Abstract
Studies of the effect of hormone therapy on cognitive function in menopausal women have been equivocal, in part due to differences in the type and timing of hormone treatment. Here we cognitively tested aged female rhesus macaques on (1) the delayed response task of spatial working memory, (2) a visuospatial attention task that measured spatially and temporally cued reaction times, and (3) a simple reaction time task as a control for motor speed. After task acquisition, animals were ovariectomized (OVX). Their performance was compared with intact controls for 2 months, at which time no group differences were found. The OVX animals were then assigned to treatment with either a subcutaneous sham implant (OVX), 17-β estradiol (E) implant (OVX+E) or E implant plus cyclic oral progesterone (OVX+EP). All groups were then tested repeatedly over 12 months. The OVX+E animals performed significantly better on the delayed response task than all of the other groups for much of the 12 month testing period. The OVX+EP animals also showed improved performance in the delayed response task, but only at 30 s delays and with performance levels below that of OVX+E animals. The OVX+E animals also performed significantly better in the visuospatial attention task, particularly in the most challenging invalid cue condition; this difference also was maintained across the 12 month testing period. Simple reaction time was not affected by hormonal manipulation. These data demonstrate that chronic, continuous administration of E can exert multiple beneficial cognitive effects in aged, OVX rhesus macaque females.
SIGNIFICANCE STATEMENT Hormone therapy after menopause is controversial. We tested the effects of hormone replacement in aged rhesus macaques, soon after surgically-induced menopause [ovariectomy (OVX)], on tests of memory and attention. Untreated ovarian-intact and OVX animals were compared with OVX animals receiving estradiol (E) alone or E with progesterone (P). E was administered in a continuous fashion via subcutaneous implant, whereas P was administered orally in a cyclic fashion. On both tests, E-treated animals performed better than the other 3 experimental groups across 1 year of treatment. Thus, in this monkey model, chronic E administered soon after the loss of ovarian hormones had long-term benefits for cognitive function.
Keywords: aging, cognition, hormones
Introduction
Human studies have reported negative impacts on cognition due to ovariectomy (OVX) (Rocca et al., 2007; Sherwin, 2012), perimenopause (Frackiewicz and Cutler, 2000; Weber et al., 2013), and menopause (LeBlanc et al., 2001; Maki and Hogervorst, 2003; Sherwin, 2006). Hormone therapy (HT) ameliorates some clinical symptoms, such as hot flashes and osteoporosis (de Villiers et al., 2013), but the effects on cognition are inconsistent (Fischer et al., 2014), complicated by variations in subject age, time of initiation, hormone preparation, and route and duration of administration (Voytko, 2009; Gibbs, 2010; Maki, 2013). For example, women who received HT in their 50s showed no benefits compared with those receiving placebo treatment (Espeland et al., 2013), whereas the Cache County study found preserved cognitive function in women receiving HT immediately following menopause (Shao et al., 2012). HT can also reduce the risk for Alzheimer's disease (Paganini-Hill and Henderson, 1994; for review, see Rocca et al., 2014), although not when treatment is delayed (Rapp et al., 2003b; Espeland et al., 2004).
While rodent models show positive effects on cognitive function when estrogen replacement is initiated soon after OVX (Daniel, 2006; Acosta et al., 2013), they differ markedly from women in their hormonal patterns. The nonhuman primate (NHP) is an attractive and highly translational alternative model (Lacreuse et al., 2015), being a long-lived, close genetic relative, with a complex brain and cognitive ability (Kohama et al., 2012). Furthermore, the reproductive physiology of female NHPs is very similar to that of women, showing characteristic monthly menstrual cycles (Jewitt and Dukelow, 1972; Knobil, 1974; Goodman et al., 1977), cycle irregularity during perimenopause (Gilardi et al., 1997; Downs and Urbanski, 2006), and cessation of cyclicity and ovarian estrogen production after menopause (Johnson and Kapsalis, 1995; Walker, 1995; Gilardi et al., 1997; Downs and Urbanski, 2006). Additionally, there are significant anatomical differences that exist between the frontal lobes of primates and rodents. Specifically, the lateral prefrontal cortex (PFC) subregions, which in humans are critical to support working memory (WM) and attentional control, cannot be clearly delineated from other frontal cortical areas in the rodent brain (Uylings et al., 2003; Seamans et al., 2008), but in monkeys the lateral PFC is widely accepted as anatomically and functionally homologous to humans (Petrides and Pandya, 2002; Petrides et al., 2002). Critically, cognitive performance dependent on PFC function declines in NHPs after OVX, whereas estrogen therapy ameliorates these deficits in memory and attention in both young (Voytko, 2002; Voytko and Tinkler, 2004) and aged monkeys (Rapp et al., 2003a; Tinkler and Voytko, 2005; Lacreuse, 2006; Voytko et al., 2008). Furthermore, spatial learning has been reported to be impaired in monkeys during the perimenopausal or postmenopausal period compared with age-matched premenopausal monkeys (Roberts et al., 1997). Complicating the issue, however, is that one recent study of a variety of forms of HT administration in aged OVX monkeys reported no positive effects on cognition (Baxter et al., 2013). However, factors, such as the age of the animal and time since OVX, can modulate hormone effects on cognition (Voytko and Tinkler, 2004; Tinkler and Voytko, 2005).
Therefore, the present study examined memory and attention in aged rhesus monkeys to determine both the acute and chronic impact of HT using naturally occurring sex steroids delivered at physiological levels. In the first phase of the study, acutely OVX females were compared with age-matched intact, cycling females. In the second or HT phase, the OVX females were divided into groups receiving either subcutaneous implants of 17-β estradiol (OVX+E), E implants plus cyclic oral progesterone (OVX+EP), or placebo treatment (OVX group). Repeated blocks of cognitive testing were performed during an initial post-OVX 2 month period, followed by 12 months of testing during the HT phase.
Materials and Methods
Animals.
The study used 28 female rhesus macaque monkeys (Macaca mulatta), with an age range of 18–25 years and experimentally naive to cognition studies. The monkeys were screened with physical and ophthalmoscopic examinations to establish the absence of age-related health disorders that might interfere with testing. They were also selected to show normal menstrual cyclicity at the initiation of the study, based on mense records and plasma levels of E and P measured every 3 d throughout at least one complete menstrual cycle. Animals were paired (n = 20) or singly housed (n = 8); the latter consisted of animals that failed at least four attempts at social housing due to excessive aggression, and were distributed across the different treatment groups. The potential influence of phytoestrogens found in commercial monkey chow was avoided by feeding all animals a specially prepared semipurified diet low in phytoestrogens (similar to that reported by Voytko, 2000) throughout both training and data collection. The diet was prepared in the Oregon National Primate Research Center diet kitchen bimonthly and kept frozen until use. It was fed to the animals twice daily at ∼8:00 A.M. or at the completion of cognitive testing and at 3:00 P.M., and was supplemented daily with fresh fruit or vegetables. Drinking water was available ad libitum. All protocols were approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee and were conducted in accordance with National Research Council's Guide for the Care and Use of Laboratory Animals.
Experimental groups and hormone replacement.
All animals were gonad-intact while undergoing initial training on each of the behavioral tasks. Once they achieved a preset criterion for each task, 6 animals remained as intact controls and were monitored for menstrual cycles (Fig. 1), and 22 animals were bilaterally OVX by laparoscopy as previously described (Fanton, 2005). In Phase 1 of the study, these two groups were cognitively tested at 2 weeks, 1 month, and 2 months after OVX, to assess the acute role of gonadal steroid loss. In Phase 2, HT was initiated 2.5 months after OVX. The OVX animals were randomly assigned to receive estradiol (OVX+E; n = 7), estradiol and progesterone (OVX+EP; n = 7), or placebo (OVX; n = 8). There was no difference in age between intact (19.9 ± 0.3 years), OVX (21.2 ± 1.0), OVX+E (22.2 ± 0.7 years), or OVX+EP (21.5 ± 1.0) groups (p = 0.36, one-way ANOVA). E was administered via subscapular subcutaneous implantation of SILASTIC capsules (Dow-Corning), which were replaced at 3+ month intervals, or whenever serum E concentration levels fell <80 pg/ml. In the OVX+EP group, 40 mg of micronized progesterone (Sigma) was administered daily via oral ingestion of a favored food item for an 11 day period in each 28 day block. The OVX group received placebo implants containing cholesterol, and the OVX and OVX+E groups were given the same preferred food items without progesterone on the same schedule as the OVX+EP group.
Figure 1.
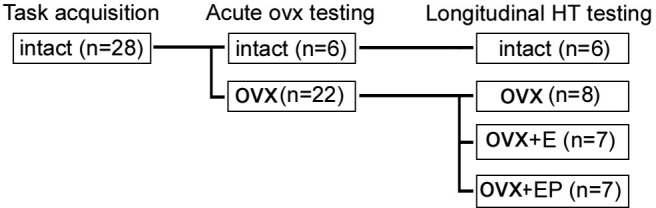
Experimental design: Gonad-intact, aged rhesus females were trained to criteria during task acquisition. All but 6 of the animals were then bilaterally ovariectomized, and all 28 were retested after 1 week, 1 month, and 2 months during the acute OVX testing phase. Longitudinal hormone replacement began thereafter, and all groups were retested at 1 week and at 1, 2, 4, 6, 9, and 12 months.
Serum hormone assays and menses.
During the training period, blood samples and daily mense data were collected from the animals to verify menstrual cyclicity. Animals were trained for unsedated blood collection to avoid effects of repeated sedation on cognitive function. All animals were initially sampled every third day over several menstrual cycles, and normal cyclicity was verified by E and P levels within the normal range and showing the cyclic pattern found in gonad-intact rhesus monkeys (Downs and Urbanski, 2006). After OVX, complete ovarian removal was verified by the absence of the ovarian hormones after 1 week.
During the experimental phase, intact controls were sampled during the late follicular and mid-luteal phases of their menstrual cycle, with timing established by cycle patterns observed during baseline training and confirmed by daily mense data. For animals in the OVX, OVX+E, and OVX+EP groups, blood samples were collected monthly for confirmation of steroid levels. For the OVX+EP treatment group, P levels were assayed on the initial day of the 11 day block of administration, with P given at 8:00 A.M. and blood collected between 10:00 A.M. and 2:00 P.M. Serum was stored frozen at −4°C until assayed for E and P by electrochemiluminescence using the Immulite 2000 platform (Siemens Healthcare Diagnostics), as previously reported (Eghlidi et al., 2010). The limit of sensitivity of the E and P assays was 20 pg/ml and 0.2 ng/ml, respectively; the intra-assay and interassay coefficients of variation were <15% for both assays.
Behavioral testing apparatus and procedures.
Training and testing were conducted in five sound-insulated computerized behavioral testing chambers that each contained a 17 inch Elo-Touch Systems CRT Touchmonitor. Each testing chamber contained a modified cage (24 inch L × 24 inch W × 32 inch H) with 3-inch-spaced bars in the front to allow access to the touchscreen and was equipped with a light, two fans, and a 1 inch closed-circuit video camera for unobtrusive monitoring of the subjects' behavior. The monkeys were unrestrained and had free access to the touchscreen. Food rewards were delivered by either Med Associates Mini M&M dispensers or custom-built universal food dispensers mounted on top of the testing chambers. The animals were rewarded on all tasks with preferred foods, generally a mixture of candy, nuts, pasta, and/or the low-phytoestrogen diet. Monkeys were not calorically restricted but generally were tested in the morning after an overnight fast and were fed their first meal at the conclusion of testing. Task stimulus presentation and reward contingencies were controlled individually for each chamber by a suite of custom programs run on Apple iMac computers.
Touchscreen training.
The monkeys were first trained to touch a physical target held outside their home cage. Then they were acclimated to the testing chamber and trained to transfer targeting to the touchscreen for manually controlled rewards. Once the animals were able to target the screen without assistance, they were placed on an automated training program that rewarded touches to a large red square in random locations on the screen, and the size of the square was then slowly decreased over time. Criterion was reached when the animal could touch a 3 inch × 3 inch square with 90% accuracy over 80 trials.
Delayed response task.
This task assesses spatial WM and is similar to that described previously (Voytko et al., 1994; Voytko, 2000). A trial began with one red square appearing on either the right or left side of the monitor (“cue phase”). Following a touch response to the square, a delay period began during which the screen was blank. At the end of the delay, two red squares appeared on the right and left sides of the monitor (“choice phase”), and the correct strategy to receive a reward was to touch the square in the same location as the square presented during the cue phase. A 3 s intertrial interval (ITI) was used after correct responses (trials), and a 5 s ITI after incorrect responses. Training began with a 0 s delay, with delays increasing by 1 s each time 85% correct responses were made in 80 sequential trials, until a 5 s delay was reached. After attaining the 5 s delay, the monkeys were tested on a variable delay version of the task in which delays of 1, 5, 15, and 30 s were randomly presented within each daily session of 80 trials. Choice accuracy was examined for 5 d for a total of 100 trials per delay.
Every attempt was made to prevent motor cueing during delayed response testing. Monkeys were trained not to leave their hand on the screen during the delay, and they were monitored continuously during testing, via video cameras mounted in the testing chambers, to capture any motor-mediated strategies. During the testing phase, animals were not observed using any positional cueing behaviors.
Visuospatial cueing task.
This task assesses the ability to orient and shift visuospatial attention. The procedure was similar to one described previously (Baxter and Voytko, 1996; Voytko, 2002). At the start of a trial, a green square with a white asterisk appeared at the center of the monitor screen for a variable duration of 1–3 s, and the monkey was required to touch and hold the central symbol as long as it was present. At the end of the delay, a small white circle (“cue”) appeared to the left or right of the central symbol for 200 ms, during which time the monkey was trained to continue touching the central symbol. At the end of the 200 ms period, the central symbol disappeared and a green square (“target”) appeared on the left or right side of the monitor. The correct strategy to receive a reward was to stop touching the square (“release”) and touch the target within a short time window. A 3 s ITI was used, and each daily session consisted of 120 trials. The dependent measure recorded for each trial was reaction time (RT), defined as the time to stop touching the center square at target appearance (release time). This measure is considered a measure of central processing time and more appropriate than the time to hit the target, which also includes a motor speed component.
Different aspects of attention were evaluated by comparison of RTs to four types of trials, presented in pseudorandom order: (1) cue placement was termed “valid” when it was on the same side of the screen where the target would appear on a given trial; this was the most common condition, appearing on 70% of all trials, and therefore the cue usually predicted target location; (2) a cue was “invalid” when it appeared on the side opposite to the target; (3) in the “neutral” cue condition, cues appeared simultaneously on both the left and right and therefore provided temporal information regarding appearance of the target but no spatial information; and (4) on “no-cue” trials, no advanced cues were provided. The proportion of trial types within a session was 70% valid, 10% invalid, 10% neutral, and 10% no-cue trials. Testing occurred for 5 d for a total of 420 valid trials, and 60 trials each of invalid, neutral, and no cue conditions. The inclusion of different trial types afforded the ability to assess several components of attention and target detection. “Validity effects” were derived from invalid cue RT − valid cue RT, and measured the speed benefit provided by accurate advanced spatial cueing of target location. “Alerting effects” equaled no-cue RT − neutral cue RT, and reflected the speed advantage achieved by cueing of the time of target appearance but not its location. Validity effects could be further dissected into “benefits” of the valid cue, equal to neutral cue RT − valid cue RT, and “costs” of the invalid cue, measured by neutral cue RT − invalid cue RT. Costs reflected the added time needed to reorient and shift spatial attention after an inaccurate cue.
Simple RT task.
This task assesses general response time and motor speed and is identical to one described previously (Baxter and Voytko, 1996; Voytko, 2002). The start of each trial was marked by the appearance of a centrally located green square with a white asterisk. The monkey was trained to touch the square continuously for a variable delay of 1–3 s. At the end of the delay, the center square was removed and a green square (target) appeared, always on the monkey's preferred side of the monitor screen. The correct strategy was to touch the target to receive a reward. The window of opportunity to respond to the target was initially 1000 ms and progressively decreased in 100 ms increments until it was the shortest time in which the monkey could respond correctly in 17 of 20 trials. If the animal was <85% correct, the window of opportunity increased by 100 ms for the next 20 trials. The final shortest time achieved was referred to as the fastest RT. A 3 s ITI was used, and each session consisted of 100 trials. Monkeys were tested for one session at each time point.
Schedule of cognitive testing.
During data collection, animals were tested for 11 consecutive days on a battery consisting of 3 tasks: 1 d of simple RT, 5 d of visuospatial cueing, and 5 d of delayed response testing. These behavioral batteries were collected during Phase 1 for all groups at 1 week, 1 month, and 2 months after OVX surgery (n = 22) and compared with intact controls (n = 6). For Phase 2 (HT), the same testing battery was repeated at 1 week and 1, 2, 4, 6, 9, and 12 months after the start of hormone treatment. All testing batteries occurred during the follicular phase for the intact group, and during the E-only part of the artificial cycles for the OVX+EP group, so that P was low during testing for all groups.
Statistical analyses.
For each cognitive task, separate analyses were conducted for Phase 1 to examine the acute effects of OVX, and for Phase 2 to evaluate effects of hormone treatment. Data are represented as percentage change from initial values, which takes advantage of each individual functioning as its own baseline, thereby reducing variation. Therefore, for Phase 1, the percentage change was based upon the initial baseline values for the task before OVX; for the effects of HT in Phase 2, the last values collected at 2 months after OVX, just before initiation of HT, were used as the baseline reference.
Performance in each task was analyzed with a mixed-model, repeated-measures ANCOVA, which included age as a continuous covariate, using SAS version 9.4 (SAS). For the delayed response task, percentage correct was evaluated as a function of hormone treatment as a between-group factor and time after OVX or initiation of HT and delay as within-group factors. RTs in the visuospatial cueing task were analyzed using hormone treatment as a between-group factor and time and cue condition as within-group factors. For the simple RT task, effects of treatment were evaluated over time. In a typical experiment using repeated measures, two measurements taken at adjacent times are more highly correlated than two measurements taken several time points apart; therefore, we used a first-order autoregressive (AR1) covariance structure to account for within-subject correlation. When appropriate, comparisons among treatment groups, delays and cue conditions and among treatment groups at each time point were performed using contrast tests. Because mixed-model analysis holds even with missing data as long as the missing data occur completely at random, no imputation/deletion was applied (Overall and Tonidandel, 2007; Howell, 2008); data were missing for only one subject (E group) at one time point (9 months) due to an acute illness. Differences were considered statistically significant at p < 0.05.
Results
Serum hormone levels
During the training phase, all 28 animals were confirmed to have normal menstrual cycles by ovarian hormone assays and mense records. All had E levels at follicular day 10–12 consistent with a periovulatory rise, as well as low P (Table 1); during the luteal phase, they exhibited high P and moderately low E levels, signifying that an ovulatory event had occurred. The same pattern was also evident in the intact animals during Phase 2, signifying that intact controls continued to have menstrual cycles (Table 1).
Table 1.
Serum hormone levels for intact controls during the two cognitive testing phasesa
| Follicular phase |
Luteal phase |
|||
|---|---|---|---|---|
| E (pg/ml) | P (ng/ml) | E (pg/ml) | P (ng/ml) | |
| Phase 1 | 139.4 ± 22.6 | 0.20 ± 0.04 | 38.1 ± 2.8 | 2.81 ± 0.63 |
| Phase 2 | 107.2 ± 12.6 | 0.36 ± 0.04 | 42.1 ± 2.0 | 3.00 ± 0.35 |
aSamples were collected during day 10–12 of the follicular phase and also during the luteal phase of the menstrual cycle, to capture rising levels of E and P, respectively. Hormone values corroborate visual mense data that indicate that intact animals maintained menstrual cycles during Phase 1 (comparison with OVX) and Phase 2 (longitudinal HT) components of the study. Values reflect the means of individual animals, which were then averaged (overall mean ± SEM).
During Phase 1, OVX animals destined for the OVX, OVX+E, and OVX+EP groups all had serum E levels <20 pg/ml and serum P levels <0.5 ng/ml, consistent with the lack of a source of ovarian steroid hormones (Table 2). During Phase 2 (HT), E levels averaged ∼125 pg/ml for both the OVX+E and OVX+EP groups. This concentration of E is consistent with levels found during the late-follicular phase when E is rising to its periovulatory peak (Downs and Urbanski, 2006). P assays confirmed that P was present above OVX levels only in the OVX+EP group during the 11 d of oral P administration; we note that levels were supraphysiological, probably exacerbated by blood sampling 2–6 h after administration of the hormone.
Table 2.
Serum hormone levels for OVX, OVX+E, and OVX+EP treatment groups during the two cognitive testing phasesa
| Treatment group | Phase 1 |
Phase 2 |
||
|---|---|---|---|---|
| E (pg/ml) | P (ng/ml) | E (pg/ml) | P (ng/ml) | |
| OVX | 11.2 ± 1.7 | 0.32 ± 0.08 | 14.7 ± 2.5 | 0.15 ± 0.05 |
| OVX+E | 9.4 ± 1.2 | 0.20 ± 0.06 | 126.5 ± 11.3 | 0.18 ± 0.06 |
| OVX+EP | 10.7 ± 1.3 | 0.18 ± 0.04 | 123.6 ± 5.5 | 11.33 ± 0.79 |
aAs expected, during Phase 1 (acute OVX testing), both E and P levels were low in all groups. Following the initiation of Phase 2 (HT), E levels in the OVX+E and OVX+EP groups were elevated to levels similar to days 10–12 of the follicular phase in intact controls. P levels were also elevated in the OVX+EP group, whereas OVX controls continued to have low serum E and P levels. Values reflect the means of individual animals, which were then averaged by treatment group (overall mean ± SEM).
Behavioral data
Delayed response
Analysis of the delayed response results at baseline showed the expected decline in performance with increasing delay (F(3,81) = 149.88, p = 0.0001), but no effect of age (F(1,23) = 0.12; p = 0.73). For Phase 1 of the experiment, the change from baseline in percentage correct at each delay across all time points was compared between the intact group (n = 6) and all OVX animals (n = 22). There was no significant group difference in delayed response performance between OVX and intact animals (F(1,25) = 0.43, p = 0.52), no effect of delay (F(3,78) = 1.98, p = 0.12), and no effect of time of testing across the 1 week, 1 month, and 2 month time points (F(2,52) = 0.01, p = 0.99; Fig. 2, left).
Figure 2.
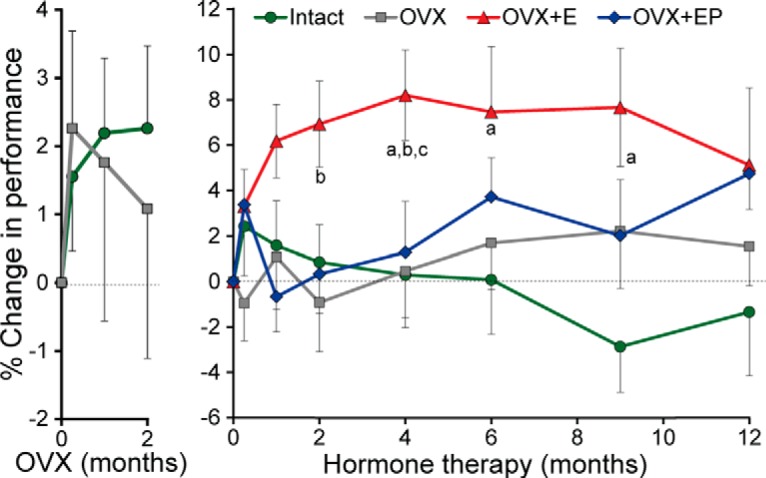
The percentage change in delayed response performance, during the 2 month, post-OVX washout period and during 12 months of HT. During the washout period, the percentage change was calculated from baseline values, whereas for the HT period, comparisons were reset at the start of HT. Left, During the 2 month post-OVX washout phase, there was no difference between intact or OVX groups. Right, During the HT period, the OVX+E animals performed significantly better than the gonad-intact, OVX, or OVX+EP groups. aSignificantly different from intact (p < 0.05, or at a greater significance level). bSignificantly different from OVX (p < 0.05, or at a greater significance level). cSignificantly different from OVX+EP (p < 0.05, or at a greater significance level). Note different y-axis scales for the left and right panels.
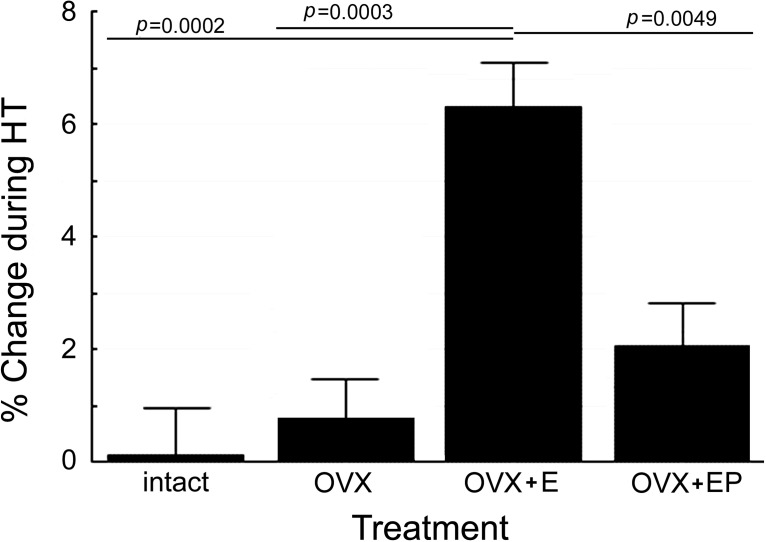
For Phase 2, as shown in Figure 3, there was a significant main effect of hormone treatment (F(3,23) = 8.10, p = 0.0007). Pairwise comparisons revealed significantly better performance in the E group than in intact (t(23) = 4.37, p = 0.0002), OVX (t(23) = 4.22, p = 0.0003), or OVX+EP animals (t(23) = 3.11, p = 0.005), with the latter three treatments overlapping statistically (all p values > 0.05) (Fig. 2, right). A significant treatment × delay interaction (F(9,72) = 5.89, p = 0.0001) reflected the result that performance at the 1 s delay was not different between treatment groups, whereas the OVX+E group showed significantly better performance than the other three groups at 15 and 30 s delays (Fig. 4), consistent with an effect on WM. At the 30 s delay, the OVX+EP group performed at an intermediate level, significantly better than intact (t(72) = 2.91, p = 0.0048) and OVX (t(72) = 3.46, p = 0.0009) animals, but also significantly below the level of performance in the OVX+E animals (t(72) = −2.41, p = 0.0183). There was no effect of the time of testing (F(6,143) = 0.14, p = 0.99), and no treatment × time interaction (F(18,143) = 0.50, p = 0.95), indicating that the positive effect of E was maintained over the 1 year duration of treatment. Other tested interactions also were not significant.
Figure 3.
The effect of HT on overall delayed response performance. When just examining the effect of treatment, collapsing the data across delay and time, the OVX+E group significantly performed better than the intact, OVX, or OVX+EP-treated animals. In all pairwise comparison, none of the other three groups was significantly different from each other in performance.
Figure 4.
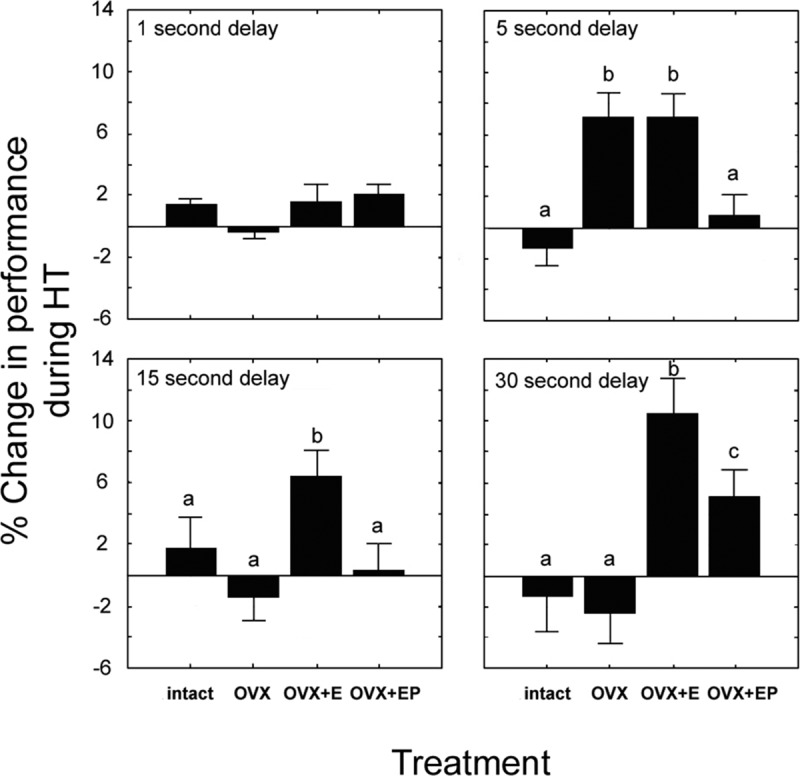
The interaction of delay and treatment on delayed response performance. A significant interaction of delay × treatment was found (p < 0.0001), and comparisons are presented for each delay. Although there were no group differences in performance for the 1 s delay, at 5 s delays both the OVX and OVX+E groups outperformed gonad-intact and OVX+EP animals. For the 15 s delay, the OVX+E group performed significantly better than all other groups. At the 30 s delay, OVX+E again resulted in significantly improved performance versus all other groups. However, in this case, the OVX+EP group performance was superior to that of gonad-intact and OVX animals, but still below that of OVX+E animals. All significant comparisons exceeded p < 0.05, with different letters for any treatment group denoting a significant pairwise difference. Data represent mean ± SEM.
Visuospatial cueing
In Phase 1 of the study (Fig. 5, left column), there was no significant overall difference (main effect) in cued RTs between intact and OVX animals (F(1,25) = 0.01, p = 0.94). There also were no significant interactions for treatment × cue condition (F(3,78) = 1.04, p = 0.38) or treatment × time (F(2,52) = 0.11, p = 0.89).
Figure 5.
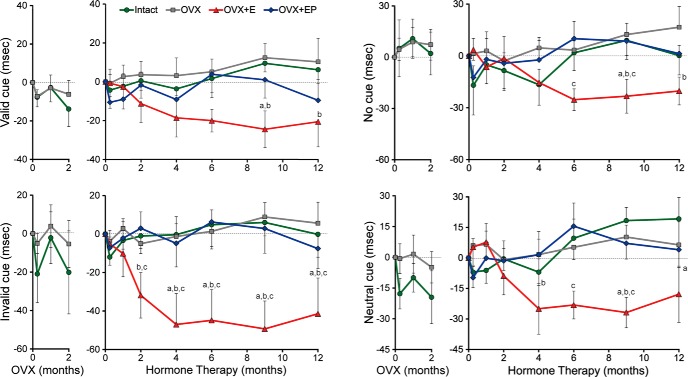
The effect of HT on RT for each cue condition during the 2 month, post-OVX period (left panel of each graph) and during 12 months of HT (right panel). Results are shown separately for valid, invalid, no cue, and neutral cue conditions, with the y-axis representing the change in RT from the baseline condition for each phase of the study. There was no effect of treatment during the 2 month OVX period (Phase 1) for any of the cue conditions. However, in the Phase 2 test of HT, post hoc analyses at each time point revealed that the OVX+E group had faster RTs in the invalid cue condition starting at 2 months, with neutral cues starting at 4 months, and in invalid or no cue conditions starting at 9 months, with all effects persisting through 12 months. aSignificantly different from intact (p < 0.05). bSignificantly different from OVX (p < 0.05). cSignificantly different from OVX+EP (p < 0.05). Data represent mean ± SEM.
During Phase 2 (Fig. 5, right column), there was a significant main effect of hormone treatment (F(3,23) = 9.47, p = 0.0003), as well as a main effect of cue condition (F(3,72) = 8.10, p = 0.0001). Pairwise comparisons showed that the OVX+E groups had overall faster RTs than the other three groups: intact (t(23) = −2.91, p < 0.0079), OVX (t(23) = −5.16, p < 0.0001), and OVX+EP (t(23) = −3.86, p = 0.0008). There was no significant main effect of time (F(6,143) = 0.71, p = 0.64) but a significant treatment × time interaction (F(18,143) = 0.71, p = 0.04), reflecting a progressive reduction in RT in the E group over the first few months of treatment. Furthermore, there was a significant treatment × cue interaction (F(9,72) = 4.00, p = 0.0004). On valid cue trials, RTs were significantly faster in the OVX+E than in the OVX group (t(72) = −3.40, p = 0.0011). For invalid cue trials, the OVX+E group had significantly faster RTs than all other treatment groups: intact (t(72) = −4.93, p < 0.0001), OVX (t(72) = −6.32, p < 0.0001), and OVX+EP (t(72) = −5.86, p < 0.0001). Neutral cue trials showed similar significant differences between OVX+E and all other treatment groups: intact (t(72) = −2.07, p = 0.0416), OVX (t(72) = −3.09, p = 0.0028), and OVX+EP (t(72) = −2.63, p = 0.0106). For the no cue condition, significant differences were found between OVX+E versus OVX (t(72) = −3.15, p = 0.0024), OVX+E versus OVX+EP (t(72) = −2.08, p = 0.0409), and intact versus OVX groups (t(72) = −2.61, p = 0.0112). There were no significant effects for the derived measures, including the validity effect (valid − invalid trial RT), alerting effect (no cue − neutral cue RT), costs (invalid − neutral cue RT), or benefits (valid − neutral trial RT; data not shown). Pairwise comparisons for the effects of treatment for each cue condition at each time point are shown in Figure 5. Only the OVX+E group showed consistently faster RTs across time and cue conditions. These improvements were observed beginning at 2 months of HT for invalid cues and 4 months for neutral cues, but not until 9 and 12 months for the valid and neutral cues.
Simple RT
During Phase 1, no differences in simple RT were found between the intact and OVX groups (F(1,25) = 1.11, p = 0.30), among time points (F(2,52) = 1.33, p = 0.27) or for their interaction (F(2,52) = 1.17, p = 0.32). Similarly, in Phase 2, there was no significant effect of treatment (F(3,23) = 1.02, p = 0.40) or treatment × time interaction (F(18,144) = 0.70, p = 0.81). However, there was a significant main effect of time (F(6,144) = 2.37, p = 0.03), as mean values increased at 12 months (data not shown).
Discussion
This study demonstrated positive, sustained effects of E replacement on spatial WM and spatially cued RT in aged, OVX monkeys. Notable experimental features were the subject's age range (18–25 years), maintenance on a low-phytoestrogen diet to minimize exposure to estrogenic compounds, use of natural hormones, continuous E administration with or without intermittent oral P, initiation of HT 2.5 months after OVX, testing during the follicular phase of intact cycling females and during the E-only phase of the OVX+EP group, and longitudinal testing over a 12 month period of HT. The use of the natural hormones, 17β estradiol and progesterone, differs from many clinical studies, but supports the use of bioidentical steroids for relief of postmenopausal symptoms (McBane et al., 2014; Mirkin et al., 2015). In contrast, negative effects of commonly used hormone preparations, such as conjugated equine estrogens and in particular medroxyprogesterone, have been documented in both women and experimental models (Nilsen and Brinton, 2002; Rapp et al., 2003b). Our results are consistent with other studies showing benefits of early postmenopausal treatment (Hale et al., 2014). The E and P levels tested were relatively high, so we cannot make conclusions about effects of lower levels.
The effect of E on the delayed response task was delay-dependent, with greater performance improvements at the longer 15 and 30 s delays but not at the shortest 5 s delay. This pattern of effects is interpreted as a specific effect on WM, as the short delays provide an internal control for effects on motivation, motor ability, and other nonspecific performance factors.
Previous studies support similar positive effects of ovarian hormones on delayed response performance in macaques. Old, irregularly cycling or acyclic rhesus monkeys performed more poorly on the delayed response task than regularly cycling aged animals (Roberts et al., 1997). Therefore, either regular menstrual cycles and/or a threshold level of ovarian steroids maintained across time may be optimal for cognitive function. Rapp et al. (2003a) reported that aged OVX rhesus females receiving cyclic intramuscular injections of estradiol cypionate showed delayed response performance superior to OVX controls and comparable with young animals. In a study of 22-year-old rhesus monkeys, E implants provided immediately after OVX maintained delayed response ability after 2 months, whereas performance in a placebo group declined (Tinkler and Voytko, 2005). In contrast, Baxter et al. (2013) failed to detect any positive E effects on delayed response, with or without oral P. This study compared E administered by implants or via E injections, but compared with Rapp et al. (2003a), used a longer time interval between E injections, did not have a cyclic E-treated group unopposed by P, and had only 5 subjects completing the study in the E implant group. Our study used a 12 month longitudinal design, which may have assisted in revealing a positive E effect compared with previous studies that measured performance at only one time point during HT. Clearly, the differential effects of chronic versus cyclic E and the interactive effects of E and P need to be explored further.
In contrast to most of the studies of older monkeys, E has not been effective in improving delayed response performance in young OVX NHPs (Hao et al., 2007; Voytko et al., 2008). Therefore, young animals are resilient to the loss of ovarian hormones in performance of these tasks, whereas in older animals loss of E generally impairs performance.
The current study also found a positive and sustained effect of OVX+E on cued RT in a test of visuospatial attention. An effect of E was seen for all cue conditions, but in the invalid cue condition the effect emerged most rapidly (by 2 months of HT) and was largest in magnitude (40–55 ms, compared with 25–40 ms for other cue conditions). These results suggest that estrogen heightens general levels of attention or alertness but may have a strong effect in the presence of invalid cues, which require disengagement from the initial locus of attention and reorienting to the correct locus of the target; however, the invalid cue condition also results in the longest RTs, which could alone account for the greater effect. The OVX+E group also showed faster RTs than the other three groups in the neutral (double cue) condition, which may also require disengagement. In any case, the effect of treatment cannot be ascribed to differences in motor speed because a simple RT task found no differences among the groups, and thus the effects can be attributed to differences in central processing speed.
Unlike delayed response, effects of OVX+E on this visuospatial cueing task have been found in both old and young monkeys. In young adult cynomolgus monkeys, OVX was associated with poorer performance on invalid cues at 2 months after surgery, whereas E therapy produced faster response times to neutral, valid, and invalid cues compared with placebo controls (Voytko, 2002). In middle-aged OVX monkeys, placebo-treated monkeys had slower release times on all trial types than E-implant-treated monkeys (Tinkler and Voytko, 2005). A larger study in aged female rhesus used E implants plus cyclic injections of estradiol valerate to mimic the periovulatory surge (Voytko et al., 2008, 2009); placebo and E groups did not differ on RTs to neutral, valid, and invalid cues, but the placebo group had smaller benefits and five times greater costs than E-treated monkeys following 2 weeks of HT, indicating inefficient attentional processing. As in our study, simple RT was not affected by OVX or by HT in any of the previous studies; thus, the effects seen in the cued RT tasks cannot be due to simple alterations in motor function. The benefits of E on visuospatial attentional processing were also observed in a study of young women, with RTs on most trials decreased during the periovulatory, high E phase of the menstrual cycle (Beaudoin and Marrocco, 2005).
Is there a common mechanism for the positive E-effects on the delayed response and visuospatial attention tasks? The DR task of spatial WM is mediated primarily by dorsolateral PFC (Bauer and Fuster, 1976; Bachevalier and Mishkin, 1986; D'Esposito and Postle, 1999, Levy and Goldman-Rakic, 2000), as shown by its sensitivity to lesions or disruption of this area, as well as by the selective, performance-associated activity of neurons in this area during task performance (Goldman-Rakic, 1995). The visuospatial cueing task assesses the ability to orient and shift visuospatial attention and is supported by frontoparietal networks (Mesulam, 1981; Alivisatos and Milner, 1989; Petersen et al., 1989; Corbetta et al., 1993; Nobre et al., 1997; Koski et al., 1998; Hopf and Mangun, 2000; Nobre et al., 2000; Hopfinger et al., 2001; Thiel et al., 2004; Vossel et al., 2006). Given that the monkey PFC is sensitive to the manipulation of ovarian hormones (Kritzer and Kohama, 1999; Gibbs et al., 2002; Tinkler et al., 2004; Wang et al., 2004), especially during middle and old age (Kompoliti et al., 2004; Hao et al., 2006; Browne et al., 2009), it is plausible that the effects of E treatment we observed on both tasks are related to mechanisms of hormonal action in this region.
Additional studies in monkeys that examined a role for E in the modulation of cognitive function are equivocal and difficult to compare with our results due to the use of different tasks, animal ages, and E formulations (Lacreuse et al., 2000, 2001, for review, see Lacreuse, 2006; Lacreuse et al., 2015). The clinical picture is also complex, as hormone formulations, routes of administration, and other factors vary across studies (Maki, 2012). However, some clinical studies in perimenopausal and postmenopausal women support our observations that memory and attention are E sensitive (Kopera, 1972; Anderson et al., 1987; Oldenhave and Netelenbos, 1994; Frackiewicz and Cutler, 2000; Woods et al., 2000).
The interaction of E and P cotreatments warrants some comment, as P attenuated the positive effects of E on both cognitive tasks. Because synthetic progestins also have negative effects on target tissues in menopausal women, notably in the WHI studies (for review, see Gurney et al., 2014), it can be interpreted that the native ligand behaves in a similar manner. However, we also report that our P levels were in the high physiological range, which may have led to unexpected secondary effects. Hence, one needs to interpret the results from the OVX+EP group with caution.
In conclusion, this study shows positive, sustained (12 months) effects of continuous estrogen replacement on cognition in middle-aged, OVX monkeys. Positive effects with initiation of treatment 2.5 months after OVX is consistent with the hypothesis that treatment is effective early in menopause, as opposed to after a long delay. In addition, the chemically defined bioidentical ovarian steroid hormones, 17β estradiol and progesterone, were used in order to provide a hormonal milieu in which to examine the chronic HT effects on brain function. Together, the data demonstrate the benefits of HT on both spatial WM and cued RT in aged, OVX rhesus macaque females, suggesting that it may be possible to achieve similar cognitive benefits in postmenopausal women.
Footnotes
This work was supported by National Institutes of Health Grants AG-019100, AG-024978, AG-026472, AG-036670, and OD-011092. We thank Josephine Gold, Laurie Brown, Dana Myers, Neal Young, Ashley Hoster, Kathryn Hooper, Jesse Brown, and Sasha Hart for expert animal testing and technical assistance.
The authors declare no competing financial interests.
References
- Acosta JI, Hiroi R, Camp BW, Talboom JS, Bimonte-Nelson HA. An update on the cognitive impact of clinically used hormone therapies in the female rat: models, mazes, and mechanisms. Brain Res. 2013;1514:18–39. doi: 10.1016/j.brainres.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alivisatos B, Milner B. Effects of frontal or temporal lobectomy on the use of advance information in a choice reaction time task. Neuropsychologia. 1989;27:495–503. doi: 10.1016/0028-3932(89)90054-7. [DOI] [PubMed] [Google Scholar]
- Anderson E, Hamburger S, Liu JH, Rebar RW. Characteristics of menopausal women seeking assistance. Am J Obstet Gynecol. 1987;156:428–433. doi: 10.1016/0002-9378(87)90298-5. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bauer RH, Fuster JM. Delayed-matching and delayed-response deficit from cooling dorsolateral prefrontal cortex in monkeys. J Comp Physiol Psychol. 1976;90:293–302. doi: 10.1037/h0087996. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Voytko ML. Spatial orienting of attention in adult and aged rhesus monkeys (Macaca mulatta) Behav Neurosci. 1996;110:898–904. doi: 10.1037/0735-7044.110.5.898. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Roberts MT, Gee NA, Lasley BL, Morrison JH, Rapp PR. Multiple clinically relevant hormone therapy regimens fail to improve cognitive function in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2013;34:1882–1890. doi: 10.1016/j.neurobiolaging.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin J, Marrocco R. Attentional validity effect across the human menstrual cycle varies with basal temperature changes. Behav Brain Res. 2005;158:23–29. doi: 10.1016/j.bbr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Browne C, Tobin JR, Voytko ML. Effects of two years of conjugated equine estrogens on cholinergic neurons in young and middle-aged ovariectomized monkeys. Brain Res. 2009;1264:13–23. doi: 10.1016/j.brainres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13:1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinology. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR. The dependence of span and delayed-response performance on prefrontal cortex. Neuropsychologia. 1999;37:1303–1315. doi: 10.1016/S0028-3932(99)00021-4. [DOI] [PubMed] [Google Scholar]
- de Villiers TJ, Gass ML, Haines CJ, Hall JE, Lobo RA, Pierroz DD, Rees M. Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16:203–204. doi: 10.3109/13697137.2013.771520. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macacaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151:3783–3794. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Shumaker SA, Leng I, Manson JE, Brown CM, LeBlanc ES, Vaughan L, Robinson J, Rapp SR, Goveas JS, Lane D, Wactawski-Wende J, Stefanick ML, Li W, Resnick SM. Long-term effects on cognitive function of postmenopausal hormone therapy prescribed to women aged 50 to 55 years. JAMA Intern Med. 2013;173:1429–1436. doi: 10.1001/jamainternmed.2013.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanton JW. Rigid endoscopy. In: Wolfe-Coote S, editor. The laboratory primate. London: Elsevier Academic; 2005. pp. 293–316. [Google Scholar]
- Fischer B, Gleason C, Asthana S. Effects of hormone therapy on cognition and mood. Fertil Steril. 2014;101:898–904. doi: 10.1016/j.fertnstert.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackiewicz EJ, Cutler NR. Women's health care during the perimenopause. J Am Pharm Assoc. 2000;40:800–811. doi: 10.1016/S1086-5802(16)31127-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Does short-term estrogen therapy produce lasting benefits in brain? Endocrinology. 2010;151:843–845. doi: 10.1210/en.2009-1453. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Nelson D, Anthony MS, Clarkson TB. Effects of long-term hormone replacement and of tibolone on choline acetyltransferase and acetylcholinesterase activities in the brains of ovariectomized, cynomologus monkeys. Neuroscience. 2002;113:907–914. doi: 10.1016/S0306-4522(02)00239-7. [DOI] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goodman AL, Descalzi CD, Johnson DK, Hodgen GD. Composite pattern of circulating LH, FSH, estradiol, and progesterone during the menstrual cycle in cynomolgus monkeys. Proc Soc Exp Biol Med. 1977;155:479–481. doi: 10.3181/00379727-155-39834. [DOI] [PubMed] [Google Scholar]
- Gurney EP, Nachtigall MJ, Nachtigall LE, Naftolin F. The Women's Health Initiative trial and related studies: 10 years later. A clinician's view. J Steroid Biochem Mol Biol. 2014;142:4–11. doi: 10.1016/j.jsbmb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Hale GE, Robertson DM, Burger HG. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. 2014;142:121–131. doi: 10.1016/j.jsbmb.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR. Shifting visual attention in space: an electrophysiological analysis using high spatial resolution mapping. Clin Neurophysiol. 2000;111:1241–1257. doi: 10.1016/S1388-2457(00)00313-8. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Woldorff MG, Fletcher EM, Mangun GR. Dissociating top-down attentional control from selective perception and action. Neuropsychologia. 2001;39:1277–1291. doi: 10.1016/S0028-3932(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Howell DC. Best practices in the analysis of variance. In: Osborne JW, editor. Best practices in quantitative methods. Thousand Oaks, CA: Sage; 2008. pp. 341–357. [Google Scholar]
- Jewitt DA, Dukelow WR. Cyclicity and gestation length of Macaca fascicularis. Primates. 1972;13:327–330. doi: 10.1007/BF01730578. [DOI] [Google Scholar]
- Johnson RL, Kapsalis E. Ageing, infecundity and reproductive senescence in free-ranging female rhesus monkeys. J Reprod Fertil. 1995;105:271–278. doi: 10.1530/jrf.0.1050271. [DOI] [PubMed] [Google Scholar]
- Knobil E. On the control of gonadotropin secretion in the female rhesus monkey. Recent Prog Horm Res. 1974;30:1–46. doi: 10.1016/b978-0-12-571130-2.50005-5. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Rosene DL, Sherman LS. Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age. 2012;34:1093–1110. doi: 10.1007/s11357-011-9357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompoliti K, Chu Y, Polish A, Roberts J, McKay H, Mufson EJ, Leurgans S, Morrison JH, Kordower JH. Effects of estrogen replacement therapy on cholinergic basal forebrain neurons and cortical cholinergic innervation in young and aged ovariectomized rhesus monkeys. J Comp Neurol. 2004;472:193–207. doi: 10.1002/cne.20050. [DOI] [PubMed] [Google Scholar]
- Kopera H. Estrogens and psychic functions. Front Hormone Res. 1972;2:118–133. [Google Scholar]
- Koski LM, Paus T, Petrides M. Directed attention after unilateral frontal excisions in humans. Neuropsychologia. 1998;36:1363–1371. doi: 10.1016/S0028-3932(98)00018-9. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, Kohama SG. Ovarian hormones differentially influence immunoreactivity for dopamine beta-hydroxylase, choline acetyltransferase, and serotonin in the dorsolateral prefrontal cortex of adult rhesus monkeys. J Comp Neurol. 1999;409:438–451. doi: 10.1002/(SICI)1096-9861(19990705)409:3<438::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Moss MB. Cognitive function in aged ovariectomized female rhesus monkeys. Behav Neurosci. 2000;114:506–513. doi: 10.1037/0735-7044.114.3.506. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Verreault M, Herndon JG. Fluctuations in spatial recognition memory across the menstrual cycle in female rhesus monkeys. Psychoneuroendocrinology. 2001;26:623–639. doi: 10.1016/S0306-4530(01)00017-8. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Mong JA, Hara Y. Neurocognitive effects of estrogens across the adult lifespan in nonhuman primates: state of knowledge and new perspectives. Horm Behav. 2015;74:157–166. doi: 10.1016/j.yhbeh.2015.03.001. [DOI] [PubMed] [Google Scholar]
- LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: a systematic review and meta-analysis. JAMA. 2001;285:1489–1499. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Maki PM. Minireview: effects of different HT formulations on cognition. Endocrinology. 2012;153:3564–3570. doi: 10.1210/en.2012-1175. [DOI] [PubMed] [Google Scholar]
- Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki P, Hogervorst E. HRT and cognitive decline. Best Pract Res Clin Endocrinol Metab. 2003;17:105–122. doi: 10.1016/S1521-690X(02)00082-9. [DOI] [PubMed] [Google Scholar]
- McBane SE, Borgelt LM, Barnes KN, Westberg SM, Lodise NM, Stassinos M. Use of compounded bioidentical hormone therapy in menopausal women: an opinion statement of the women's health practice and research network of the American College of Clinical Pharmacy. Pharmacotherapy. 2014;34:410–423. doi: 10.1002/phar.1394. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mirkin S, Amadio JM, Bernick BA, Pickar JH, Archer DF. 17β-Estradiol and natural progesterone for menopausal hormone therapy: REPLENISH phase 3 study design of a combination capsule and evidence review. Maturitas. 2015;81:28–35. doi: 10.1016/j.maturitas.2015.02.266. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;1431:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Mesulam MM, Frackowiak RS, Frith CD. Functional localization of the system for visuospatial attention using positron emission tomography. Brain. 1997;120:515–533. doi: 10.1093/brain/120.3.515. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Oldenhave A, Netelenbos C. Pathogenesis of climacteric complaints: ready for the change? Lancet. 1994;343:649–653. doi: 10.1016/S0140-6736(94)92641-7. [DOI] [PubMed] [Google Scholar]
- Overall JE, Tonidandel S. Analysis of data from a controlled repeated measurements design with baseline-dependent dropouts. Methodology. 2007;3:58–66. doi: 10.1027/1614-2241.3.2.58. [DOI] [Google Scholar]
- Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Robinson DL, Currie JN. Influences of lesions of parietal cortex on visual spatial attention in humans. Exp Brain Res. 1989;76:267–280. doi: 10.1007/BF00247887. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative cytoarchitecture analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci U S A. 2002;99:5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003a;2:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D. Effect of estrogen plus progestin on global cognitive function in postmenopausal women. JAMA. 2003b;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8:2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen and dementia: a 2014 update. Mol Cell Endocrinol. 2014;389:7–12. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Lapish CC, Durstewitz D. Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res. 2008;14:249–262. doi: 10.1007/BF03033814. [DOI] [PubMed] [Google Scholar]
- Shao H, Breitner JC, Whitmer RA, Wang J, Hayden K, Wengreen H, Corcoran C, Tschanz J, Norton M, Munger R, Welsh-Bohmer K, Zandi PP. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County study. Neurology. 2012;79:1846–1852. doi: 10.1212/WNL.0b013e318271f823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138:1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive functioning in women: lessons we have learned. Behav Neurosci. 2012;126:123–127. doi: 10.1037/a0025539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Zilles K, Fink GR. Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: an event-related fMRI study. Neuroimage. 2004;21:318–328. doi: 10.1016/j.neuroimage.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Voytko ML. Estrogen modulates cognitive and cholinergic processes in surgically menopausal monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:423–431. doi: 10.1016/j.pnpbp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004;469:507–521. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vossel S, Thiel CM, Fink GR. Cue validity modulates the neural correlates of covert endogenous orienting of attention in parietal and frontal cortex. Neuroimage. 2006;32:1257–1264. doi: 10.1016/j.neuroimage.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Voytko ML. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys. Behav Neurosci. 2000;114:1078–1087. doi: 10.1037/0735-7044.114.6.1078. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Estrogen and the cholinergic system modulate visuospatial attention in monkeys. Behav Neurosci. 2002;116:187–197. doi: 10.1037/0735-7044.116.2.187. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Women's cognitive health special edition. Age (Dordr) 2009;31:189–190. doi: 10.1007/s11357-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer's disease and menopause. Front Biosci. 2004;9:1899–1914. doi: 10.2741/1370. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Olton DS, Richardson RT, Gorman LK, Tobin JR, Price DL. Basal forebrain lesions in monkeys discrupt attention but not learning and memory. J Neurosci. 1994;14:167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience. 2008;154:1205–1217. doi: 10.1016/j.neuroscience.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ML. Menopause in female rhesus monkeys. Am J Primatol. 1995;35:59–71. doi: 10.1002/ajp.1350350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng CM, Zhou J, Smith A, Weickert CS, Perlman WR, Becker KG, Powell D, Bondy CA. Estradiol alters transcription factor gene expression in primate prefrontal cortex. J Neurosci Res. 2004;76:306–314. doi: 10.1002/jnr.20076. [DOI] [PubMed] [Google Scholar]
- Weber MT, Rubin LH, Maki PM. Cognition in perimenopause: the effect of transition stage. Menopause. 2013;20:511–517. doi: 10.1097/GME.0b013e31827655e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NF, Mitchell ES, Adams C. Memory functioning among midlife women: observations from the Seattle midlife women's health study. Menopause. 2000;7:257–265. doi: 10.1097/00042192-200007040-00008. [DOI] [PubMed] [Google Scholar]