlöhner S, Voelker W, Ertl G, Strotmann JM. Impact of myocardial fibrosis in patients with symptomatic severe aortic stenosis. Circulation. 2009;120:577–584. doi: 10.1161/CIRCULATIONAHA.108.847772. [DOI] [PubMed] [Google Scholar]
- 26.Azevedo CF, Nigri M, Higuchi ML, Pomerantzeff PM, Spina GS, Sampaio RO, Tarasoutchi F, Grinberg M, Rochitte CE. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J Am Coll Cardiol. 2010;56:278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Chang HJ, Choi JH, Yang PS, Lee SE, Heo R, Shin S, Cho IJ, Kim YJ, Shim CY, Hong GR, Chung N. Late gadolinium enhancement in cardiac MRI in patients with severe aortic stenosis and preserved left ventricular systolic function is related to attenuated improvement of left ventricular geometry and filling pressure after aortic valve replacement. Korean Circ J. 2014;44:312–319. doi: 10.4070/kcj.2014.44.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaudino M, Alessandrini F, Glieca F, Luciani N, Cellini C, Pragliola C, Morelli M, Canosa C, Nasso G, Possati G. Survival after aortic valve replacement for aortic stenosis: does left ventricular mass regression have a clinical correlate? Eur Heart J. 2005;26:51–57. doi: 10.1093/eurheartj/ehi012. [DOI] [PubMed] [Google Scholar]
- 29.Tsang W, Veronesi F, Sugeng L, Weinert L, Takeuchi M, Jeevanandam V, Lang RM. Mitral valve dynamics in severe aortic stenosis before and after aortic valve replacement. J Am Soc Echocardiogr. 2013;26:606–614. doi: 10.1016/j.echo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Dahl JS, Videbaek L, Poulsen MK, Pellikka PA, Veien K, Andersen LI, Haghfelt T, Møller JE. Effect of candesartan treatment on left ventricular remodeling after aortic valve replacement for aortic stenosis. Am J Cardiol. 2010;106:713–719. doi: 10.1016/j.amjcard.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 31.Palmieri V, Dahlöf B, DeQuattro V, Sharpe N, Bella JN, de Simone G, Paranicas M, Fishman D, Devereux RB. Reliability of echocardiographic assessment of left ventricular structure and function: the PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34:1625–1632. doi: 10.1016/s0735-1097(99)00396-4. [DOI] [PubMed] [Google Scholar]