Abstract
Objective. The aim of this systematic review was to investigate the advances in the study of medicinal plants and their biologic effects on periodontitis in animal models. Study Design. A systematic search was conducted by three independent researchers, who screened articles published up to March/2016, to identify the studies that contained sufficient and clear information on the association of the medicinal plants and periodontitis in murine models. The searches were performed using PubMed, Cochrane, and Science Direct databases. Results. After a critical analysis of titles and abstracts, 30 studies were finally eligible for analysis. The studies presented a great diversity of the experiment designed regarding the methods of induced periodontitis and the evaluation of the medicinal plants efficacy. None of the studies described the possible toxic effects associated with the administration of the plant material to animals and whether they could prevent damage to organs caused by systemic effect of induced periodontitis. Gel-based formulations containing plant substances are seen as an interesting strategy to treat periodontitis. Conclusions. In this systematic review, the state-of-the-art knowledge on the medicinal plants and the induced periodontitis was critically evaluated and discussed from the experiment designed to the possible clinical application.
1. Introduction
Periodontitis is one of the most extensive oral problems that affect human population, resulting from an inflammatory response against microorganisms involved with plaque accumulation on the subgingival dental surface. The development of the process depends on the interaction between the bacteria in the site of infection. The presence of an oral biofilm composed by bacteria and their products includes also lipopolysaccharides and proteinases that are responsible for the progression of periodontitis. Bacteria stimulate host immunopathological and inflammatory mechanisms that result in the destruction of the periodontal tissue [1].
Different strategies have been used to treat periodontal diseases. Mechanical therapy and surgical procedures reduce microbial burden, being effective in the control of the periodontitis progression. Nevertheless, this regulation is not always satisfactory, possibly due to the prominent role of immunogenetic response on periodontal destruction. In some cases, adjunctive therapies may be required [2]. Thus, the discovery and development of potential therapeutic drugs with the ability to regulate the host immune and bacteria-mediated inflammatory interactions are a valuable approach for the prevention and treatment of the periodontal disease [2–4]. In this context, plants can represent an interesting source of molecules with a potential activity against periodontal disease progression. The growing incidence of periodontitis, the increased resistance of oral bacteria to antibiotics, and the adverse effects of some drugs used in dentistry all motivate the search for safe and effective molecules to treat and prevent the disease [5].
Medicinal plants have been fundamental for thousands of years to provide bioactive molecules used to treat different types of human infirmities, such as inflammation, pain, and tumors. Also, they can be a source of compounds to be tested in the treatment of periodontal diseases. Nowadays, there is an increasing number of scientific investigation exploring plant extracts or purified molecules in periodontal diseases [3]. Several of these studies were performed using animal models since biochemical, histological, and anatomical features are similar to humans [3, 4, 6, 7].
The aim of this systematic review was to investigate the advances in the study of medicinal plants and the development of induced periodontitis in animal models. Data were described and discussed in order to evaluate the limitation and also the perspectives of the application of these agents in the treatment of the periodontal disease.
2. Material and Methods
A systematic search was conducted by three independent researchers who screened articles published up to March/2016 in order to identify the studies that contained sufficient and clear information on the association of the medicinal plants and periodontitis in murine models. The searches were performed using PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Cochrane (http://www.cochranelibrary.com/), and Science Direct (http://www.sciencedirect.com/) databases using the key words plant, periodontitis, and rats and search details “plant” [All Fields] AND “periodontitis” [All Fields] AND “rats” [All Fields]. The articles selected by each researcher were compared to remove duplicate records and 109 different articles were initially evaluated. After a critical analysis of titles and abstracts, 79 articles were excluded such as articles that were not in English, articles that were not fully available, or those that did not report an association between medicinal products and murine model of periodontitis. Hence, 30 studies were finally eligible for a qualitative analysis for this review (Figure 1).
Figure 1.
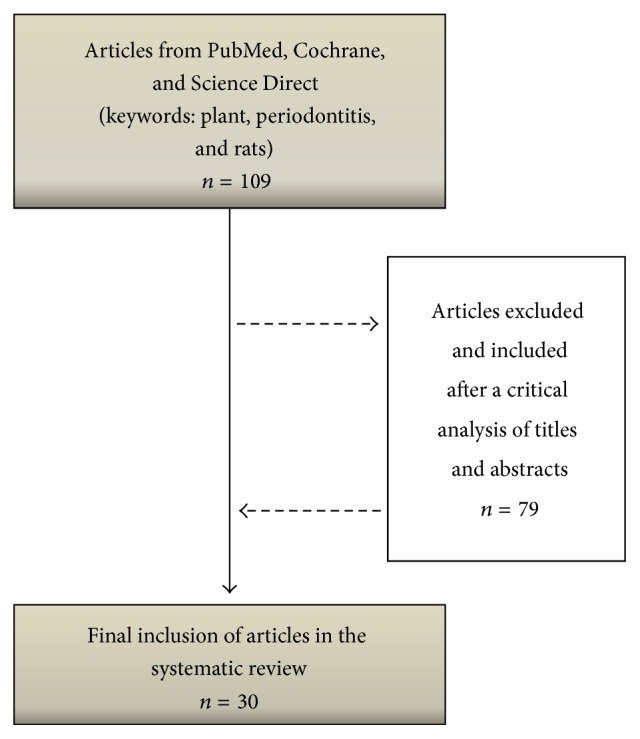
Search flowchart and selection of articles for the review of the literature.
3. Results and Discussion
Table 1 summarize plant species, plant material, route of administration, animal used, type of induction and time of analysis, and the ability of the medicinal plant to reduce alveolar bone loss related to 30 selected articles.
Table 1.
List of medicinal plants, experimental methods, and their biological effects on induced periodontitis.
| Plant species | Plant material | Route of administration | Animal used (gender) |
Type of induction/time of analysis | Antibacterial effect1 | Bone loss/method2 | Anti-inflammatory activity3 | Reference |
|---|---|---|---|---|---|---|---|---|
| Panax notoginseng and Rehmannia radix | Mixture of extracts (9 : 1 weight) | Oral | Rattus norvegicus (male) | Injecting E. coli endotoxin (LPS)/28 days | Not evaluated | Reduced ABC/µCT | Reduced the in vitro release of TNF-α from human monocytic cells and hGF cells | Almeida et al. [2] |
|
| ||||||||
| Ocimum sanctum | Tulsi extract | Topical | Rattus norvegicus (male and female) | Silk ligature/9 days | Not evaluated | Not reduced ABC/Stereo | Presented anti-inflammatory activity in another experimental model | Hosadurga et al. [4] |
|
| ||||||||
| Scutellaria baicalensis | Baicalin | Oral | Rattus norvegicus (male) | Nylon ligature/7 days | Not evaluated | Reduced ABC/histology | Not evaluated | Cai et al. [6] |
|
| ||||||||
| Rhizoma coptidis, Hydrastis canadensis, and Cortex phellodendri | Berberine | Oral | Rattus norvegicus (male) | Silk ligature/8 days | Not evaluated | Reduced ABC/µCT | Not evaluated | Tu et al. [7] |
|
| ||||||||
| Cucurbita pepo, Mentha piperita, Crataegus spp., Rosmarinus officinalis, Capsicum annuum, and Achillea millefolium | LongoVital® | Oral | Rattus norvegicus (male and female) | Injecting A. viscosus and P. gingivalis/63 days | Not evaluated | Reduced ABC/Radio | Not evaluated | Klausen et al. [8] |
|
| ||||||||
| Mangifera indica | Mangiferin (Sigma-Aldrich Co.) |
Oral | Rattus norvegicus (male) | Cotton ligature/1, 4, and 7 days | Not evaluated | Reduced ABC/histology | Inhibited COX-2 expression, the rolling, and adhesion of leukocytes in the periodontal tissue | Carvalho et al. [9] |
|
| ||||||||
| Curcuma longa | Curcumin (Sigma-Aldrich Co.) |
Gavage | Rattus norvegicus (male) | Nylon ligature/30 days | Not evaluated | Reduced ABC/histology | Reduced the expression of TNF-α in gingival tissues | Zhou et al. [10] |
|
| ||||||||
| Magnolia officinalis | Magnolol (Sigma-Aldrich Co.) |
Oral | Rattus norvegicus (male) | Silk ligature/9 days | In vitro activity against P. gingivalis and A. actinomycetemcomitan | Reduced ABC/µCT | Inhibited neutrophil migration, MPO activity, and COX-2 and iNOS expression in gingival tissues | Lu et al. [11] |
|
| ||||||||
| Rhizoma drynariae and Rehmannia glutinosa | Bu-Shen-Gu-Chi-Wan (JiuZhiTang Pharmaceutical) |
Oral | Rattus norvegicus (female) | Injecting P. gingivalis and ligature/28 days | Not evaluated | Improved the mineral density of the bone/µCT | Reduced levels of IL-1β, TNF-α, and inflammatory cell infiltration in the periodontal tissues | Yang et al. [12] |
|
| ||||||||
| Pinus pinaster | Pycnogenol® (Tradepia Co.) |
Oral | Balb/c (male) | Injecting P. gingivalis/34 days | Antibacterial activity against P. gingivalis | Reduced ABC/Stereo | Not evaluated | Sugimoto et al. [13] |
|
| ||||||||
| Vaccinium macrocarpon | Aqueous extract containing tannin and phenolic compounds | Oral | Mus musculus (female) | Injecting P. gingivalis and F. nucleatum/42 days | Anti-adhesive properties against P. gingivalis and F. nucleatum. Increased the phagocytosis of P. gingivalis | Not evaluated | Reduced in vivo levels of TNF-α | Polak et al. [15] |
|
| ||||||||
| Curcuma longa | Curcumin | Topical | Rattus norvegicus | Silk ligature/28 days | Not evaluated | Did not reduce ABC/Stereo | Exhibited anti-inflammatory activity in another experimental model | Hosadurga et al. [16] |
|
| ||||||||
| Camellia sinensis | Extract containing Catechin | Topical | Rattus norvegicus (male) | Injecting E. coli (LPS) and S. griseus (proteases)/56 days | Not evaluated | Did not reduce ABC/histology | Reduced inflammatory cell infiltration and levels of TNF-α
in the periodontal lesion |
Maruyama et al. [19] |
|
| ||||||||
| Spatholobus suberectus | Aqueous extract | Oral | Mus musculus (male) | Injecting P. gingivalis /42 days | In vitro antibacterial activity against P. gingivalis | Reduced ABC/Stereo | Not evaluated | Toyama et al. [20] |
|
| ||||||||
| Carapa guianensis | Andiroba oil | Oral | Rattus norvegicus (male) | Cotton ligature/50 days | Not evaluated | Did not reduce ABC/histology | Reduced the quantity of inflammatory cells in histology | Carmona et al. [21] |
|
| ||||||||
| Protium heptaphyllum | α-amyrin and β-amyrin | Oral | Rattus norvegicus (male) | Nylon ligature/1 day | Not evaluated | Not evaluated | Reduced gingival TNF-α levels and MPO activity | Holanda Pinto et al. [22] |
|
| ||||||||
| Camellia sinensis | Extract containing Catechin | Oral | Rattus norvegicus (male) | Nylon ligature/7, 14, and 28 days | Not evaluated | Reduced ABC/histology | Reduced in vivo levels of TNF-α | Cho et al. [23] |
|
| ||||||||
| Ipomoea alba | Mixture of dichloromethane and methanol extract | Topical | Rattus norvegicus (male) | Cotton ligature/11 days | In vitro antibacterial activity against S. mutans and E. faecalis | Did not reduce ABC/Stereo | Not evaluated | Barrella et al. [24] |
|
| ||||||||
| Lippia sidoides and Myracrodruon urundeuva | Mixture of leaf essential oil and hydroalcoholic solution from bark | Topical | Rattus norvegicus (male) | Nylon ligature/11 days | Prevented the growth of oral microorganisms from gingival tissue | Reduced ABC/histology | Reduced MPO activity and inhibited TNF-α and IL-1β production in gingival tissue | Botelho et al. [25] |
|
| ||||||||
| Lippia sidoides | Carvacrol | Topical | Rattus norvegicus (male) | Nylon ligature/11 days | Prevented the growth of oral microorganisms from gingival tissue | Reduced ABC/AFM | Reduced MPO activity in gingival tissue | Botelho et al. [26] |
|
| ||||||||
| Hypericum Perforatum | Methanolic extract | Oral | Rattus norvegicus (male) | Silk ligature /8 days | Not evaluated | Reduced ABC/Stereo | Reduced inflammatory cell infiltration, vascular permeability, expression of NF-κB, and MPO activity in gingivomucosal tissue | Paterniti et al. [27] |
|
| ||||||||
| Cordia verbenacea | Essential oil | Topical | Rattus norvegicus (male) | Cotton ligature/11 days | Reduced in vivo frequency of P. gingivalis and A. actinomycetemcomitans | Reduced ABC/histology | Increment in the in vivo levels of IL-10 | Pimentel et al. [28] |
|
| ||||||||
| Camellia sinensis | Extract containing Catechin | Topical | Rattus norvegicus (male) | Injecting E. coli (LPS)/10 and 20 days | Not evaluated | Reduced ABC/histology | Reduced inflammatory cell infiltration | Yoshinaga et al. [29] |
|
| ||||||||
| Theobroma cacao | Cocoa extract containing flavonoids | Oral | Rattus norvegicus (male) | Cotton ligature/28 days | Not evaluated | Reduced ABC/histology | Reduced/oxidized glutathione ratio and neutrophil infiltration | Tomofuji et al. [30] |
|
| ||||||||
| Mikania laevigata | Ethanol extract | Subcutaneous | Rattus norvegicus (male) | Nylon ligature/30 days | Not evaluated | Reduced the furcation region/histology | Reduced neutrophil accumulation in the gingival tissue | Benatti et al. [31] |
|
| ||||||||
| Pimpinella anisum, Illicium verum, and Anethum foeniculum | Anethole | Intraperitoneal | Rattus norvegicus (male) | Injecting E. coli (LPS)/10 days | Not evaluated | Not evaluated | Reduced serum levels of IL-1β and TNF-α | Moradi et al. [32] |
|
| ||||||||
| Curcuma spp. | Modified curcumin | Gavage | Rattus norvegicus (male) | Injecting E. coli (LPS)/14 days | Not evaluated | Reduced ABC/Stereo, and µCT | Reduced serum level of IL-1β | Elburki et al. [33] |
|
| ||||||||
| Syringa vulgaris | Product of fermentation |
Oral | Rattus norvegicus (male) | Silk ligature/8 days | Not evaluated | Reduced ABC/Stereo | Reduced NF-κB and iNOS expression, MPO activity, and other inflammatory parameters in gingivomucosal tissue | Paola et al. [34] |
|
| ||||||||
| Ginkgo biloba | Ginkgo biloba extract | Oral | Rattus norvegicus (male) | Silk ligature/11 days | Not evaluated | Reduced ABC/histology | Not evaluated | Sezer et al. [35] |
|
| ||||||||
| Curcuma spp. | Curcumin | Gavage | Rattus norvegicus (male) | Cotton ligature/15 days | Not evaluated | Did not reduce ABC/µCT | Reduced the inflammatory cell infiltrate to gingival tissue | Guimarães et al. [36] |
1 Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Streptococcus mutans, Streptococcus sanguinis, Enterococcus faecalis, and Fusobacterium nucleatum. 2ABC: alveolar bone crest, µCT: microcomputed tomography, AFM: atomic force microscopy, Stereo: stereomicroscopy, Radio: radiography. 3TNF-α: tumor necrosis factor α, hGF: hepatocyte growth factor, MPO: myeloperoxidase activity, IL-1β: interleukin-1 beta, IL-10: interleukin-10, NF-κB: nuclear factor kappa-β, COX-2: ciclooxigenase-2, and iNOS: inducible nitric oxide synthase.
The majority of articles (43.3%) described studies conducted with plant extracts, 40% with purified compounds and 16.6% with a mixture of two or more plant extracts. Among them, six articles described experiments developed using a commercial product [8–13], containing purified compounds or a mixture of plant extracts. None of authors mentioned the process of obtaining selected plant material as an important limitation for development of the research.
In general, studies were preferentially conducted using Rattus norvegicus (90%) of different strains Wistar, Lewis, or Sprague-Dawley. This specie is one of the mostly used for in vivo experimental models, including the pathogenesis of periodontal disease because they offer some advantages such as price, easy handling, and the possibility of microbiological, macroscopic, and histological evaluation [14]. Other studies were conducted using Mus musculus Balb/c (6.6%) or C57BL/6. Rodents were preferred as the animal model since they present biochemical, histological, and anatomical features similar to humans [14].
Taking into consideration the sex of the animal, most authors chose to use males (86.6%). Two articles described the use of females [12, 15] and two studies were conducted using males and females [4, 8]. One study did not describe the sex of the animals [16]. Most in vivo studies which investigate the biological effect of plant substances favor the use of male animals. This choice is based on the fact that female hormones should interfere in the development and progression of the disease [17]. None of the articles analyzed was related to the hormonal influence on the biological activities investigated. However, studies conducted with females could clarify the effect of their hormones on the specific pathways involved in the periodontitis progression.
Regarding the common type of periodontitis-induction, in 66.6% of the articles, the process was caused by ligature (silk: 23.3%, nylon: 23.3%, and cotton: 20%). This method is widely used because it facilitates the accumulation of biofilm. This procedure increases the infiltration of inflammatory cells and the production of chemical mediators that lead to the degradation of the tissues around the teeth contributing to the destruction of the periodontal tissues [1]. In addition to periodontitis induced by ligature, 16.6% of articles described the gingival injection of bacteria, such as P. gingivalis and 16.6% described the administration of E. coli endotoxin (LPS) or S. griseus (proteases) to promote the periodontal disease. The inoculation of bacteria or their subproducts leads to an inflammatory response different from that promoted by periodontitis induced through induction with ligature [18].
Some of the studies analyzed described that long periods of experimental design were utilized to properly investigate the severity of tissue destruction during periodontal disease treatment: 63 days [8]; 56 days [19]; 42 days [20]; and 50 days [21]. However, the time of analysis changed substantially. More than 55% of the studies performed experiments during one to two weeks, 26.6% for three to four weeks, and 13.3% during six weeks or more. One study was conducted with an acute periodontitis rat model in 24 hours [22]. These variations in the experimental periods may be due to factors such as the type and location of the ligature, the bacteria species or their sub-products injected for the induction of the disease and the specie (strain), and age and weight of the animal used. These factors are generally associated with the objective of the study and the expected results.
More than 66% of studies chose the oral administration to evaluate the efficacy of the plant material. The topical administration was described by 26.6% of the studies and 6.9% treated animals through subcutaneous or intraperitoneal cavity injections. The route of administration is an important parameter to influence the efficacy of the material since it can interfere with the sufficient amount of substance available to promote the biological effects. As observed, different results were seen for alveolar bone loss of animals submitted to the treatment with an extract containing Catechin obtained from Camellia sinensis. Although the route of administration had not influenced the anti-inflammatory activity of the material, a significant reduction of bone loss was observed when given orally [23] and no difference was seen after its topical treatment [19].
Periodontal disease initiation and progression occur as a consequence of the host response to microorganisms of the dental biofilm [1]. Therefore, the antibacterial effect is an important factor in the periodontal therapy. Only 26.6% of the studies investigated the capacity of the material to present antibacterial activity. In the study developed by Barrella et al. [24] the organic extract obtained from Ipomoea alba showed significant in vitro activity against Streptococcus mutans, S. sanguinis, and Enterococcus faecalis. The commercial product Magnolol obtained from Magnolia officinalis exhibited intense inhibition of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans growth in a dependent dose [11] and the aqueous extract from Vaccinium macrocarpon containing tannin and phenolic compounds inhibited the adhesion of Fusobacterium nucleatum and Porphyromonas gingivalis [15]. Study developed by Botelho et al. [25] and Botelho et al. [26] with Lippia sidoides observed the decrease of salivary bacterial levels and this event was followed by an increment in clinical scores of gingival bleeding. Except for the work published by Klausen et al. [8] which did not evaluate the alveolar bone loss, all the articles that described the identification of antibacterial activity also demonstrated that plant material treatment induced a significant reduction of alveolar bone loss.
The alveolar bone loss is one of the most important parameters evaluated in the induced periodontal disease. From 30 selected articles, 27 evaluated the ability of the plant material to promote a significant reduction of the bone loss. Carvacrol purified from Lippia sidoides, the extract containing Catechin obtained from Camellia sinensis, the essential oil of Cordia verbenacea, and the methanolic extract of Hypericum perforatum are examples of 77% of studies that observed a significant reduction of the bone loss [23, 26–28]. On the other hand, in 23% of studies such as andiroba oil from Carapa guianensis, as well as the mixture of dichloromethane and methanol extracts from Ipomoea alba and Longo Vital, the authors did not observe a significant effect [8, 21, 24]. Moreover, it was interesting to note that different studies using the same plant material have reached different results regarding alveolar bone loss. According to Yoshinaga et al. [29] the topical treatment of animals with the extract containing Catechin (Camellia sinensis) promoted a significant reduction of bone loss in periodontitis induced by E. coli (LPS). On the other hand, the same material did not present a significant reduction in the alveolar bone loss against periodontitis induced by injection of E. coli (LPS) and S. griseus (proteases) [19]. Different efficacy was also observed in studies developed with curcumin (Curcuma longa). The topical administration of curcumin did not reduce the alveolar bone loss [16] while its oral administration reduced this parameter significantly [10]. The methodologies used to evaluate the alveolar bone loss were histology (44.0%), stereomicroscopy (25.9%), microcomputed tomography (22.2%), atomic force microscopy (3.7%), and radiography (3.7%). Histology, stereomicroscopy, and microcomputed tomography are favorable methods once they yield precise information concerning the evaluation of periodontal tissue destruction.
It is noteworthy that the reduction of the inflammatory process may be associated with a reduced bone loss [12, 30, 31]. Because of this, a reduction of inflammatory process is usually investigated in periodontal disease. Taking the selected articles into account, 76.6% of them evaluated inflammatory parameters associated with induced periodontitis. The common strategies used to observe the ability of the plant material to inhibit the inflammatory process in periodontal tissue were the evaluation of migration of inflammatory cells (30%), measurement of proinflammatory cytokines (TNF-α: 30% and IL1-β: 13.3%) [2, 10, 12, 15, 19, 22, 23, 25, 32, 33], respectively, and dosage of myeloperoxidase (MPO) activity (20%) [11, 22, 25–27, 34]. Other molecular markers of inflammatory process, such as iNOS (13.0%) [11, 34], NF-κB (8.7%) [27, 34], COX-2 (8.7%) [9, 11], and IL-10 (4.3%) [28], were also investigated. Although the presence of the anti-inflammatory effect reflects a possible efficacy against periodontitis, 13% of the authors who found anti-inflammatory properties did not observe a significant reduction of alveolar bone loss in the periodontitis assays. The anti-inflammatory activity was not analyzed in the 23.3% [6–8, 13, 20, 24, 35] of the studies. The andiroba oil (Carapa guianensis), the extract from Camellia sinensis (containing Catechin), and Curcumin (Curcuma spp.) were able to reduce the number of inflammatory cells in the gingival tissue. However, they were not effective to protect from destructive periodontal process [19, 21, 36]. According to the authors, these negative results may be related to the time delay to reach levels that are high enough for the biological effects of administered substances in the experimental models adopted. Further researches are necessary to observe how much time is appropriate to manage the substances for the recovery of bone loss.
Although there is a close relation between the bone reabsorption and the inflammatory response, we suggest that the negative results of some of substances analyzed should be attributed to the fact that they do not act in the osteoclastogenesis process. This information is supported by the personal observation conducted using sulphated polysaccharides recovered from red marine algae Gracilaria caudata. Our data revealed that the treatment of experimental animals with sulphated polysaccharides improved clinical and inflammatory parameters. However, no significant effect was observed in the reduction of alveolar bone loss (unpublished data).
It is important to mention that, although many plant substances may have deleterious effects to different animal organs [37], none of the evaluated studies has investigated the possible toxicological effects associated with the administration of the medicinal plants. In addition, several studies have documented that induced periodontitis is followed by significant changes of morphological structures and biochemical functions of different organs [38, 39]. Yet again, none of studies investigated the ability of these medicinal plants to prevent changes in organs promoted by a systemic action of the induced periodontitis.
Finally, 8 of the 30 selected articles conducted their experiments with a gel-based formulation containing the plant material investigated. In these experiments, the authors administered the gel topically, generally three times a day. The main idea behind these investigations is to reveal the further potential of the combined gel preparation to combat periodontal disease in closer than clinical situations. This was the outlook investigated in the studies published by [40, 41]. The authors evaluated the efficiency of a green tea Catechin gel as an adjunct on human periodontal therapy. In first work [40], the authors observed a significant reduction on pockets and inflammation during the 4 weeks of the clinical trial. In the second work [41], it was demonstrated that when used as an adjunct to periodontal treatment, green tea gel could provide benefit in reducing bleeding on probing and gingival inflammation at 1st and 3rd months of evaluation. What makes these studies even more relevant is the fact that the findings on complementary products for the treatment of human periodontitis still can be enhanced.
4. Conclusion
In conclusion, the selected studies presented a large diversity of experimental designs, concerning the type of induction, time of analysis, and methods used for the evaluation of alveolar bone loss, anti-inflammatory, and antibacterial activities. None of the studies evaluated the possible toxic effects associated with the administration of the material analyzed or their ability to prevent damages to organs caused by systemic effects of induced periodontitis. Gel-based formulations present an interesting strategy to treat periodontitis; however, further studies are necessary to clarify its usefulness in the clinical situation.
Acknowledgments
This research is supported by the Federal University of Piauí (UFPI-Edital PIBIC 2014/2015 and BIAMA 03/2014), CNPq (455104/2014-0). The authors thank teacher Abilio Borghi for the grammar review of the paper.
Competing Interests
The authors declare that they have no conflict of interests.
References
- 1.Bascones-Martínez A., Muñoz-Corcuera M., Noronha S., Mota P., Bascones-Ilundain C., Campo-Trapero J. Host defence mechanisms against bacterial aggression in periodontal disease: basic mechanisms. Medicina Oral, Patologia Oral y Cirugia Bucal. 2009;14(12):e680–e685. doi: 10.4317/medoral.14.e680. [DOI] [PubMed] [Google Scholar]
- 2.De Almeida J., Ervolino E., Bonfietti L. H., et al. Adjuvant therapy with sodium alendronate for the treatment of experimental periodontitis in rats. Journal of Periodontology. 2015;86(10):1166–1175. doi: 10.1902/jop.2015.150166. [DOI] [PubMed] [Google Scholar]
- 3.Huang R.-Y., Lu S.-H., Su K.-W., et al. Diacerein: a potential therapeutic drug for periodontal disease. Medical Hypotheses. 2012;79(2):165–167. doi: 10.1016/j.mehy.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 4.Hosadurga R. R., Rao S. N., Edavanputhalath R., et al. Evaluation of the efficacy of 2% Ocimum sanctum gel in the treatment of experimentalperiodontitis. International Journal of Pharmaceutical Investigation. 2015;5(1):35–42. doi: 10.4103/2230-973x.147231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardila C. M., López M. A., Guzmán I. C. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Medicina Oral, Patologia Oral y Cirugia Bucal. 2010;15(6):e947–e951. doi: 10.4317/medoral.15.e947. [DOI] [PubMed] [Google Scholar]
- 6.Cai X., Li C., Du G., Cao Z. Protective effects of baicalin on ligature-induced periodontitis in rats. Journal of Periodontal Research. 2008;43(1):14–21. doi: 10.1111/j.1600-0765.2007.00989.x. [DOI] [PubMed] [Google Scholar]
- 7.Tu H.-P., Fu M. M. J., Kuo P.-J., et al. Berberine's effect on periodontal tissue degradation by matrix metalloproteinases: an in vitro and in vivo experiment. Phytomedicine. 2013;20(13):1203–1210. doi: 10.1016/j.phymed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Klausen B., Apostolopoulos A., Stoltze K., Nörgaard F. Effect of LongoVital treatment on development of periodontal disease in rats. Scandinavian Journal of Dental Research. 1993;101(1):33–36. doi: 10.1111/j.1600-0722.1993.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho R. R., Pellizzon C. H., Justulin L., Jr., et al. Effect of mangiferin on the development of periodontal disease: involvement of lipoxin A4, anti-chemotaxic action in leukocyte rolling. Chemico-Biological Interactions. 2009;179(2-3):344–350. doi: 10.1016/j.cbi.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 10.Zhou T., Chen D., Li Q., Sun X., Song Y., Wang C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontologica Scandinavica. 2013;71(2):349–356. doi: 10.3109/00016357.2012.682092. [DOI] [PubMed] [Google Scholar]
- 11.Lu S.-H., Huang R.-Y., Chou T.-C. Magnolol ameliorates ligature-induced periodontitis in rats and osteoclastogenesis: in vivo and in vitro study. Evidence-Based Complementary and Alternative Medicine. 2013;2013:12. doi: 10.1155/2013/634095.634095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang H., Wen Q., Xue J., Ding Y. Alveolar bone regeneration potential of a traditional Chinese medicine, Bu-Shen-Gu-Chi-Wan, in experimental periodontitis. Journal of Periodontal Research. 2014;49(3):382–389. doi: 10.1111/jre.12117. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto H., Watanabe K., Toyama T., et al. Inhibitory effects of French pine bark extract, pycnogenol®, on alveolar bone resorption and on the osteoclast differentiation. Phytotherapy Research. 2015;29(2):251–259. doi: 10.1002/ptr.5245. [DOI] [PubMed] [Google Scholar]
- 14.Struillou X., Boutigny H., Soueidan A., Layrolle P. Experimental animal models in periodontology: a review. The Open Dentistry Journal. 2010;4(1):33–47. doi: 10.2174/1874210601004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polak D., Naddaf R., Shapira L., Weiss E. I., Houri-Haddad Y. Protective potential of non-dialyzable material fraction of cranberry juice on the virulence of P. gingivalis and F. nucleatum mixed infection. Journal of Periodontology. 2013;84(7):1019–1025. doi: 10.1902/jop.2012.120331. [DOI] [PubMed] [Google Scholar]
- 16.Hosadurga R. R., Rao S., Jose J., Rompicharla N. C., Shakil M., Shashidhara R. Evaluation of the efficacy of 2% curcumin gel in the treatment of experimental periodontitis. Pharmacognosy Research. 2014;6(4):326–333. doi: 10.4103/0974-8490.138287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Figuero E., Carrillo-De-Albornoz A., Herrera D., Bascones-Martínez A. Gingival changes during pregnancy: I. Influence of hormonal variations on clinical and immunological parameters. Journal of Clinical Periodontology. 2010;37(3):220–229. doi: 10.1111/j.1600-051x.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 18.Garcia de Aquino S., Manzolli Leite F. R., Stach-Machado D. R., Francisco da Silva J. A., Spolidorio L. C., Rossa C., Jr. Signaling pathways associated with the expression of inflammatory mediators activated during the course of two models of experimental periodontitis. Life Sciences. 2009;84(21-22):745–754. doi: 10.1016/j.lfs.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama T., Tomofuji T., Endo Y., et al. Supplementation of green tea catechins in dentifrices suppresses gingival oxidative stress and periodontal inflammation. Archives of Oral Biology. 2011;56(1):48–53. doi: 10.1016/j.archoralbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 20.Toyama T., Todoki K., Takahashi Y., et al. Inhibitory effects of Jixueteng on P. gingivalis-induced bone loss and osteoclast differentiation. Archives of Oral Biology. 2012;57(11):1529–1536. doi: 10.1016/j.archoralbio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Carmona G. B., Teixeira R. K. C., Brito M. V. H., et al. Effect of andiroba oil on periodontitis in wistar rats. Acta Cirurgica Brasileira. 2013;28(6):430–434. doi: 10.1590/S0102-86502013000600005. [DOI] [PubMed] [Google Scholar]
- 22.Holanda Pinto S. A., Pinto L. M. S., Cunha G. M. A., Chaves M. H., Santos F. A., Rao V. S. Anti-inflammatory effect of α, β-Amyrin, a pentacyclic triterpene from Protium heptaphyllum in rat model of acute periodontitis. Inflammopharmacology. 2008;16(1):48–52. doi: 10.1007/s10787-007-1609-x. [DOI] [PubMed] [Google Scholar]
- 23.Cho A.-R., Kim J.-H., Lee D.-E., et al. The effect of orally administered epigallocatechin-3-gallate on ligature-induced periodontitis in rats. Journal of Periodontal Research. 2013;48(6):781–789. doi: 10.1111/jre.12071. [DOI] [PubMed] [Google Scholar]
- 24.Barrella G. E., Suffredini I. B., Ribeiro F. V., Cirano F. R., Pimentel S. P. Evaluation of the effect of an organic extract obtained from Ipomoea alba L. on experimental periodontitis in rats. Brazilian Oral Research. 2012;26(2):158–164. doi: 10.1590/s1806-83242012000200012. [DOI] [PubMed] [Google Scholar]
- 25.Botelho M. A., Rao V. S., Carvalho C. B. M., et al. Lippia sidoides and Myracrodruon urundeuva gel prevents alveolar bone resorption in experimental periodontitis in rats. Journal of Ethnopharmacology. 2007;113(3):471–478. doi: 10.1016/j.jep.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Botelho M. A., Martins J. G., Ruela R. S., et al. Protective effect of locally applied carvacrol gel on ligature-induced periodontitis in rats: a tapping mode AFM study. Phytotherapy Research. 2009;23(10):1439–1448. doi: 10.1002/ptr.2798. [DOI] [PubMed] [Google Scholar]
- 27.Paterniti I., Briguglio E., Mazzon E., et al. Effects of Hypericum Perforatum, in a rodent model of periodontitis. BMC Complementary and Alternative Medicine. 2010;10, article 73 doi: 10.1186/1472-6882-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pimentel S. P., Barrella G. E., Casarin R. C. V., et al. Protective effect of topical Cordia verbenacea in a rat periodontitis model: immune-inflammatory, antibacterial and morphometric assays. BMC Complementary and Alternative Medicine. 2012;12(1, article 224):1–8. doi: 10.1186/1472-6882-12-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshinaga Y., Ukai T., Nakatsu S., et al. Green tea extract inhibits the onset of periodontal destruction in rat experimental periodontitis. Journal of Periodontal Research. 2014;49(5):652–659. doi: 10.1111/jre.12147. [DOI] [PubMed] [Google Scholar]
- 30.Tomofuji T., Ekuni D., Irie K., et al. Preventive effects of a cocoa-enriched diet on gingival oxidative stress in experimental periodontitis. Journal of Periodontology. 2009;80(11):1799–1808. doi: 10.1902/jop.2009.090270. [DOI] [PubMed] [Google Scholar]
- 31.Benatti B. B., Campos-Júnior J. C., Silva-Filho V. J., et al. Effects of a Mikania laevigata extract on bone resorption and RANKL expression during experimental periodontitis in rats. Journal of Applied Oral Science. 2012;20(3):340–346. doi: 10.1590/S1678-77572012000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moradi J., Abbasipour F., Zaringhalam J., et al. Anethole, a medicinal plant compound, decreases the production of pro-inflammatory TNF-α and IL-1β in a rat model of LPS-induced periodontitis. Iranian Journal of Pharmaceutical Research. 2014;13(4):1319–1325. [PMC free article] [PubMed] [Google Scholar]
- 33.Elburki M. S., Rossa C., Guimaraes M. R., et al. A novel chemically modified curcumin reduces severity of experimental periodontal disease in rats: Initial observations. Mediators of Inflammation. 2014;2014:10. doi: 10.1155/2014/959471.959471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paola R. D. I., Oteri G., Mazzon E., et al. Effects of verbascoside, biotechnologically purified by Syringa vulgaris plant cell cultures, in a rodent model of periodontitis. Journal of Pharmacy and Pharmacology. 2011;63(5):707–717. doi: 10.1111/j.2042-7158.2011.01262.x. [DOI] [PubMed] [Google Scholar]
- 35.Sezer U., Kara M. İ., Erciyas K., et al. Protective effects of ginkgo biloba extract on ligature-induced periodontitis in rats. Acta Odontologica Scandinavica. 2013;71(1):38–44. doi: 10.3109/00016357.2011.650195. [DOI] [PubMed] [Google Scholar]
- 36.Guimarães M. R., Coimbra L. S., de Aquino S. G., Spolidorio L. C., Kirkwood K. L., Rossa C., Jr. Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo . Journal of Periodontal Research. 2011;46(2):269–279. doi: 10.1111/j.1600-0765.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ezuruike U. F., Prieto J. M. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. Journal of Ethnopharmacology. 2014;155(2):857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- 38.Tomofuji T., Ekuni D., Yamanaka R., et al. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. Journal of Periodontology. 2007;78(10):1999–2006. doi: 10.1902/jop.2007.070056. [DOI] [PubMed] [Google Scholar]
- 39.Tomofuji T., Sanbe T., Ekuni D., et al. Oxidative damage of rat liver induced by ligature-induced periodontitis and chronic ethanol consumption. Archives of Oral Biology. 2008;53(12):1113–1118. doi: 10.1016/j.archoralbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Chava V. K., Vedula B. D. Thermo-reversible green tea catechin gel for local application in chronic periodontitis: a 4-week clinical trial. Journal of Periodontology. 2013;84(9):1290–1296. doi: 10.1902/jop.2012.120425. [DOI] [PubMed] [Google Scholar]
- 41.Rattanasuwan K., Rassameemasmaung S., Sangalungkarn V., Komoltri C. Clinical effect of locally delivered gel containing green tea extract as an adjunct to non-surgical periodontal treatment. Odontology. 2016;104(1):89–97. doi: 10.1007/s10266-014-0190-1. [DOI] [PubMed] [Google Scholar]


