Abstract
In spite of many years of research, the pathomechanism of depression has not yet been elucidated. Among many hypotheses, the immune theory has generated a substantial interest. Up till now, it has been thought that depression is accompanied by the activation of inflammatory response and increase in pro-inflammatory cytokine levels. However, recently this view has become controversial, mainly due to the family of small proteins called chemokines. They play a key role in the modulation of peripheral function of the immune system by controlling immune reactions, mediating immune cell communication, and regulating chemotaxis and cell adhesion. Last studies underline significance of chemokines in the central nervous system, not only in the neuromodulation but also in the regulation of neurodevelopmental processes, neuroendocrine functions and in mediating the action of classical neurotransmitters. Moreover, it was demonstrated that these proteins are responsible for maintaining interactions between neuronal and glial cells both in the developing and adult brain also in the course of diseases.
This review outlines the role of chemokine in the central nervous system under physiological and pathological conditions and their involvement in processes underlying depressive disorder. It summarizes the most important data from experimental and clinical studies.
Keywords: Antidepressant drugs, chemokine, chemokine receptors, depression, neuroinflammation, neuroplasticity, neuroendocrinology, neurotransmission
1. Introduction
Depression is a highly prevalent disorder characterized by a cluster of symptoms including disturbances of mood and affect, anhedonia and psychomotor symptoms with the greatest severity in youth and the elderly. Currently, the World Health Organization (WHO) estimated that approximately 350 million of people worldwide suffer from depression. Over the years, many different directions have been proposed and explored to investigate the mechanisms of the onset of affective disorders, like major depression, bipolar disorder or mania, but the actual pathogenesis of depression has not yet been elucidated.
Due to multiplicity and diversity of symptoms as well as various clinical pictures, it is difficult to find a common mechanism that could be responsible for the development of this disorder. During the progress of depression, multiple molecular, cellular, structural, and functional changes occur in the brain, what makes the task of explaining them by one hypothesis so challenging.
An initial theory of depression postulated that the changes of monoaminergic neurotransmission are responsible for these disorder [1-3], whereas next hypothesis pointed to an imbalance of excitatory and inhibitory signaling in the brain [4, 5], hyperactivity of the hypothalamus-pituitary-adrenal (HPA) axis [6-8] or malfunction in the growth and neurotrophic factor systems [9]. Since many years, the immune and endocrine systems have been accepted to play a key role in the regulation of the nervous system function and to co-operate with it in maintaining brain homeostasis. For this reason, the immune hypothesis is of paramount importance among many potential theories of the pathogenesis of depression. Its peculiarity consists mainly in its ability to combine all previous hypotheses of depression, mostly based on multifarious functions of immune cells and mediators.
Interestingly, the components of the immune system may influence directly and indirectly the development of depression via several neurobiological processes. The impairment of proper regulation within the immune system, particularly in the brain, interacts negatively with many pathways leading to: dysfunction of monoaminergic system e.g. reduction of serotonin level, production of neurotoxic tryptophan-like by-products (3-hydroxykynurenine (3-HK) and quinolinic acid (QA)), disturbances in HPA axis activity (e.g. hypercortisolemia and reduced glucocorticoid receptor density), defective neurogenesis (e.g. apoptosis and reduced neurotrophin production), neurocircuitry malfunction (cortical-striatal-limbic circuits) as well as neuroimmune deterioration [10-16].
This paper reviews data evidencing a new role of some cytokines with chemotactic property, called chemokines in the pathogenesis of depressive disorders. In the light of experimental data indicating their involvement in neuromodulation, regulation of neuroinflammation, brain cell communication and modulation of neuroendocrine functions, we aim to discuss important actions of chemokines in the brain and their direct or indirect impact on neuronal as well as glial cells.
1.1. The Role of Inflammatory Processes in the Pathomechanism of Depression
It is well known that inflammation plays a role in cardiovascular disorders, diabetes and neurodegenerative diseases. Currently, a growing body of data has suggested that the list of disorders where inflammation is substantially involved should be supplemented with neuropsychiatric diseases, including depression. Several studies both in animal models and in humans revealed a strong link between the onset and progression of depression and the alterations in the levels of inflammatory mediators, including cytokines and chemokines in blood, serum and brain tissues.
One of the earliest results showing that depression may be associated with inflammatory processes came from the work of Maes and collaborators [17-19]. They demonstrated in depressed patients, the increased levels of inflammatory biomarkers, such as pro-inflammatory cytokines (e.g. interleukin 1β (IL-1β), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), interferon ɣ (IFN-ɣ)) and altered secretion of anti-inflammatory cytokines (e.g. interleukin 4 (IL-4), interleukin 10 (IL-10)). Moreover, increased concentration of acute-phase proteins and up-regulation of cellular markers of immune activation was found. Following these initial reports, also other papers confirmed the immune theory of depression. Zorilla and collaborators [20] and Dowlati and collaborators [21] described increased levels of cytokines: IL-6, TNF-α and enhanced c-reactive protein (CRP) levels in blood samples from depressed patients. Clinical studies also showed that in patients suffering from depression the levels of IL-1β in plasma and cerebrospinal fluid (CSF) were increased and there was a positive correlation between serum concentration of the cytokine and depression severity [22, 23]. Moreover, the Song’s group [24] demonstrated in patients with depression not only elevated IL-1β, but also decreased IL-10 levels in serum. However, some evidences indicated that IL-1β levels in the periphery and in CSF remained unchanged in patients with major depressive disorder (MDD) [21, 25, 26]. These discrepancies may result from the heterogeneous clinical picture in depressive patients.
Also studies in animal models of depression revealed changes in the function of the immune system. For instance, in rats exposed to repeated intermittent lipopolysaccharide (LPS) injections (neurobehavioral model of chronic depression), the thymus weight and proliferative activity of lymphocytes were significantly reduced. These changes were accompanied by alterations in IFN-γ and IL-10 synthesis in the periphery [27].
Moreover, using the prenatal stress model of depression, we demonstrated increased levels of pro-inflammatory cytokines: IL-1β, TNF-α and IFN- γ in both structures engaged in the pathogenesis of depression: hippocampus and frontal cortex [28, 29]. Prenatal stress procedure also modified response to peripheral inflammation induced by systemic administration of bacterial LPS [29, 30]. Moreover, experiments in the restraint stress model in mice revealed a higher expression of IL-1β and TNF-α in the hippocampus [31]. Further, in the animal model of depression based on chronic mild stress (CMS), where adult animals are exposed to the stress, the levels of IL-1β and IL-6 in the brain and IL-6 and TNF-α levels in the serum were increased [32].
The immune theory of depression was further confirmed by studies utilizing antidepressant drugs, which may have anti-inflammatory properties via affecting the expression of pro- and anti-inflammatory factors [33-36]. Studies revealed that the elevated levels of pro-inflammatory cytokines, like interleukin 12 (IL-12) or IFN-γ were reduced after treatment with drugs from the group of selective serotonin reuptake inhibitors (SSRI). Additionally, one of the SSRIs - sertraline has been shown to increase the serum concentration of anti-inflammatory cytokines: IL-4 and transforming growth factor-β1 (TGF-β1) in patients suffering from depression [37]. Further research also reported that elevated levels of most cytokines (interleukin 1 receptor antagonist (IL-1Ra), IL-6, interleukin 7 (IL-7), interleukin 8 (IL-8), IL-10, granulocyte-colony stimulating factor (G-CSF) and IFN-γ) were reduced after 12 weeks of treatment with different antidepressant drugs [38].
The next important line of evidence that supports the key role of inflammation in the pathogenesis of depressive disorders indicated that the administration of inflammatory factors (e.g. cytokines or cytokine release inducers) in animals as well as in humans can induce depression-like symptoms. For example, in patients, chronic treatment with interferon α (IFN-α) has been found to cause depressive behaviors [39-41].
2. Chemokines - background
In the late 1980s scientists isolated the signaling molecules, termed chemokines (chemotactic cytokines) that allowed leukocytes to communicate with one another and to recognize and destroy invading pathogens. Since then the view on chemokines in the brain has substantially changed. Apart from their traditional role in chemotaxis of immune cells, chemokines fulfill a lot of other functions, like regulation of migration [42, 43], proliferation of neuronal stem/progenitor cells [44], control of axon sprouting and elongation [45], synaptic pruning processes [46, 47], control of blood-brain barrier (BBB) permeability and regulation of infiltration and activation states of central and peripheral immune cells [48, 49]. They can also act as neuromodulators affecting pre- as well as post-synaptic systems [50-52]. In addition, chemokines regulate neuroendocrine functions, thus producing important actions in the brain via a direct or indirect impact on neuronal as well as glial cells [52, 43].
Altogether, these observations greatly contributed to recognizing that the chemokine system consisting of ligands and receptors may be considered as the third major communication system of the brain [53]. For this reason, the immune theory of depression once again came into the spotlight. Based on the above data it appeared that chemokines are the main proteins responsible for the regulation of inflammatory processes by controlling interactions between the immune and nervous system cells. This pathway seems to be important for maintaining or restoring brain homeostasis also in pathological conditions, including depression.
2.1. Chemokines - An Overview
Chemokines are a group of small (8-14kDa) polypeptides. Since the discovery of the first protein with chemotactic activity, the chemokine family has included over 50 chemokines and 20 receptors [54]. Chemokines nomenclature is based on the class and a numerical designation, e.g. Chemokine (C-C Motif) Ligand 3 (CCL3), C-X-C motif chemokine 10 (CXCL10, IP-10) [55, 56]. It simplified the system that existed previously whereby chemokines were named according to their function and, therefore, could have several different names, e.g. Chemokine (C-C Motif) Ligand 2 (CCL2) was originally named monocyte chemoattractant protein 1 (MCP-1) [57, 58].
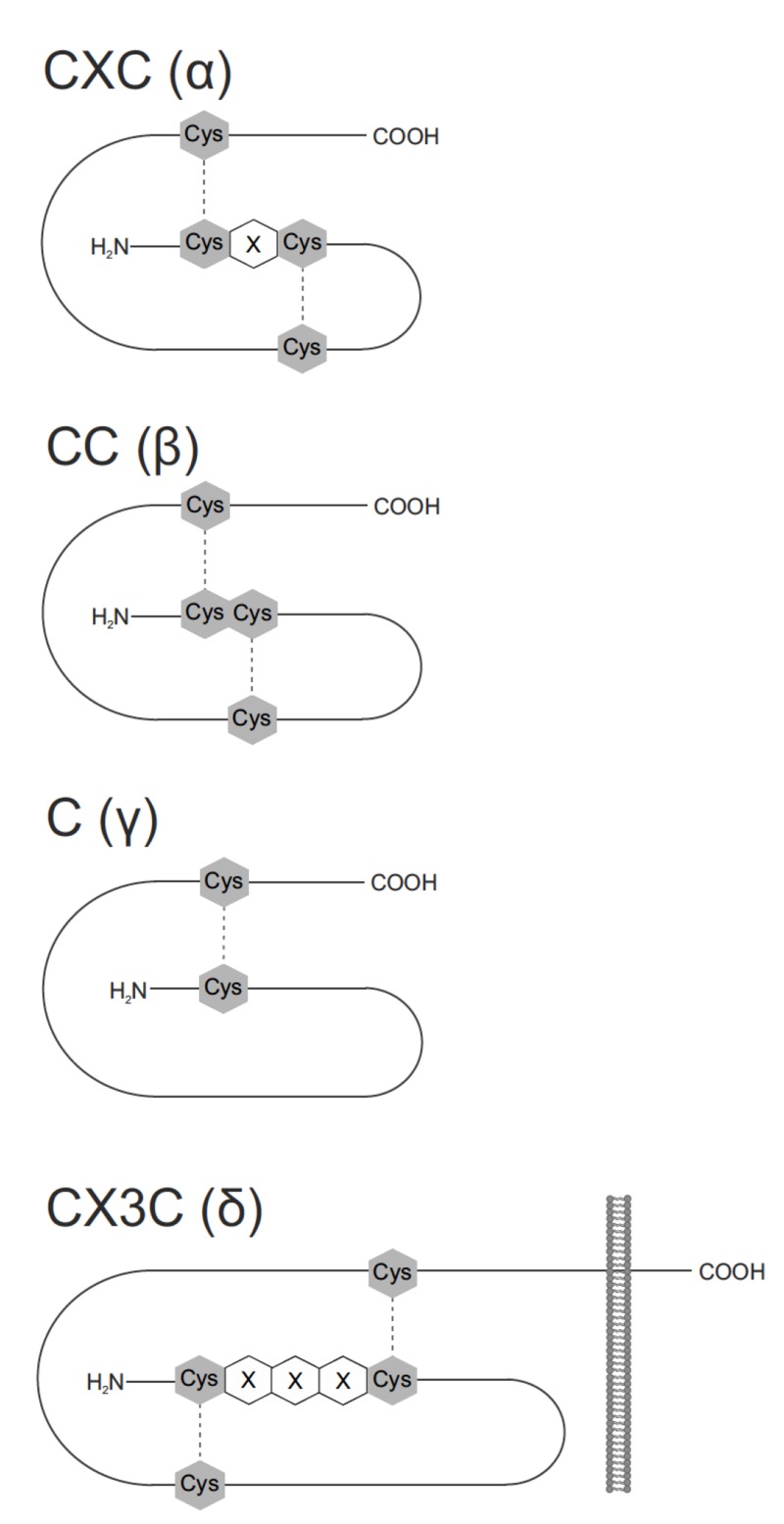
Recently, chemokines have been divided into four groups: C, CC, CXC and CX3C, based on the position of the key cysteine residues (N-terminal region of the protein) that participate in disulfide bonding (Fig. 1). CC and CXC are the two largest groups. In the CC group, comprising ca. 28 members, the first two cysteines are adjacent to each other. In contrast, in the CXC group (ca. 16 members) they are separated by two amino acid residues. In the case of C group, there is only one cysteine in the N-terminal domain, while in the smallest CX3C family, three amino acid residues separate first two cysteine groups [59].
Fig. (1).
Chemokine families. Chemokines are classified in four distinct subclasses: C (γ), CC(β), CXC (α), and CX3C(δ) according to the number and spacing of their cysteine residues in their N-terminus. Cys – cysteine residue, X – amino acid residue, disulfide bridges are shown as dotted lines.
Moreover, genomic organization seems to be helpful to organize this large superfamily [60]. It has been found that the majority of human CXC chemokines are encoded at chromosomal location 4q12-21, while most of CC chemokines was found at 17q11-21 and these loci are often syntenic in other mammalian species [49].
It has been demonstrated that a lot of chemokines are synthesized as proteins composed of a sequence of 20-25 amino acids [61]. After synthesis, they are next secreted from the cell. Interestingly, two chemokines: C-X3-C motif ligand 1 (CX3CL1, fractalkine) and C-X-C motif chemokine 10 (CXCL16, SR-PSOX) are expressed as transmembrane components and can be secreted after cleavage by members of the A Disintegrin And Metalloprotease (ADAM) family of enzymes. Therefore, they may act at a distance as soluble chemoattractants. Membrane-bound CX3CL1 and CXCL16 can also serve as adhesion molecules for receptor-bearing cells [62-64].
Regarding the structure of chemokines, all proteins have similar tertiary structure: a disordered N-terminus of 6-10 amino acids, long loop, known also as the N loop, a 310-helix and a three-stranded beta-sheet, and finally a C-terminal alpha helix [65].
2.2. Chemokine Receptors as a Target for the Chemokine Action
Chemokines mediate their biological effects via interactions with 7-transmembrane G protein-coupled receptors (GPCRs). Studies showed that these proteins have two main sites of interaction with their receptors: the flexible N-terminal domain and the rigid loop. Moreover, model composed of two steps, for chemokine receptor binding and activation has been demonstrated. First, the N-terminus and extracellular loops of the receptor binds to the core domain of the chemokine ligand. Next, the N-terminus of the chemokine enters directly into the helical bundle of the receptor [52, 66, 67].
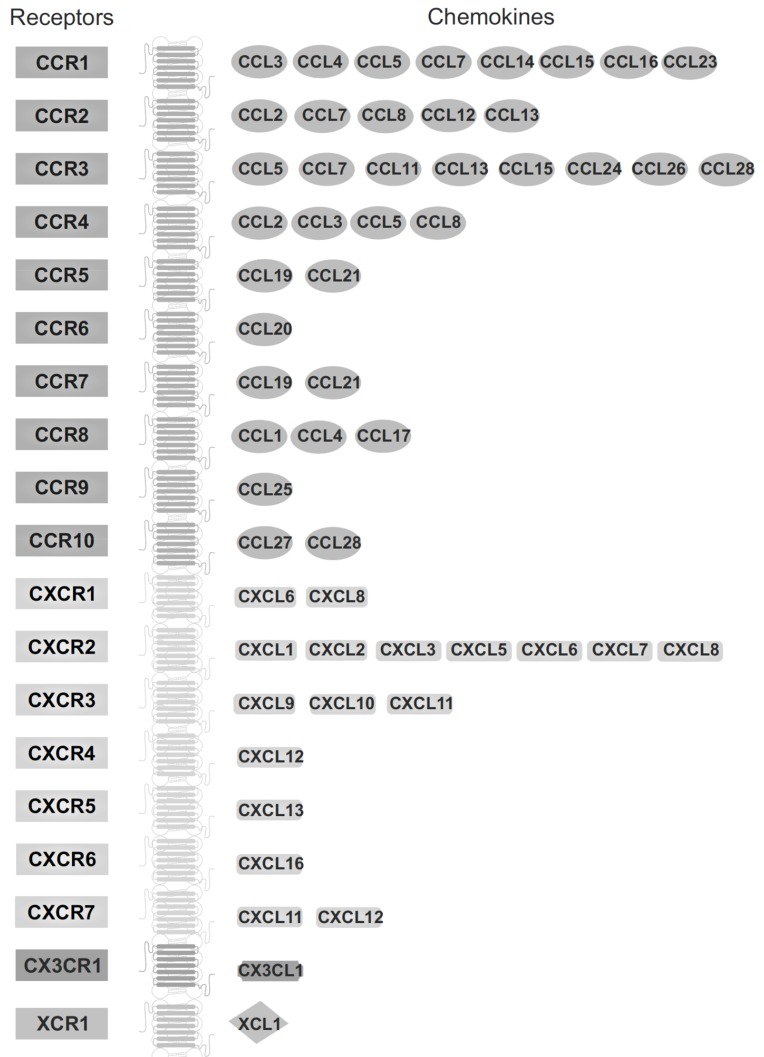
Chemokine receptors have 340-370 amino acids and their amino acid sequences show 25-80% identity [52]. Receptors for chemokines are divided into subtypes according to the chemokine group they preferentially bind, e.g. CC chemokines bind to CC receptors. As yet, only one exception has been found, namely Chemokine (C-C Motif) Ligand 21 (CCL21), that in addition to Chemokine (C-C Motif) Ligand 7 (CCR7), also binds to C-X-C motif chemokine 3 (CXCR3) [58]. The name of the receptor consists of the chemokine subfamily name (C, CC, CXC, CX3C) followed by the letter “R” (“receptor”) and a number in the chronological order in which it was identified. It is very interesting that one chemokine can bind to more than one receptor, but likewise each receptor subtype binds multiple chemokines (Fig. 2).
Fig. (2).
Chemokine receptors and their ligands. Chemokine receptors belong to the superfamily of seven-transmembrane-domain G-protein-coupled receptors (GPCRs). Interestingly, some chemokines can bind to more than one receptor. Also, certain receptors can bind to multiple chemokines, while other chemokine receptors bind to a single ligand.
All chemokine receptors belong to the family of GPCRs. Generally, the G-proteins activated by chemokine receptors belong to Gαi family (class A GPCRs) and are pertussis toxin sensitive [55, 69]. Although recently, it has been shown that C-X-C motif chemokine 7 (CXCR7), the newly discovered receptor for C-X-C motif chemokine 12 (CXCL12, SDF-1, stromal cell-derived factor 1), does not lead to the activation of Gαi signaling and may act as a β-arrestin-based receptor [70].
After activation by a chemokine, chemokine receptors may stimulate various intracellular targets, e.g. adenylcyclase, phospholipases, GTP-ases, such as Rho, Rac, and Cdc42 and pathways of major kinases, like phosphatidyl inositol-3 kinase (PI3-K) and mitogen-activated protein kinases (MAPKs) [71, 72].
3. The role of chemokines in the brain
Considering the diversity of intracellular signaling pathways activated by chemokines, these protein systems can influence at once a broad spectrum of cellular processes. Therefore recently, their impact on both physiological and pathological processes has been strongly underlined.
Chemokines are divided according to their role into three categories based on biological activity: those responsible for the maintenance of homeostasis, involved in the onset of inflammatory processes and dual-function chemokines. Inflammatory chemokines are enhanced during inflammation and are considered to take part in leukocyte recruitment to inflamed areas. Unlike, homeostatic chemokines are constitutively expressed and mediate migration or homing. At last, dual-function chemokines cannot be assigned to either of the above-mentioned categories without ambiguity [73].
In the brain, the glial cells (microglia and astrocytes) are the main source of chemokines, but also neuronal cells may express some chemokines, like CCL2, CCL3, CXCL10, CCL21 and CX3CL1 [68, 74]. The expression of chemokines in normal physiological conditions is hardly observable but inflammatory stimuli significantly enhance the production of these proteins [75, 76]. So far, it has been postulated that only two chemokines: CX3CL1 and CXCL12 are expressed constitutively in the central nervous system [77-79]. Interestingly, also the expression of chemokine receptors, widely present in the normal brain, like CCR2, CCR5, CXCR2, CXCR3, CXCR4 and CX3CR1 can change in dependence on pathological conditions, which indicates that in the brain these proteins are engaged in the response to pathological stimuli.
3.1. Chemokines-Brain Development and Neuronal Plasticity
Recently, it has been suggested that chemokines are important players in the regulation of neurodevelopmental processes and neuronal plasticity [80, 81]. Their activity is slightly different between respective brain structures, nevertheless essential in hippocampus and frontal cortex, two structures strongly involved in the pathogenesis of depressive disorders. Especially, the role of chemokine CXCL12 and its receptor (CXCR4) in the migration of neurons to their final destination is highlighted. Data demonstrated that CXCR4 and CXCL12 control the migration of proliferating cells of the dentate gyrus (DG), elongation of axons and branching within hippocampal neurons as well as migration of GABA-ergic interneurons to the cortex and gonadotropin-releasing hormone (GnRH) neurons from the vomeronasal organ to their destination within the hypothalamus [45, 79, 82, 83]. CXCL12 is present in the cerebral cortex along the migration stream of interneurons, whereas the CXCR4 of migrating interneurons [84-86]. What is important in CXCR4 knock-out mice, where the stream migration is disrupted, interneurons prematurely exit the migratory streams and invade the cortical plate, leading to abnormal interneuron distribution [86, 87].
An increasing body of evidence implicated dysregulation of hippocampal neurogenesis as an important factor in the pathophysiology of several psychiatric disorders including depression [88]. It has been found that C-X-C motif chemokine 13 (CXCL13) and its receptor (CXCR5) may be involved in the maturation and proliferation of subgranular zone cells in the hippocampal dentate gyrus [89]. Furthermore, another chemokine-C-X-C motif chemokine 14 (CXCL14), highly expressed especially in the hippocampus, may regulate synaptic inputs to the adult neural progenitor cells (NPCs) as well as hippocampal integrity in mature mammals [90]. Recently, studies in CX3CR1 knock-out animals have revealed that CX3CL1-CX3CR1 signaling regulates the development and plasticity of neuronal circuits, with consequences on the brain connectivity, adult hippocampal neurogenesis, memory, learning and the behavioral performance [91-94]. Interestingly, CX3CL1-CX3CR1 system also determines the course of “synaptic pruning” [46]. Synaptic pruning is an activity-dependent developmental program in which many synapses that form in early stages are eliminated, while remaining are maintained and strengthened. In relation, Paolicelli and collaborators [92] demonstrated that mice lacking CX3CR1 possessed more synapses and reduced number of microglial cells in hippocampus during weeks 2-4 of postnatal development confirming in that way the importance of CX3CL1-CX3CR1 signaling in the development of neuronal networks.
Altogether, these evidences substantiate the hypothesis that chemokines play an important role in the proper neurogenesis and neuronal plasticity, which are essential for normal functioning of the brain, while their distortion has been suggested to belong to the causes of depression.
3.2. Chemokines and Neurotransmission
Further support to an important role of chemokines in the pathomechanism of depression comes from the fact that these proteins modulate the release of some brain neurotransmitters. For example, CX3CR1 and CX3CL1 are co-localized with serotonin (5-HT) neurons of the dorsal raphne nucleus. Electrophysiological experiments have shown that CX3CL1 inhibits 5-HT neurotransmission in an indirect way by up-regulating the sensitivity of 5-HT dorsal raphne nucleus neurons to GABA inputs [95].
Similarly to CX3CL1, also CCL2 co-localizes with classical neurotransmitters, like acetylcholine in the substantia innominata and in the oculomotor nucleus as well as with dopamine in the substantia nigra [76, 96]. The suggested mechanism of CCL2 action is based on increases in the spinal neuronal excitability by suppression of the effect of GABA on these cells. On the other hand, results obtained from patch-clamp recordings have shown that CCL2 increases α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptor currents in spinal neurons and modulates glutamate secretion via a presynaptic effect [97]. Data revealed that another chemokine belonging to CC family namely CCL5 can regulate glutamate release in the cortex and spinal cord via intensifying the basal secretion while inhibiting the depolarization-evoked release of the excitatory amino acid [98, 99].
Likewise, CXCL12 has been shown to presynaptically modulate glutamatergic and GABA-ergic transmission in the rat substantia nigra, consequently leading to the modulation of downstream dopaminergic neurons [100]. Moreover, the paper by Melik-Parsadaniantz and Rostene [76] showed that CXCL12, as a stimulatory factor of dopamine neurotransmission, might be involved in the regulation of locomotor behavior in animals.
3.3. Chemokines and Neuroinflammation
Chemokines and their receptors are thought to be important mediators of the inflammatory processes. Actually, the monocyte chemotactic protein (MCP) family members including e.g. CCL2, CCL7, CCL8 and CCL13 are involved in the immune/inflammatory response and are considered to be important mediators of neuroinflammation in the central nervous system [101]. CCL2 exerts mainly pro-inflammatory action through the regulation of chemotaxis of monocyte-derived macrophages and other inflammatory cells to the disturbed brain areas [102]. CCL2 and its receptor CCR2 were demonstrated to be expressed on astrocytes, neurons, microglia and neuronal progenitor cells under basal as well as inflammatory conditions [103, 104]. Importantly, the regulatory role of CCL2 in the microglia is commonly accepted [102]. Data demonstrated that CCL2 treatment of microglia led to the increase in migration and proliferation of these cells but not to a direct induction of its pro-inflammatory phenotype. On the other hand, some data demonstrated that the CCL2-treated microglia showed enhanced pro-inflammatory activity probably indirectly via the migration of P2X4 purinergic receptors to the cell surface membrane [105]. The pro-inflammatory role of the CCL2 in the pathogenesis of neurodegenerative processes (e.g. Alzheimer’s disease (AD)) has also been reported. The enhanced level of CCL2 may induce monocyte differentiation as well as monocyte and leukocyte recruitment, from the blood to the brain through the disrupted blood-brain barrier (BBB), thus increasing neuroinflammatory processes and hastening the AD pathogenesis [106].
Importantly, several data postulate a dichotomy between the pro- and anti-inflammatory potential of CCL2 action mainly in the periphery [107]. There are some data revealing also a beneficial property of CCL2 in the brain but the mechanism of CCL2 action remains open to further investigation [73, 76]. One of them involves the intensification of anti-inflammatory cytokine, e.g. the IL-4 expression [108]. Furthermore, some data have demonstrated that CCL2/CCR2 axis, may be implicated in neuronal communication and even in neuronal regeneration [109, 110]. Indeed, the CCL2/CCR2 axis participates in recovery after ischemia as well as in maintenance of the neurovascular unit. CCL2 produced by activated glial cells plays a role in neurogenesis after stroke. It can attract neuroblasts derived from neuroprogenitor cells (NPCs) from the subventricular zone toward the ischemic region [61]. Another study also reported functional benefits of the changes in the CCL2/ CCR2 signaling in a model of combined radiation/traumatic brain injury. In this study, hippocampal-dependent cognitive impairment was reduced in CCR2-deficient mice. It is worth emphasizing that the contribution of other chemokines of the CC family to the neuroinflammation has been extensively reviewed. For instance, in CCR7-deficient mice, the impaired cell-mediated immune function and some behavioral changes have recently been described [111]. Some data indicated also the disturbances in the expression of chemokine receptor CXCR2 to participate in posttraumatic inflammation and secondary degeneration. In line with this suggestion, CXCR2 knockout mice (CXCR2-/-) showed the reduced tissue damage and milder neuronal loss in comparison to wild-type mice [112].
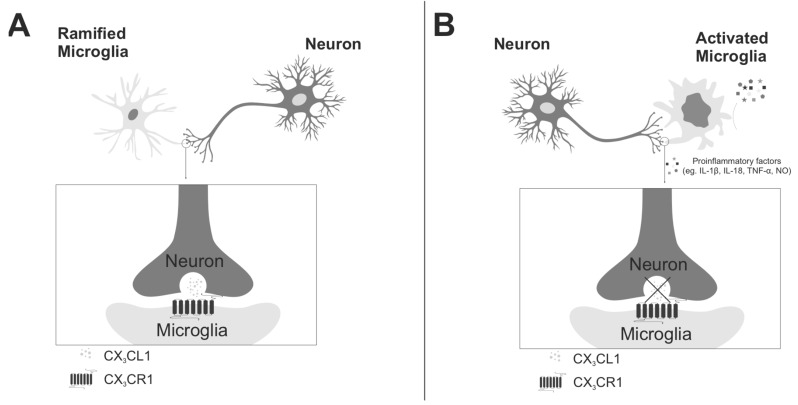
In recent years, peculiarity of fractalkine (CX3CL1) and its only receptor (CX3CR1) has focused interest in the context of neuroinflammation (Fig. 3). Fractalkine is produced mainly by neurons and acts on its receptor expressed on microglia what establishes a unique signaling system between cells within the central nervous system [113]. Due to specific features of this system, it is able to regulate glial activity: the cytokine and chemokine release and phagocytic activity of these cells [114-116]. Moreover, it has been shown that microglial cells play a key role in developmental synaptic pruning, while fractalkine has an important impact on the development of neurons, their maturation and creation of neuronal network, by regulating microglial activation [92, 93, 117-119]. Cardona and collaborators [120] demonstrated that in many models of neurodegenerative disorders, the loss of neuron-microglia interactions by disturbances in the fractalkine and its receptor signaling resulted in microglial activation and, consequently, in worsening of the disease. Our recent studies in the prenatal stress model (an animal model of depression) have shown an altered expression of fractalkine in the hippocampus and frontal cortex of adult animals [our unpublished data]. It was accompanied by exacerbation of inflammatory activation in these brain structures [29]. These results seem to confirm that the fractalkine-fractalkine receptor system may be an important regulator of neuroinflammation.
Fig. (3).
Role of fractalkine (CX3CL1) and its receptor (CX3CR1) in the neuron-microglia interactions. A) in the healthy brain fractalkine is mainly expressed on neurons and its receptors on microglial cells. The crucial role of this system is to keep microglia in a quiescent ‘inactive’ state. B) the disruption of fractalkine-fractalkine receptor signaling leads to prolonged activation of microglia which become ameboid-shaped (short processes, large soma). The activated microglia release a variety of pro-inflammatory factors such as: IL-1β, TNF-α, IL-18 or nitric oxide (NO). The aberrant activation of microglia caused by CX3CL1-CX3CR1 disturbances triggers to enhanced and prolonged neuroinflammation which may lead to the development of depressive disorders.
Moreover, recent studies indicate that, besides CX3CL1, also CXCL16 contributes to the cross-talk between neurons, microglia and astrocytes to promote neuroprotection especially during ischemia. It has been shown that the treatment with exogenous CXCL16 diminished the ischemic volume in an in vivo model of permanent middle cerebral artery occlusion (pMCAO). It is very interesting that CX3CL1 acts on microglial cells and activates CXCL16 secretion by these cells, which may suggest a synergistic action of these particular protein systems in the brain [121].
3.4. Chemokines and Neuroendocrine Functions
The role of chemokines in neuroendocrine function is mostly related to their implication in the stress responses [122]. Initially, it was revealed that CXCL8 mRNA was expressed in the rat paraventricular nucleus (PVN), where the corticotropin-releasing hormone (CRH) is produced. Moreover, expression of CXCL8 was also found in the hippocampus, where negative feedback to the hypothalamo-pituitary-adrenal axis (HPA) is generated. Therefore, it might imply that CXCL8 is involved in the stress-related neuroendocrine system [123].
It is worth emphasizing that some data showed the implication of chemokines, like CXCL12, in the migration of gonadotropin releasing hormone (GnRH) neurons. Importantly, GnRH neurons migrate from nasal regions to the hypothalamus to regulate reproduction in adults. Migrating GnRH neurons are CXCR4 positive [124] and in mice lacking CXCR4 a decreased GnRH neuron migration has been shown [124, 125].
The obtained data demonstrat the expression of cytokine-induced neurotrophil chemoattractant (CINC, CXCL1) in the hypothalamus and in the pituitary [96]. Moreover, after the immune challenge induced by LPS administration, an enhanced expression of CXCL1 and interferon γ-inducible protein (IP-10, CXCL10) was reported in the PVN [126, 127]. In addition, an immobilization stress procedure up-regulated CXCL1 mRNA expression in the parvocellular and magnocellular subdivision of the PVN [126]. Painful stress induced by a noxious stimulation with formalin injection enhanced CINC immunoreactivity in the posterior pituitary and external layers of the median eminence [128].
A significant role of some chemokine-chemokine receptor systems in the regulation of stress response is correlated with the action of these proteins on glucocorticoids. It was discovered that high dose of methylprednisolone reduced expression of CCL2 in the ischemic rat brain [79]. In activated microglial cells dexamethasone also inhibits CCL2 secretion. The destabilization of CCL2 mRNA by glucocorticoids can be mediated by the glucocorticoid receptor (GR) [59].
Besides the involvement of chemokines in stress-related neuroendocrine function, they also play an important role in the regulation of body temperature, feeding or water balance. Several studies demonstrated that chemokines, such as: CXCL8, CCL3, CCL4, CLL5 induced hyperthermia [129-131]. Instead, icv administration of CXCL4 suppressed the 2-h night-time and total daily food intake. More recently it has also been shown that CXCL12 is constitutively expressed in melanine-concentrating hormone (MCH)-expressing neurons, which are involved in food intake [132]. CXCL12 is suggested to be important not only in the regulation of nutrition but also water balance, as it can blunt the autoregulation of AVP release in vitro and counteract angiotensin-II induced plasma AVP release in vivo [122].
4. The evidences for disturbances in the chemokine network in depressive disorders: experimental studies
There are only several papers showing the changes in the chemokine-chemokine receptor systems in animal models of depression. The majority of results come from the models of this disease based on stress procedures. Specifically, it was reported that immobilization stress increased the CXCL1 mRNA expression in the parvocellular and magnocellular subdivision of the PVN, but not in the supra optic nucleus (SON). Immunostaining showed an increase in the CXCL1 in the posterior pituitary [133]. In addition, painful stress induced by a noxious stimulation consecutively to subcutaneous injection of formalin into the hind footpad, increased immunoreactivity of the CXCL1 in the posterior pituitary and external layers of the median eminence and the secretion of CINC/CXCL1 in the peripheral blood [128]. Our recently published data demonstrated that prenatally stressed rats exhibited enhanced protein levels of CCL2 and CXCL12 in the hippocampus and frontal cortex [28]. Moreover, we observed the alteration in the expression of chemokine receptors, such as CCR2 and CXCR4 in the same structures, caused by maternal stress during pregnancy in male offspring. Contrarily, in an animal model of depression based on the chronic unpredictable mild stress, no significant changes in CCL2 level in the hippocampus were found [134]. Girotti’s group [135] revealed no effect of chronic intermittent cold stress (CIC stress) on basal expression of CXCL1 or CCL2 24h after the termination of stress. Nevertheless, CIC stress enhanced the release of the both CXCL1 and CCL2 chemokines in plasma as well as in the hypothalamus and prefrontal cortex in response to LPS administration.
A significant role of some chemokine-chemokine receptor system in the regulation of stress response was confirmed by the studies of Harrison and collaborators [111] who discovered that CCR7(-/-) mice after maternal separation exhibited significantly lower serum corticosterone concentrations compared to non-separated mice. Mice lacking CCR7 spent also less time in the open space during an open field test and more time in the closed arm of the elevated zero maze compared to their wild-type (WT) controls suggesting that CCR7 may be involved in social behavior and stress response following maternal separation [111]. In addition, the CCR7 and CCR6 have been shown to participate in other mechanisms regulating brain function, which may be responsible for behavioral disturbances related to neuropsychiatric disorders, like depression [136].
A lot of recent studies which underlined the significance of chemokine disturbances in the pathomechanism of depressive-like behaviors in animals are based on experimental treatment with different activators of the immune system. Indeed, rats administered intraperitoneally (i.p) with CXCL1 showed the reduced spontaneous exploratory activity, changes in the open field test, and reduced burrowing behavior [137]. On the other hand, acute treatment with LPS increased CXCL1 and CCL2 levels in the prefrontal cortex [135]. What is more, the impact of chemokine system, in induction of “sickness behavior”, was demonstrated in the CX3CR1 knock-out model. In that study, the lack of CX3CR1 receptor in mice was shown to be connected with an increased duration of sickness behavior based on the tail suspension test in response to a peripheral LPS challenge [138]. Moreover, depression-like behavior in CX3CR1-/- mice was associated with persistent microglial activation in the hippocampus and prefrontal cortex as well as 2,3-indoleamine dioxygenase (IDO) activation (enzyme responsible for breakdown of serotonin precursors to neurotoxic metabolites), and was ameliorated by competitive inhibition of 2,3-IDO [139]. In another set of experiments where the polyinosinic:polycytidylic acid (poly (I:C)) and IFN-α injection in mice were used for induction of the depressive-like behaviors, up-regulated expression of CXCL1, CXCL10 as well as CCL5 was observed in the hippocampus and frontal cortex [140].
Taken together, the results of studies on experimental animal models of depression conducted so far clearly demonstrated changes in the chemokine-chemokine receptor systems in the brain structures engaged in pathogenesis of this disease paralleled with behavioral disturbances which confirms the hypothesis about the role of these protein systems in the pathomechanism of depression.
5. The evidence for disturbances in the chemokine network in depressive disorders: clinical studies
Studies confirming chemokine malfunction in patients suffering from depression are scarce and inconsistent (Table 1). Most of them are based on the chemokine expression only in blood, plasma or CSF. Some of them postulated to use the changes in chemokine levels as biomarkers of depression [142, 143].
Table 1.
The evidence for disturbances in the chemokine network: clinical studies.
| Illness Classification | Results |
|---|---|
| MDD (major depressive disorder) |
• increased plasma level of CXCL8 [24] • increased blood level of CCL2 [37] • increased serum level of CCL2, CCL3, CCL11 [143] • increased blood level of CCL2 [144] • decreased blood level of CCL2, CCL3 [145] • increased blood level of CXCL10 [165] • increased plasma level of CXCL12, CCL5, CXCXR4, CCR5 [166] • increased serum level of CXCL10 [164] |
| MSD (moderate severe depression) |
• increased plasma level of CCL3 [156] |
| Suicidal attempts | • decreased serum level of CCL2, CCL5 [148] • increased serum level of CCL11 [148] • no changes in CSF level of CCL2 [149] • decreased CSF level of CCL11, CCL3, CCL2 [159] • decreased blood level of CCL2, CCL5 [146] • increased blood level of CCL11 [146] • decreased CSF level of CCL2, CCL4, CCL11, CCL13, CCL17 [146] • no changes in CSF and serum level of CXCL8 [161] • no changes in serum level of CXCL8 [162] |
| BD (bipolar disorder) |
• no changes in serum level of CCL2, CCL3, CCL24, CXCL9, and CXCL11 [147] • decreased level of CCL24 [147] • decreased tissue (post-mortem) level of CCL3 [157] • no association with CCL2 promoter – A2518G polymorphism [151-153] • increased mRNA level of CCL2 isolated monocytes [154] |
| OCD (obsessive-compulsive disorder) |
• increased plasma level of CCL3, CXCL8, CCL24 [158] • increased plasma level of CCL11, CXCL10, CCL24 [160] • decrease plasma level of CXCL8 [160] |
Distorted levels of chemokines belonging to CC and CXC families are described most often. In the CC family, CCL2 is the most notable for being a major monocyte-attracting chemokine. The Simon’s group [143] has shown elevated level of CCL2 in serum of 49 patients with a current Major Depressive Episode and primary Major Depressive Disorder, in comparison to 49 age (within 3 years) and gender matched healthy control subjects. However, it should be mentioned that data on CCL2 level are equivocal. Although most of them have described an increased CCL2 expression in serum in patients with depression [37, 144], there are also studies reporting a reduced [145, 146] or unchanged [147] level of this protein. In MDD suffering patients with suicidal ideation (SI), a lower serum level of CCL2 was described by Grassi-Olivier’s group [148] or no differences were observed [149]. Additionally, Grassi-Olivier’s group [148] has demonstrated that lower level of CCL2 was found in MDD patients with suicidal ideation compared not only to control subjects but also to MDD patients without SI, suggesting distinct immunological profiles of these two groups of patients. Interestingly, Black and Miller [148] have shown a decrease in CCL2 in CSF of patients who attempted suicide. In another study in patients suffering from bipolar disorder (BD), the elevated CCL2 level correlated with the length of illness and the age of patients [150], while the other found no association [147]. Further to these results, several studies reporting analysis of the CCL2 promoter – A2518G polymorphism (rs1024611) in various ethnic groups have unanimously demonstrated no significant association [151-153]. The utility of serum CCL2 as a marker of bipolar disorder will therefore require further investigation. However, an alternative approach has demonstrated a potential role of CCL2 in the heritability of bipolar disorder. Data from patients with BD and their offspring found that the expression of CCL2 mRNA in isolated monocytes was up-regulated in both patients with BD and their offspring, who developed mood disorders [154].
Importantly, studies on the use of antidepressant drugs have not clarified the changes in CCL2 levels. A majority of data obtained so far showed that most of antidepressant drugs, e.g. venlafaxine, reduced the level of this chemokine increased by the disease [144, 155], but some, like sertraline, did not induce such changes [37].
CCL3 (MIP-1α, macrophage inflammatory protein -1α) is also a member of the CC family. Up till now, only a few studies have shown the correlation between CCL3 and depressive disorders. Some data [143] revealed the increase in plasma CCL3 level in depressed patients. The same relationship was observed by Merendino’s group [156]. On the other hand, in patients suffering from BD, only one report revealed no differences in serum CCL3 level in patients in comparison to healthy control subjects [147]. However, post-mortem examination of tissues from BD patients evidenced down-regulation of CCL3 expression [157]. In patients with obsessive-compulsive disorders (OCD), plasma level of CCL3 was increased. Moreover, alterations in this immunological factor, responsible for the enhancement of neuronal activity, were inversely correlated with the severity of depression [158].
A link between CCL11 (Eotaxin) and depression has been suggested by some research. Apart from studies where no differences in serum level of this chemokine were shown [147], several studies reported a positive association between depression and CCL11 [143, 148]. Among them, a higher level of CCL11 was found in serum of MDD patients with suicidal ideation [146, 158]. These results were supported also by Grassi-Oliveira [148], who observed elevated CCL11 level in depressed patients with suicidal ideation. However, Janelidze’s group [159] found that patients who had attempted suicide within the previous two weeks showed lower levels of the chemokine CCL11 in the CSF compared to healthy controls what also was confirmed later [146]. Furthermore, Barbosa and collaborators [160] found that BD patients demonstrated increased plasma levels of CCL11 and its level was associated with BD trait, similarly as in the case of CXCL10.
Most data on chemokines of the CXC family have focused on CXCL8 which is a chemokine peripherally inducing transmigration and degranulation of neurotrophils. However, data on alterations in CXCL8 level are incoherent. Results showed a decreased plasma level of CXCL8 in BD patients, which was associated with BD trait [160]. In contrast, there are also data revealing its increased plasma level in depressive and manic states [24] while another study showed no changes in BD euthymic patients [147]. The increased plasma expression of CXCL8 has been also noted in OCD patients [158]. Contrarily, no association between serum or CSF level was revealed in suicide attempters and suicide victims [149, 161]. On the other hand, Black and Miller [146] have shown that there is a significant decrease in patients with suicidality versus control subject but there are no differences between patients with suicidality compared to patients without suicidality. Importantly, the increased level of serum CXCL8 [24] or no differences [164] have been also observed in MDD patients.
Another chemokine of the CXC family namely CXCL10 is a potent promoter of chemotaxis of activated Th1 cells and also an angiostatic factor with anti-fibrotic properties. Literature data indicate that its elevation is congruent with the increased leukocyte counts in peripheral blood that have been shown to be dependent on severity and treatment outcome in MDD patients [163]. Wong and collaborators [164] discovered the increased level of CXCL10 in serum in patients suffering from MDD. Moreover, the CXCL10 level was diminished after antidepressant treatment. Similarly, the study investigating the plasma CXCL10 level in BD type I patients in different mood states (e.g. euthymia and mania) revealed a significant increase in CXCL10 [160]. Additionally elevated level of this chemokine was associated with BD trait. The observation of the alterations in CXCL10 level in BD patients is compatible with results of Brietzke group [147], showing the enhanced serum CXCL10 level in patients suffering from bipolar disorder (euthymic). Also other reports highlighted the increased CXCL10 level in MDD patients [167], which has been show to decrease in response to antidepressant [164]. However, in OCD patients, there was an inverse relationship between the severity of depression and the CXCL10 level [158].
It should be added that in depressed patients the serum levels of other chemokines were also changed. For example: CCL24 and CXCL9 levels were mostly decreased [142, 147, 160]. Fontenelle and collaborators [158] have shown that OCD subjects with greater severity of hoarding displayed lower level of CCL24.
Finally, the data pointed that the levels of CX3CL1 [156], CXCL4 [41, 166] and CCL5 [146, 166] were altered.
Analysis of clinical literature data on the chemokine network has indicated that the changes in chemokine levels were observed principally in the periphery. Moreover, for now, it appears that there is no sufficient evidence for distinguishing or classification of depressive disorders, with a complex and variable clinical picture, based on disturbances in the chemokine network. Further, clinical picture of depression was complicated by other factors, like condition of the immune system, comorbid diseases, patient’s age, pharmacotherapy used, which should be regarded as a limitation of currently available studies.
On the other hand, the studies on animal models of the CNS diseases have provided substantial arguments for a key role of chemokines in the brain and, hopefully, new insight into these processes will help us to understand better depressive disorders.
6. Conclusions
The studies cited above provide evidences that the network of chemokines and their receptors play an important role in the regulation of peripheral as well as of the central nervous system function. In the light of a wide variety of chemokine actions, including brain development, plasticity, cell-to-cell communication, neurotransmission, neuroendocrinology function, inflammatory processes and behavioral regulation, these proteins may be considered as possible target in the treatment of depression disease or attractive biomarkers of psychiatric disorders. If only the methodological limitations of studies on this protein network, especially in human diseases, are overcome, future works may better characterize the role of chemokines as crucial regulators of the brain function. Detailed research may also help develop new therapeutic strategies both for prevention and more efficient treatment of depressive disorders.
Funding Statement
This research was supported by grant no. 2013/09/B/ NZ7/04096, National Science Center, Poland. Joanna Ślusarczyk, Ewa Trojan, Jakub Chwastek are holders of scholarships from the KNOW, sponsored by the Ministry of Science and Higher Education, Poland.
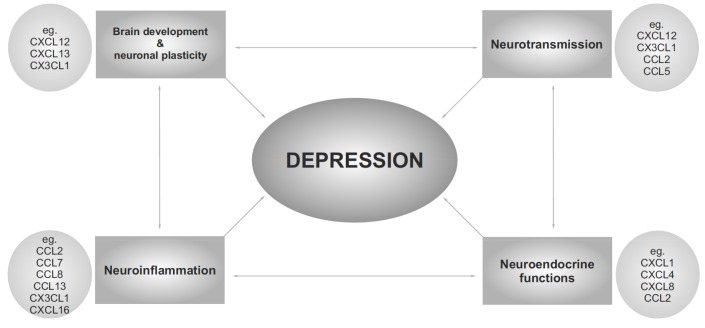
Fig. (4).
Chemokines are suggested to be involved in the pathophysiology of depressive disorders because of their impact on neurotransmission, neurodevelopmental processes and neuroplasticity, regulation of neuroinflammation and modulation of neuroendocrine functions.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
All authors have no financial interests or potential conflicts of interests to declare.
REFERENCES
- 1.Bunney W.E., Jr, Davis J.M. Norepinephrine in depressive reactions. A review. Arch. Gen. Psychiatry. 1965;13(6):483–494. doi: 10.1001/archpsyc.1965.01730060001001. [http://dx.doi.org/10.1001/archpsyc.1965.01730060001001]. [PMID: 5320621]. [DOI] [PubMed] [Google Scholar]
- 2.Schildkraut J.J. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry. 1965;122(5):509–522. doi: 10.1176/ajp.122.5.509. [http://dx.doi.org/10.1176/ajp.122.5.509]. [PMID: 5319766]. [DOI] [PubMed] [Google Scholar]
- 3.Elhwuegi A.S. Central monoamines and their role in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2004;28(3):435–451. doi: 10.1016/j.pnpbp.2003.11.018. [http://dx.doi.org/10.1016/j.pnpbp.2003.11.018]. [PMID: 15093950]. [DOI] [PubMed] [Google Scholar]
- 4.Trullas R., Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185(1):1–10. doi: 10.1016/0014-2999(90)90204-j. [http://dx.doi.org/10.1016/0014-2999(90) 90204-J]. [PMID: 2171955]. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res. Brain Res. Rev. 2009;61(2):105–123. doi: 10.1016/j.brainresrev.2009.05.005. [http://dx.doi.org/10.1016/j.brainresrev.2009.05. 005]. [PMID: 19481572]. [DOI] [PubMed] [Google Scholar]
- 6.Pariante C.M. Depression, stress and the adrenal axis. J. Neuroendocrinol. 2003;15(8):811–812. doi: 10.1046/j.1365-2826.2003.01058.x. [http://dx.doi.org/10. 1046/j.1365-2826.2003.01058.x]. [PMID: 12834443]. [DOI] [PubMed] [Google Scholar]
- 7.Thomson F., Craighead M. Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem. Res. 2008;33(4):691–707. doi: 10.1007/s11064-007-9518-3. [http://dx.doi.org/10.1007/s11064-007-9518-3]. [PMID: 17960478]. [DOI] [PubMed] [Google Scholar]
- 8.Pariante C.M., Lightman S.L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [http://dx.doi.org/10.1016/j.tins.2008.06.006]. [PMID: 18675469]. [DOI] [PubMed] [Google Scholar]
- 9.Duman R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [http://dx.doi.org/10.1385/NMM:5:1:011]. [PMID: 15001809]. [DOI] [PubMed] [Google Scholar]
- 10.Mello A.F., Mello M.F., Carpenter L.L., Price L.H. Update on stress and depression: the role of the hypothalamic-pituitary-adrenal (HPA) axis. Rev. Bras. Psiquiatr. 2003;25(4):231–238. doi: 10.1590/s1516-44462003000400010. [http://dx.doi.org/10.1590/S1516-44462003000400010]. [PMID: 15328550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [http://dx.doi.org/10.1016/0278-5846(94)00101-M]. [PMID: 7708925]. [DOI] [PubMed] [Google Scholar]
- 12.Clark J.A., Pai L.Y., Flick R.B., Rohrer S.P. Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biol. Psychiatry. 2005;57(8):943–946. doi: 10.1016/j.biopsych.2005.01.013. [http://dx.doi.org/10.1016/j.biopsych.2005.01.013]. [PMID: 15820718]. [DOI] [PubMed] [Google Scholar]
- 13.Miller A.H. Depression and immunity: a role for T cells? Brain Behav. Immun. 2010;24(1):1–8. doi: 10.1016/j.bbi.2009.09.009. [http://dx.doi.org/10.1016/ j.bbi.2009.09.009]. [PMID: 19818725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubera M., Obuchowicz E., Goehler L., Brzeszcz J., Maes M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(3):744–759. doi: 10.1016/j.pnpbp.2010.08.026. [http://dx.doi.org/10.1016/j. pnpbp.2010.08.026]. [PMID: 20828592]. [DOI] [PubMed] [Google Scholar]
- 15.Piser T.M. Linking the cytokine and neurocircuitry hypotheses of depression: a translational framework for discovery and development of novel anti-depressants. Brain Behav. Immun. 2010;24(4):515–524. doi: 10.1016/j.bbi.2010.02.006. [http://dx.doi.org/10.1016/j.bbi.2010.02.006]. [PMID: 20193757]. [DOI] [PubMed] [Google Scholar]
- 16.Bloch M.H., Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2011;50(10):991–1000. doi: 10.1016/j.jaac.2011.06.008. [http://dx.doi.org/10.1016/j.jaac.2011.06.008]. [PMID: 21961774]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M., Bosmans E., Suy E., Vandervorst C., DeJonckheere C., Raus J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1 beta and soluble interleukin-2 receptor production. Acta Psychiatr. Scand. 1991;84(4):379–386. doi: 10.1111/j.1600-0447.1991.tb03163.x. [http://dx.doi.org/10.1111/j.1600-0447.1991.tb03163.x]. [PMID: 1746291]. [DOI] [PubMed] [Google Scholar]
- 18.Maes M., Lambrechts J., Bosmans E., Jacobs J., Suy E., Vandervorst C., de Jonckheere C., Minner B., Raus J. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol. Med. 1992;22(1):45–53. doi: 10.1017/s0033291700032712. [http://dx.doi.org/10.1017/S0033291700032712]. [PMID: 1574566]. [DOI] [PubMed] [Google Scholar]
- 19.Maes M., Stevens W., DeClerck L., Peeters D., Bridts C., Schotte C., Meltzer H., Scharpé S., Cosyns P. Neutrophil chemotaxis, phagocytosis, and superoxide release in depressive illness. Biol. Psychiatry. 1992;31(12):1220–1224. doi: 10.1016/0006-3223(92)90341-v. [http://dx.doi. org/10.1016/0006-3223(92)90341-V]. [PMID: 1327195]. [DOI] [PubMed] [Google Scholar]
- 20.Zorrilla E.P., Luborsky L., McKay J.R., Rosenthal R., Houldin A., Tax A., McCorkle R., Seligman D.A., Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav. Immun. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [http://dx.doi.org/10.1006/brbi.2000.0597]. [PMID: 11566046]. [DOI] [PubMed] [Google Scholar]
- 21.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [http://dx.doi. org/10.1016/j.biopsych.2009.09.033]. [PMID: 20015486]. [DOI] [PubMed] [Google Scholar]
- 22.Levine J., Barak Y., Chengappa K.N., Rapoport A., Rebey M., Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [http://dx. doi.org/10.1159/000026615]. [PMID: 10559698]. [DOI] [PubMed] [Google Scholar]
- 23.Owen B.M., Eccleston D., Ferrier I.N., Young A.H. Raised levels of plasma interleukin-1beta in major and postviral depression. Acta Psychiatr. Scand. 2001;103(3):226–228. doi: 10.1034/j.1600-0447.2001.00162.x. [http://dx.doi.org/10.1034/j.1600-0447.2001.00162.x]. [PMID: 11240580]. [DOI] [PubMed] [Google Scholar]
- 24.Song C., Halbreich U., Han C., Leonard B.E., Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42(5):182–188. doi: 10.1055/s-0029-1202263. [http://dx.doi.org/10.1055/s-0029-1202263]. [PMID: 19724980]. [DOI] [PubMed] [Google Scholar]
- 25.Brambilla F., Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr. Scand. 1998;97(4):309–313. doi: 10.1111/j.1600-0447.1998.tb10005.x. [http://dx.doi.org/10.1111/j.1600-0447.1998. tb10005.x]. [PMID: 9570493]. [DOI] [PubMed] [Google Scholar]
- 26.Martinez J.M., Garakani A., Yehuda R., Gorman J.M. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress. Anxiety. 2012;29(1):32–38. doi: 10.1002/da.20876. [http://dx.doi.org/10.1002/da.20876]. [PMID: 21898706]. [DOI] [PubMed] [Google Scholar]
- 27.Kubera M., Curzytek K., Duda W., Leskiewicz M., Basta-Kaim A., Budziszewska B., Roman A., Zajicova A., Holan V., Szczesny E., Lason W., Maes M. A new animal model of (chronic) depression induced by repeated and intermittent lipopolysaccharide administration for 4 months. Brain Behav. Immun. 2013;31:96–104. doi: 10.1016/j.bbi.2013.01.001. [http://dx.doi.org/10.1016/j.bbi.2013. 01.001]. [PMID: 23313516]. [DOI] [PubMed] [Google Scholar]
- 28.Ślusarczyk J., Trojan E., Głombik K., Budziszewska B., Kubera M., Lasoń W., Popiołek-Barczyk K., Mika J., Wędzony K., Basta-Kaim A. Prenatal stress is a vulnerability factor for altered morphology and biological activity of microglia cells. Front. Cell. Neurosci. 2015;9:82. doi: 10.3389/fncel.2015.00082. [http://dx.doi.org/10.3389/fncel.2015. 00082]. [PMID: 25814933]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szczesny E., Basta-Kaim A., Slusarczyk J., Trojan E., Glombik K., Regulska M., Leskiewicz M., Budziszewska B., Kubera M., Lason W. The impact of prenatal stress on insulin-like growth factor-1 and pro-inflammatory cytokine expression in the brains of adult male rats: the possible role of suppressors of cytokine signaling proteins. J. Neuroimmunol. 2014;276(1-2):37–46. doi: 10.1016/j.jneuroim.2014.08.001. [http:// dx.doi.org/10.1016/j.jneuroim.2014.08.001]. [PMID: 25151093]. [DOI] [PubMed] [Google Scholar]
- 30.Diz-Chaves Y., Pernía O., Carrero P., Garcia-Segura L.M. Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J. Neuroinflammation. 2012;9(1):71. doi: 10.1186/1742-2094-9-71. [http://dx.doi.org/10. 1186/1742-2094-9-71]. [PMID: 22520439]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diz-Chaves Y., Astiz M., Bellini M.J., Garcia-Segura L.M. Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav. Immun. 2013;28:196–206. doi: 10.1016/j.bbi.2012.11.013. [http://dx.doi.org/10.1016/j.bbi.2012.11.013]. [PMID: 23207108]. [DOI] [PubMed] [Google Scholar]
- 32.Xiu L.J., Lin H.M., Wei P.K. The effect of chronic mild stress on tumor-bearing rats’ behavior and its mechanism. Neurosci. Lett. 2010;473(1):1–4. doi: 10.1016/j.neulet.2009.06.031. [http://dx.doi.org/10.1016/j.neulet.2009.06.031]. [PMID: 19539710]. [DOI] [PubMed] [Google Scholar]
- 33.Lam R.W., Malhi G.S., McIntyre R.S., Demyttenaere K., Gorwood P., Michalak E.E., Hegerl U. Fatigue and occupational functioning in major depressive disorder. Aust. N. Z. J. Psychiatry. 2013;47(11):989–991. doi: 10.1177/0004867413488222. [http://dx.doi.org/10.1177/ 0004867413488222]. [PMID: 23652385]. [DOI] [PubMed] [Google Scholar]
- 34.Liu R.P., Zou M., Wang J.Y., Zhu J.J., Lai J.M., Zhou L.L., Chen S.F., Zhang X., Zhu J.H. Paroxetine ameliorates lipopolysaccharide-induced microglia activation via differential regulation of MAPK signaling. J. Neuroinflammation. 2014;11(1):47. doi: 10.1186/1742-2094-11-47. [http://dx.doi.org/10.1186/1742-2094-11-47]. [PMID: 24618100]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su F., Yi H., Xu L., Zhang Z. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience. 2015;294:60–68. doi: 10.1016/j.neuroscience.2015.02.028. [http://dx.doi.org/10.1016/ j.neuroscience.2015.02.028]. [PMID: 25711936]. [DOI] [PubMed] [Google Scholar]
- 36.Strawbridge R., Arnone D., Danese A., Papadopoulos A., Herane Vives A., Cleare A.J. Inflammation and Clinical Response to Treatment in Depression: A Meta-Analysis. Eur. Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Sutcigil L., Oktenli C., Musabak U., Bozkurt A., Cansever A., Uzun O., Sanisoglu S.Y., Yesilova Z., Ozmenler N., Ozsahin A., Sengul A. Pro- and Anti-Inflammatory Cytokine Balance in Major Depression: Effect of Sertraline Therapy. Clin. Dev. Immunol. 2007 doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dahl J., Ormstad H., Aass H.C., Malt U.F., Bendz L.T., Sandvik L., Brundin L., Andreassen O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. doi: 10.1016/j.psyneuen.2014.03.019. [http://dx.doi.org/10.1016/j.psyneuen.2014.03. 019]. [PMID: 24845179]. [DOI] [PubMed] [Google Scholar]
- 39.Raison C.L., Broadwell S.D., Borisov A.S., Manatunga A.K., Capuron L., Woolwine B.J., Jacobson I.M., Nemeroff C.B., Miller A.H. Depressive symptoms and viral clearance in patients receiving interferon-alpha and ribavirin for hepatitis C. Brain Behav. Immun. 2005;19(1):23–27. doi: 10.1016/j.bbi.2004.05.001. [http://dx.doi.org/10.1016/ j.bbi.2004.05.001]. [PMID: 15581735]. [DOI] [PubMed] [Google Scholar]
- 40.Raison C.L., Borisov A.S., Broadwell S.D., Capuron L., Woolwine B.J., Jacobson I.M., Nemeroff C.B., Miller A.H. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J. Clin. Psychiatry. 2005;66(1):41–48. doi: 10.4088/jcp.v66n0106. [http://dx.doi.org/10.4088/JCP.v66n0106]. [PMID: 15669887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musselman D.L., Lawson D.H., Gumnick J.F., Manatunga A.K., Penna S., Goodkin R.S., Greiner K., Nemeroff C.B., Miller A.H. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 2001;344(13):961–966. doi: 10.1056/NEJM200103293441303. [http://dx.doi.org/10.1056/NEJM200103293441303]. [PMID: 11274622]. [DOI] [PubMed] [Google Scholar]
- 42.Eugenin E.A., Dyer G., Calderon T.M., Berman J.W. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: possible role in NeuroAIDS. Glia. 2005;49(4):501–510. doi: 10.1002/glia.20137. [http://dx.doi.org/10. 1002/glia.20137]. [PMID: 15578658]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biber K., Vinet J., Boddeke H.W. Neuron-microglia signaling: chemokines as versatile messengers. J. Neuroimmunol. 2008;198(1-2):69–74. doi: 10.1016/j.jneuroim.2008.04.012. [http://dx.doi.org/10.1016/j.jneuroim.2008.04.012]. [PMID: 18538419]. [DOI] [PubMed] [Google Scholar]
- 44.Riek-Burchardt M., Kolodziej A., Henrich-Noack P., Reymann K.G., Höllt V., Stumm R. Differential regulation of CXCL12 and PACAP mRNA expression after focal and global ischemia. Neuropharmacology. 2010;58(1):199–207. doi: 10.1016/j.neuropharm.2009.07.032. [http://dx.doi.org/ 10.1016/j.neuropharm.2009.07.032]. [PMID: 19647005]. [DOI] [PubMed] [Google Scholar]
- 45.Pujol F., Kitabgi P., Boudin H. The chemokine SDF-1 differentially regulates axonal elongation and branching in hippocampal neurons. J. Cell Sci. 2005;118(Pt 5):1071–1080. doi: 10.1242/jcs.01694. [http://dx.doi.org/10.1242/jcs.01694]. [PMID: 15731012]. [DOI] [PubMed] [Google Scholar]
- 46.Kettenmann H., Kirchhoff F., Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77(1):10–18. doi: 10.1016/j.neuron.2012.12.023. [http:// dx.doi.org/10.1016/j.neuron.2012.12.023]. [PMID: 23312512]. [DOI] [PubMed] [Google Scholar]
- 47.Milior G., Lecours C., Samson L., Bisht K., Poggini S., Pagani F., Deflorio C., Lauro C., Alboni S., Limatola C., Branchi I., Tremblay M.E., Maggi L. Fractalkine receptor deficiency impairs microglial and neuronal responsiveness to chronic stress. Brain Behav. Immun. 2015 doi: 10.1016/j.bbi.2015.07.024. [PMID: 26231972]. [DOI] [PubMed] [Google Scholar]
- 48.Dimitrijevic O.B., Stamatovic S.M., Keep R.F., Andjelkovic A.V. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J. Cereb. Blood Flow Metab. 2006;26(6):797–810. doi: 10.1038/sj.jcbfm.9600229. [http://dx.doi.org/ 10.1038/sj.jcbfm.9600229]. [PMID: 16192992]. [DOI] [PubMed] [Google Scholar]
- 49.Ransohoff R.M. Chemokines and chemokine receptors: standing at the crossroads of immunobiology and neurobiology. Immunity. 2009;31(5):711–721. doi: 10.1016/j.immuni.2009.09.010. [http://dx.doi.org/10.1016/j.immuni.2009. 09.010]. [PMID: 19836265]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh S.B., Tran P.B., Gillard S.E., Hurley R.W., Hammond D.L., Miller R.J. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J. Neurosci. 2001;21(14):5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [PMID: 11438578]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh S.B., Cho C., Miller R.J. Electrophysiological analysis of neuronal chemokine receptors. Methods. 2003;29(4):335–344. doi: 10.1016/s1046-2023(02)00357-2. [http://dx.doi.org/10.1016/S1046-2023(02)00357-2]. [PMID: 12725800]. [DOI] [PubMed] [Google Scholar]
- 52.Réaux-Le Goazigo A., Van Steenwinckel J., Rostène W. [DOI] [PubMed]; Mélik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog. Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [http://dx.doi.org/ 10.1016/j.pneurobio.2013.02.001]. [PMID: 23454481]. [DOI] [PubMed] [Google Scholar]
- 53.Adler M. W., Geller E.B., Chen X., Rogers T.J. Viewing Chemokines as a Third Major System of Communication in the Brain. Drug Addict. From Basic Res. to Ther. 2008;7(4):127–138. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimura T., Matsushima K., Tanaka S., Robinson E.A., Appella E., Oppenheim J.J., Leonard E.J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc. Natl. Acad. Sci. USA. 1987;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [http://dx.doi.org/ 10.1073/pnas.84.24.9233]. [PMID: 3480540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy P.M., Baggiolini M., Charo I.F., Hébert C.A., Horuk R., Matsushima K., Miller L.H., Oppenheim J.J., Power C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52(1):145–176. [PMID: 10699158]. [PubMed] [Google Scholar]
- 56.Zlotnik A., Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. doi: 10.1016/s1074-7613(00)80165-x. [http://dx.doi.org/10.1016/S1074-7613(00)80165-X]. [PMID: 10714678]. [DOI] [PubMed] [Google Scholar]
- 57.Furutani Y., Nomura H., Notake M., Oyamada Y., Fukui T., Yamada M., Larsen C.G., Oppenheim J.J., Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF). Biochem. Biophys. Res. Commun. 1989;159(1):249–255. doi: 10.1016/0006-291x(89)92430-3. [http://dx.doi.org/10.1016/0006-291X(89)92430-3]. [PMID: 2923622]. [DOI] [PubMed] [Google Scholar]
- 58.Mehrabian M., Sparkes R.S., Mohandas T., Fogelman A.M., Lusis A.J. Localization of monocyte chemotactic protein-1 gene (SCYA2) to human chromosome 17q11.2-q21.1. Genomics. 1991;9(1):200–203. doi: 10.1016/0888-7543(91)90239-b. [http://dx.doi.org/10.1016/0888-7543(91)90239-B]. [PMID: 2004761]. [DOI] [PubMed] [Google Scholar]
- 59.Fernandez E.J., Lolis E. Structure, function, and inhibition of chemokines. Annu. Rev. Pharmacol. Toxicol. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [http://dx.doi.org/10.1146/annurev.pharmtox.42.091901.115838]. [PMID: 11807180]. [DOI] [PubMed] [Google Scholar]
- 60.Colobran R., Pujol-Borrell R., Armengol M.P., Juan M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin. Exp. Immunol. 2007;148(2):208–217. doi: 10.1111/j.1365-2249.2007.03344.x. [http://dx.doi.org/10.1111/ j.1365-2249.2007.03344.x]. [PMID: 17437419]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones B.A., Beamer M., Ahmed S. Fractalkine/CX3CL1: a potential new target for inflammatory diseases. Mol. Interv. 2010;10(5):263–270. doi: 10.1124/mi.10.5.3. [http://dx.doi.org/10.1124/mi.10.5.3]. [PMID: 21045240]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bazan J.F., Bacon K.B., Hardiman G., Wang W., Soo K., Rossi D., Greaves D.R., Zlotnik A., Schall T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. doi: 10.1038/385640a0. [http://dx.doi.org/10.1038/385640a0]. [PMID: 9024663]. [DOI] [PubMed] [Google Scholar]
- 63.Matloubian M., David A., Engel S., Ryan J.E., Cyster J.G. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 2000;1(4):298–304. doi: 10.1038/79738. [http://dx.doi.org/ 10.1038/79738]. [PMID: 11017100]. [DOI] [PubMed] [Google Scholar]
- 64.Hundhausen C., Misztela D., Berkhout T.A., Broadway N., Saftig P., Reiss K., Hartmann D., Fahrenholz F., Postina R., Matthews V., Kallen K.J., Rose-John S., Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102(4):1186–1195. doi: 10.1182/blood-2002-12-3775. [http://dx.doi.org/10.1182/blood-2002-12-3775]. [PMID: 12714508]. [DOI] [PubMed] [Google Scholar]
- 65.Allen S.J., Crown S.E., Handel T.M. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [http://dx.doi.org/10.1146/annurev.immunol.24. 021605.090529]. [PMID: 17291188]. [DOI] [PubMed] [Google Scholar]
- 66.Gupta S.K., Pillarisetti K., Thomas R.A., Aiyar N. Pharmacological evidence for complex and multiple site interaction of CXCR4 with SDF-1α: implications for development of selective CXCR4 antagonists. Immunol. Lett. 2001;78(1):29–34. doi: 10.1016/s0165-2478(01)00228-0. [http://dx. doi.org/10.1016/S0165-2478(01)00228-0]. [PMID: 11470148]. [DOI] [PubMed] [Google Scholar]
- 67.Blanpain C., Buser R., Power C.A., Edgerton M., Buchanan C., Mack M., Simmons G., Clapham P.R., Parmentier M., Proudfoot A.E. A chimeric MIP-1alpha/RANTES protein demonstrates the use of different regions of the RANTES protein to bind and activate its receptors. J. Leukoc. Biol. 2001;69(6):977–985. [PMID: 11404385]. [PubMed] [Google Scholar]
- 68.Biber K., Zuurman M.W., Dijkstra I.M., Boddeke H.W. Chemokines in the brain: neuroimmunology and beyond. Curr. Opin. Pharmacol. 2002;2(1):63–68. doi: 10.1016/s1471-4892(01)00122-9. [http://dx.doi.org/10.1016/ S1471-4892(01)00122-9]. [PMID: 11786310]. [DOI] [PubMed] [Google Scholar]
- 69.Murphy P.M. International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002;54(2):227–229. doi: 10.1124/pr.54.2.227. [http://dx.doi.org/10.1124/pr.54.2.227]. [PMID: 12037138]. [DOI] [PubMed] [Google Scholar]
- 70.Rajagopal S., Bassoni D.L., Campbell J.J., Gerard N.P., Gerard C., Wehrman T.S. Biased agonism as a mechanism for differential signaling by chemokine receptors. J. Biol. Chem. 2013;288(49):35039–35048. doi: 10.1074/jbc.M113.479113. [http://dx.doi.org/10.1074/jbc.M113.479113]. [PMID: 24145037]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knall C., Young S., Nick J.A., Buhl A.M., Worthen G.S., Johnson G.L. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol. Chem. 1996;271(5):2832–2838. doi: 10.1074/jbc.271.5.2832. [http://dx.doi.org/10.1074/ jbc.271.5.2832]. [PMID: 8576262]. [DOI] [PubMed] [Google Scholar]
- 72.Curnock A.P., Logan M.K., Ward S.G. Chemokine signalling: pivoting around multiple phosphoinositide 3-kinases. Immunology. 2002;105(2):125–136. doi: 10.1046/j.1365-2567.2002.01345.x. [http://dx.doi.org/10.1046/j.1365-2567. 2002.01345.x]. [PMID: 11872087]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Thuc O., Blondeau N., Nahon J.L., Rovère C. The complex contribution of chemokines to neuroinflammation: switching from beneficial to detrimental effects. Ann. N. Y. Acad. Sci. 2015;1351(1):127–140. doi: 10.1111/nyas.12855. [http://dx.doi.org/10.1111/nyas.12855]. [PMID: 26251227]. [DOI] [PubMed] [Google Scholar]
- 74.Che X., Ye W., Panga L., Wu D.C., Yang G.Y. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res. 2001;902(2):171–177. doi: 10.1016/s0006-8993(01)02328-9. [http://dx.doi.org/10.1016/S0006-8993(01)02328-9]. [PMID: 11384610]. [DOI] [PubMed] [Google Scholar]
- 75.Mantovani A. Chemokines. Introduction and overview. Chem. Immunol. 1999;72:1–6. [http://dx.doi.org/10.1159/000058734]. [PMID: 10550926]. [PubMed] [Google Scholar]
- 76.Mélik-Parsadaniantz S., Rostène W. Chemokines and neuromodulation. J. Neuroimmunol. 2008;198(1-2):62–68. doi: 10.1016/j.jneuroim.2008.04.022. [http:// dx.doi.org/10.1016/j.jneuroim.2008.04.022]. [PMID: 18538863]. [DOI] [PubMed] [Google Scholar]
- 77.Limatola C., Ransohoff R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 2014;8(8):229. doi: 10.3389/fncel.2014.00229. [PMID: 25152714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Banisadr G., Fontanges P., Haour F., Kitabgi P., Rostène W., Mélik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur. J. Neurosci. 2002;16(9):1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [http://dx.doi. org/10.1046/j.1460-9568.2002.02237.x]. [PMID: 12431218]. [DOI] [PubMed] [Google Scholar]
- 79.Stumm R.K., Zhou C., Ara T., Lazarini F., Dubois-Dalcq M., Nagasawa T., Höllt V., Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J. Neurosci. 2003;23(12):5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [PMID: 12832536]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schönemeier B., Kolodziej A., Schulz S., Jacobs S., Hoellt V., Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J. Comp. Neurol. 2008;510(2):207–220. doi: 10.1002/cne.21780. [http://dx.doi.org/ 10.1002/cne.21780]. [PMID: 18615560]. [DOI] [PubMed] [Google Scholar]
- 81.Tran P.B., Banisadr G., Ren D., Chenn A., Miller R.J. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J. Comp. Neurol. 2007;500(6):1007–1033. doi: 10.1002/cne.21229. [http://dx.doi.org/10.1002/cne.21229]. [PMID: 17183554]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu M., Grove E.A., Miller R.J. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc. Natl. Acad. Sci. USA. 2002;99(10):7090–7095. doi: 10.1073/pnas.092013799. [http://dx.doi.org/10.1073/pnas.092013799]. [PMID: 11983855]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwarting G.A., Henion T.R., Nugent J.D., Caplan B., Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J. Neurosci. 2006;26(25):6834–6840. doi: 10.1523/JNEUROSCI.1728-06.2006. [http://dx.doi.org/ 10.1523/JNEUROSCI.1728-06.2006]. [PMID: 16793890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stumm R., Höllt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J. Mol. Endocrinol. 2007;38(3):377–382. doi: 10.1677/JME-06-0032. [http://dx.doi.org/ 10.1677/JME-06-0032]. [PMID: 17339400]. [DOI] [PubMed] [Google Scholar]
- 85.Stumm R., Kolodziej A., Schulz S., Kohtz J.D., Höllt V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J. Comp. Neurol. 2007;502(3):382–399. doi: 10.1002/cne.21336. [http://dx.doi.org/10.1002/cne.21336]. [PMID: 17366607]. [DOI] [PubMed] [Google Scholar]
- 86.López-Bendito G., Sánchez-Alcañiz J.A., Pla R., Borrell V., Picó E., Valdeolmillos M., Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J. Neurosci. 2008;28(7):1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI.4651-07.2008]. [PMID: 18272682]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li G., Adesnik H., Li J., Long J., Nicoll R.A., Rubenstein J.L., Pleasure S.J. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J. Neurosci. 2008;28(5):1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [http://dx.doi. org/10.1523/JNEUROSCI.4602-07.2008]. [PMID: 18234887]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eyre H., Baune B.T. Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology. 2012;37(9):1397–1416. doi: 10.1016/j.psyneuen.2012.03.019. [http://dx.doi.org/10.1016/j.psyneuen.2012.03.019]. [PMID: 22525700]. [DOI] [PubMed] [Google Scholar]
- 89.Stuart M.J., Corrigan F., Baune B.T. Knockout of CXCR5 increases the population of immature neural cells and decreases proliferation in the hippocampal dentate gyrus. J. Neuroinflammation. 2014;11(1):31. doi: 10.1186/1742-2094-11-31. [http://dx.doi.org/10.1186/1742-2094-11-31]. [PMID: 24528805]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banisadr G., Bhattacharyya B.J., Belmadani A., Izen S.C., Ren D., Tran P.B., Miller R.J. The chemokine BRAK/CXCL14 regulates synaptic transmission in the adult mouse dentate gyrus stem cell niche. J. Neurochem. 2011;119(6):1173–1182. doi: 10.1111/j.1471-4159.2011.07509.x. [http:// dx.doi.org/10.1111/j.1471-4159.2011.07509.x]. [PMID: 21955359]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao F., Xu J.M., Jiang X.H. CX3 chemokine receptor 1 deficiency leads to reduced dendritic complexity and delayed maturation of newborn neurons in the adult mouse hippocampus. Neural Regen. Res. 2015;10(5):772–777. doi: 10.4103/1673-5374.156979. [http://dx.doi.org/10. 4103/1673-5374.156979]. [PMID: 26109952]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [http://dx.doi.org/10.1126/science.1202529]. [PMID: 21778362]. [DOI] [PubMed] [Google Scholar]
- 93.Paolicelli R.C., Bisht K., Tremblay M.È. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014;8(8):129. doi: 10.3389/fncel.2014.00129. [PMID: 24860431]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sheridan G.K., Wdowicz A., Pickering M., Watters O., Halley P., O’Sullivan N.C., Mooney C., O’Connell D.J., O’Connor J.J., Murphy K.J. CX3CL1 is up-regulated in the rat hippocampus during memory-associated synaptic plasticity. Front. Cell. Neurosci. 2014;8(8):233. doi: 10.3389/fncel.2014.00233. [PMID: 25161610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heinisch S., Kirby L.G. Fractalkine/CX3CL1 enhances GABA synaptic activity at serotonin neurons in the rat dorsal raphe nucleus. Neuroscience. 2009;164(3):1210–1223. doi: 10.1016/j.neuroscience.2009.08.075. [http://dx.doi.org/ 10.1016/j.neuroscience.2009.08.075]. [PMID: 19748551]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Banisadr G., Gosselin R.D., Mechighel P., Rostène W., Kitabgi P., Mélik Parsadaniantz S. Constitutive neuronal expression of CCR2 chemokine receptor and its colocalization with neurotransmitters in normal rat brain: functional effect of MCP-1/CCL2 on calcium mobilization in primary cultured neurons. J. Comp. Neurol. 2005;492(2):178–192. doi: 10.1002/cne.20729. [http://dx.doi.org/ 10.1002/cne.20729]. [PMID: 16196033]. [DOI] [PubMed] [Google Scholar]
- 97.Gao Y.J., Zhang L., Samad O.A., Suter M.R., Yasuhiko K., Xu Z.Z., Park J.Y., Lind A.L., Ma Q., Ji R.R. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J. Neurosci. 2009;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [http://dx.doi.org/10.1523/JNEUROSCI.3623-08.2009]. [PMID: 19339605]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musante V., Longordo F., Neri E., Pedrazzi M., Kalfas F., Severi P., Raiteri M., Pittaluga A. RANTES modulates the release of glutamate in human neocortex. J. Neurosci. 2008;28(47):12231–12240. doi: 10.1523/JNEUROSCI.3212-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI. 3212-08.2008]. [PMID: 19020017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Prisco S., Merega E., Milanese M., Summa M., Casazza S., Raffaghello L., Pistoia V., Uccelli A., Pittaluga A. CCL5-glutamate interaction in central nervous system: Early and acute presynaptic defects in EAE mice. Neuropharmacology. 2013;75:337–346. doi: 10.1016/j.neuropharm.2013.07.037. [http://dx.doi.org/10.1016/j.neuropharm.2013.07.037]. [PMID: 23958452]. [DOI] [PubMed] [Google Scholar]
- 100.Guyon A., Skrzydelsi D., Rovère C., Rostène W., Parsadaniantz S.M., Nahon J.L. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J. Neurochem. 2006;96(6):1540–1550. doi: 10.1111/j.1471-4159.2006.03659.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.03659.x]. [PMID: 16476083]. [DOI] [PubMed] [Google Scholar]
- 101.Gerard C., Rollins B.J. Chemokines and disease. Nat. Immunol. 2001;2(2):108–115. doi: 10.1038/84209. [http://dx.doi.org/10.1038/84209]. [PMID: 11175802]. [DOI] [PubMed] [Google Scholar]
- 102.Hinojosa A.E., Garcia-Bueno B., Leza J.C., Madrigal J.L. CCL2/MCP-1 modulation of microglial activation and proliferation. J. Neuroinflammation. 2011;8(1):77. doi: 10.1186/1742-2094-8-77. [http://dx.doi. org/10.1186/1742-2094-8-77]. [PMID: 21729288]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stuart M.J., Baune B.T. Chemokines and chemokine receptors in mood disorders, schizophrenia, and cognitive impairment: a systematic review of biomarker studies. Neurosci. Biobehav. Rev. 2014;42:93–115. doi: 10.1016/j.neubiorev.2014.02.001. [http://dx.doi.org/10.1016/j.neubiorev.2014. 02.001]. [PMID: 24513303]. [DOI] [PubMed] [Google Scholar]
- 104.Semple B.D., Kossmann T., Morganti-Kossmann M.C. Role of chemokines in CNS health and pathology: a focus on the CCL2/CCR2 and CXCL8/CXCR2 networks. J. Cereb. Blood Flow Metab. 2010;30(3):459–473. doi: 10.1038/jcbfm.2009.240. [http://dx.doi.org/10.1038/jcbfm. 2009.240]. [PMID: 19904283]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toyomitsu E., Tsuda M., Yamashita T., Tozaki-Saitoh H., Tanaka Y., Inoue K. CCL2 promotes P2X4 receptor trafficking to the cell surface of microglia. Purinergic Signal. 2012;8(2):301–310. doi: 10.1007/s11302-011-9288-x. [http://dx.doi.org/10.1007/s11302-011-9288-x]. [PMID: 22222817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishizuka K., Kimura T., Igata-yi R., Katsuragi S., Takamatsu J., Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin. Neurosci. 1997;51(3):135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [http://dx.doi. org/10.1111/j.1440-1819.1997.tb02375.x]. [PMID: 9225377]. [DOI] [PubMed] [Google Scholar]
- 107.Flaishon L., Hart G., Zelman E., Moussion C., Grabovsky V., Lapidot Tal G., Feigelson S., Margalit R., Harmelin A., Avin-Wittenberg T., Shoseyov D., Alon R., Girard J.P., Shachar I. Anti-inflammatory effects of an inflammatory chemokine: CCL2 inhibits lymphocyte homing by modulation of CCL21-triggered integrin-mediated adhesions. Blood. 2008;112(13):5016–5025. doi: 10.1182/blood-2007-12-129122. [http://dx.doi.org/10.1182/blood-2007-12-129122]. [PMID: 18802011]. [DOI] [PubMed] [Google Scholar]
- 108.Gu L., Tseng S., Horner R.M., Tam C., Loda M., Rollins B.J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404(6776):407–411. doi: 10.1038/35006097. [http://dx.doi.org/10.1038/35006097]. [PMID: 10746730]. [DOI] [PubMed] [Google Scholar]
- 109.Zhou Y., Tang H., Liu J., Dong J., Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J. Neurochem. 2011;116(3):406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [http://dx.doi.org/10.1111/j.1471-4159.2010.07121.x]. [PMID: 21105875]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kalehua A.N., Nagel J.E., Whelchel L.M., Gides J.J., Pyle R.S., Smith R.J., Kusiak J.W., Taub D.D. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp. Cell Res. 2004;297(1):197–211. doi: 10.1016/j.yexcr.2004.02.031. [http://dx.doi.org/10.1016/j.yexcr.2004.02.031]. [PMID: 15194436]. [DOI] [PubMed] [Google Scholar]
- 111.Harrison E.L., Jaehne E.J., Jawahar M.C., Corrigan F., Baune B.T. Maternal separation modifies behavioural and neuroendocrine responses to stress in CCR7 deficient mice. Behav. Brain Res. 2014;263(4):169–175. doi: 10.1016/j.bbr.2014.01.036. [http://dx.doi.org/10.1016/j.bbr.2014.01. 036]. [PMID: 24503116]. [DOI] [PubMed] [Google Scholar]
- 112.De Paola M., Buanne P., Biordi L., Bertini R., Ghezzi P., Mennini T. Chemokine MIP-2/CXCL2, acting on CXCR2, induces motor neuron death in primary cultures. Neuroimmunomodulation. 2007;14(6):310–316. doi: 10.1159/000123834. [http://dx.doi.org/10.1159/000123834].[PMID: 18391506]. [DOI] [PubMed] [Google Scholar]
- 113.Bachstetter A.D., Morganti J.M., Jernberg J., Schlunk A., Mitchell S.H., Brewster K.W., Hudson C.E., Cole M.J., Harrison J.K., Bickford P.C., Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging. 2011;32(11):2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [http://dx.doi.org/ 10.1016/j.neurobiolaging.2009.11.022]. [PMID: 20018408]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suzumura A. Neuron-microglia interaction in neuroinflammation. Curr. Protein Pept. Sci. 2013;14(1):16–20. doi: 10.2174/1389203711314010004. [http://dx.doi.org/10. 2174/1389203711314010004]. [PMID: 23544747]. [DOI] [PubMed] [Google Scholar]
- 115.Mizuno T., Kawanokuchi J., Numata K., Suzumura A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Res. 2003;979(1-2):65–70. doi: 10.1016/s0006-8993(03)02867-1. [http:// dx.doi.org/10.1016/S0006-8993(03)02867-1]. [PMID: 12850572]. [DOI] [PubMed] [Google Scholar]
- 116.Lyons A., Lynch A.M., Downer E.J., Hanley R., O’Sullivan J.B., Smith A., Lynch M.A. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J. Neurochem. 2009;110(5):1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [http://dx.doi.org/10.1111/j.1471-4159.2009.06253.x].[PMID: 19627440]. [DOI] [PubMed] [Google Scholar]
- 117.Stephan A.H., Barres B.A., Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu. Rev. Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [http://dx.doi. org/10.1146/annurev-neuro-061010-113810].[PMID: 22715882]. [DOI] [PubMed] [Google Scholar]
- 118.Bilimoria P.M., Stevens B. Microglia function during brain development: New insights from animal models. Brain Res. 2015;1617:7–17. doi: 10.1016/j.brainres.2014.11.032. [http://dx.doi.org/10.1016/j.brainres.2014.11.032]. [PMID: 25463024]. [DOI] [PubMed] [Google Scholar]
- 119.Pagani F., Paolicelli R.C., Murana E., Cortese B., Di Angelantonio S., Zurolo E., Guiducci E., Ferreira T.A., Garofalo S., Catalano M., D’Alessandro G., Porzia A., Peruzzi G., Mainiero F., Limatola C., Gross C.T., Ragozzino D. Defective microglial development in the hippocampus of Cx3cr1 deficient mice. Front. Cell. Neurosci. 2015;9(9):111. doi: 10.3389/fncel.2015.00111. [PMID: 25873863]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cardona A.E., Pioro E.P., Sasse M.E., Kostenko V., Cardona S.M., Dijkstra I.M., Huang D., Kidd G., Dombrowski S., Dutta R., Lee J.C., Cook D.N., Jung S., Lira S.A., Littman D.R., Ransohoff R.M. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [http://dx.doi.org/10.1038/nn1715]. [PMID: 16732273]. [DOI] [PubMed] [Google Scholar]
- 121.Rosito M., Lauro C., Chece G., Porzia A., Monaco L., Mainiero F., Catalano M., Limatola C., Trettel F. Trasmembrane chemokines CX3CL1 and CXCL16 drive interplay between neurons, microglia and astrocytes to counteract pMCAO and excitotoxic neuronal death. Front. Cell. Neurosci. 2014;8(8):193. doi: 10.3389/fncel.2014.00193. [PMID: 25071451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Callewaere C., Banisadr G., Rostène W., Parsadaniantz S.M. Chemokines and chemokine receptors in the brain: implication in neuroendocrine regulation. J. Mol. Endocrinol. 2007;38(3):355–363. doi: 10.1677/JME-06-0035. [http://dx.doi.org/10.1677/JME-06-0035]. [PMID: 17339398]. [DOI] [PubMed] [Google Scholar]
- 123.Licinio J., Wong M.L., Gold P.W. Neutrophil-activating peptide-1/interleukin-8 mRNA is localized in rat hypothalamus and hippocampus. Neuroreport. 1992;3(9):753–756. doi: 10.1097/00001756-199209000-00008. [http://dx.doi.org/ 10.1097/00001756-199209000-00008].[PMID: 1421131]. [DOI] [PubMed] [Google Scholar]
- 124.Toba Y., Tiong J.D., Ma Q., Wray S. CXCR4/SDF-1 system modulates development of GnRH-1 neurons and the olfactory system. Dev. Neurobiol. 2008;68(4):487–503. doi: 10.1002/dneu.20594. [http://dx.doi.org/ 10.1002/dneu.20594]. [PMID: 18188864]. [DOI] [PubMed] [Google Scholar]
- 125.Schwarting G.A., Henion T.R., Nugent J.D., Caplan B., Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J. Neurosci. 2006;26(25):6834–6840. doi: 10.1523/JNEUROSCI.1728-06.2006. [http://dx.doi. org/10.1523/JNEUROSCI.1728-06.2006]. [PMID: 16793890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sakamoto Y., Koike K., Kiyama H., Konishi K., Watanabe K., Osako Y., Hirota K., Miyake A. Endotoxin activates a chemokinergic neuronal pathway in the hypothalamo-pituitary system. Endocrinology. 1996;137(10):4503–4506. doi: 10.1210/endo.137.10.8828513. [PMID: 8828513]. [DOI] [PubMed] [Google Scholar]
- 127.Reyes T.M., Walker J.R., DeCino C., Hogenesch J.B., Sawchenko P.E. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J. Neurosci. 2003;23(13):5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [PMID: 12843263]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsumoto K., Koike K., Miyake A., Watanabe K., Konishi K., Kiyama H. Noxious stimulation enhances release of cytokine-induced neutrophil chemoattractant from hypothalamic neurosecretory cells. Neurosci. Res. 1997;27(2):181–184. doi: 10.1016/s0168-0102(96)01144-3. [http:// dx.doi.org/10.1016/S0168-0102(96)01144-3]. [PMID: 9100261]. [DOI] [PubMed] [Google Scholar]
- 129.Davatelis G., Wolpe S.D., Sherry B., Dayer J.M., Chicheportiche R., Cerami A. Macrophage inflammatory protein-1: a prostaglandin-independent endogenous pyrogen. Science. 1989;243(4894 Pt 1):1066–1068. doi: 10.1126/science.2646711. [http://dx.doi.org/10.1126/ science.2646711]. [PMID: 2646711]. [DOI] [PubMed] [Google Scholar]
- 130.Miñano F.J., Sancibrian M., Vizcaino M., Paez X., Davatelis G., Fahey T., Sherry B., Cerami A., Myers R.D. Macrophage inflammatory protein-1: unique action on the hypothalamus to evoke fever. Brain Res. Bull. 1990;24(6):849–852. doi: 10.1016/0361-9230(90)90150-x. [http://dx.doi. org/10.1016/0361-9230(90)90150-X]. [PMID: 2196977]. [DOI] [PubMed] [Google Scholar]
- 131.Miñano F.J., Sancibrian M., Myers R.D. Fever induced by macrophage inflammatory protein-1 (MIP-1) in rats: hypothalamic sites of action. Brain Res. Bull. 1991;27(5):701–706. doi: 10.1016/0361-9230(91)90049-p. [http://dx. doi.org/10.1016/0361-9230(91)90049-P]. [PMID: 1756389]. [DOI] [PubMed] [Google Scholar]
- 132.Banisadr G., Skrzydelski D., Kitabgi P., Rostène W., Parsadaniantz S.M. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur. J. Neurosci. 2003;18(6):1593–1606. doi: 10.1046/j.1460-9568.2003.02893.x. [http://dx. doi.org/10.1046/j.1460-9568.2003.02893.x]. [PMID: 14511338]. [DOI] [PubMed] [Google Scholar]
- 133.Sakamoto Y., Koike K., Kiyama H., Konishi K., Watanabe K., Tsurufuji S., Bicknell R.J., Hirota K., Miyake A. A stress-sensitive chemokinergic neuronal pathway in the hypothalamo-pituitary system. Neuroscience. 1996;75(1):133–142. doi: 10.1016/0306-4522(96)00252-7. [http://dx.doi.org/10.1016/0306-4522(96)00252-7]. [PMID: 8923529]. [DOI] [PubMed] [Google Scholar]
- 134.Tagliari B., Tagliari A.P., Schmitz F., da Cunha A.A., Dalmaz C., Wyse A.T. Chronic variable stress alters inflammatory and cholinergic parameters in hippocampus of rats. Neurochem. Res. 2011;36(3):487–493. doi: 10.1007/s11064-010-0367-0. [http://dx.doi.org/10.1007/s11064-010-0367-0]. [PMID: 21184279]. [DOI] [PubMed] [Google Scholar]
- 135.Girotti M., Donegan J.J., Morilak D.A. Chronic intermittent cold stress sensitizes neuro-immune reactivity in the rat brain. Psychoneuroendocrinology. 2011;36(8):1164–1174. doi: 10.1016/j.psyneuen.2011.02.008. [http://dx.doi.org/ 10.1016/j.psyneuen.2011.02.008].[PMID: 21411230]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jaehne E.J., Baune B.T. Effects of chemokine receptor signalling on cognition-like, emotion-like and sociability behaviours of CCR6 and CCR7 knockout mice. Behav. Brain Res. 2014;261:31–39. doi: 10.1016/j.bbr.2013.12.006. [http://dx.doi.org/10.1016/j.bbr.2013.12.006].[PMID: 24333375]. [DOI] [PubMed] [Google Scholar]
- 137.Campbell S.J., Meier U., Mardiguian S., Jiang Y., Littleton E.T., Bristow A., Relton J., Connor T.J., Anthony D.C. Sickness behaviour is induced by a peripheral CXC-chemokine also expressed in multiple sclerosis and EAE. Brain Behav. Immun. 2010;24(5):738–746. doi: 10.1016/j.bbi.2010.01.011. [http://dx.doi.org/10.1016/j.bbi.2010.01.011].[PMID: 20138139]. [DOI] [PubMed] [Google Scholar]
- 138.Corona A.W., Huang Y., O’Connor J.C., Dantzer R., Kelley K.W., Popovich P.G., Godbout J.P. Fractalkine receptor (CX3CR1) deficiency sensitizes mice to the behavioral changes induced by lipopolysaccharide. J. Neuroinflammation. 2010;7(1):93. doi: 10.1186/1742-2094-7-93. [http://dx.doi.org/10.1186/1742-2094-7-93].[PMID: 21167054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Corona A.W., Norden D.M., Skendelas J.P., Huang Y., O’Connor J.C., Lawson M., Dantzer R., Kelley K.W., Godbout J.P. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav. Immun. 2013;31:134–142. doi: 10.1016/j.bbi.2012.08.008. [http://dx.doi.org/10.1016/j.bbi.2012.08.008].[PMID: 22926082]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hoyo-Becerra C., Liu Z., Yao J., Kaltwasser B., Gerken G., Hermann D.M., Schlaak J.F. Rapid Regulation of Depression-Associated Genes in a New Mouse Model Mimicking Interferon-α-Related Depression in Hepatitis C Virus Infection. Mol. Neurobiol. 2015;52(1):318–329. doi: 10.1007/s12035-014-8861-z. [http://dx.doi.org/10.1007/s12035-014-8861-z].[PMID: 25159480]. [DOI] [PubMed] [Google Scholar]
- 141.Ogłodek E., Szota A., Just M., Moś D., Araszkiewicz A. The role of the neuroendocrine and immune systems in the pathogenesis of depression. Pharmacol. Rep. 2014;66(5):776–781. doi: 10.1016/j.pharep.2014.04.009. [http://dx. doi.org/10.1016/j.pharep.2014.04.009].[PMID: 25149980]. [DOI] [PubMed] [Google Scholar]
- 142.Powell T.R., McGuffin P., D’Souza U.M., Cohen-Woods S., Hosang G.M., Martin C., Matthews K., Day R.K., Farmer A.E., Tansey K.E., Schalkwyk L.C. Putative transcriptomic biomarkers in the inflammatory cytokine pathway differentiate major depressive disorder patients from control subjects and bipolar disorder patients. PLoS One. 2014;9(3):e91076. doi: 10.1371/journal.pone.0091076. [http://dx.doi.org/10.1371/journal. pone.0091076].[PMID: 24618828]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simon N.M., McNamara K., Chow C.W., Maser R.S., Papakostas G.I., Pollack M.H., Nierenberg A.A., Fava M., Wong K.K. A detailed examination of cytokine abnormalities in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2008;18(3):230–233. doi: 10.1016/j.euroneuro.2007.06.004. [http://dx.doi.org/10.1016/j.euroneuro.2007.06. 004].[PMID: 17681762]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Piletz J.E., Halaris A., Iqbal O., Hoppensteadt D., Fareed J., Zhu H., Sinacore J., Devane C.L. Pro-inflammatory biomakers in depression: treatment with venlafaxine. World J. Biol. Psychiatry. 2009;10(4):313–323. doi: 10.3109/15622970802573246. [http://dx.doi.org/10.3109/ 15622970802573246].[PMID: 19921973]. [DOI] [PubMed] [Google Scholar]
- 145.Lehto S.M., Niskanen L., Herzig K.H., Tolmunen T., Huotari A., Viinamäki H., Koivumaa-Honkanen H., Honkalampi K., Ruotsalainen H., Hintikka J. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology. 2010;35(2):226–232. doi: 10.1016/j.psyneuen.2009.06.007. [http://dx.doi.org/10.1016/j.psyneuen.2009.06.007].[PMID: 19592174]. [DOI] [PubMed] [Google Scholar]
- 146.Black C., Miller B.J. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol. Psychiatry. 2015;78(1):28–37. doi: 10.1016/j.biopsych.2014.10.014. [http:// dx.doi.org/10.1016/j.biopsych.2014.10.014]. [PMID: 25541493]. [DOI] [PubMed] [Google Scholar]
- 147.Brietzke E., Kauer-Sant’Anna M., Teixeira A.L., Kapczinski F. Abnormalities in serum chemokine levels in euthymic patients with bipolar disorder. Brain Behav. Immun. 2009;23(8):1079–1082. doi: 10.1016/j.bbi.2009.04.008. [http://dx.doi.org/10.1016/j.bbi.2009.04.008]. [PMID: 19406226]. [DOI] [PubMed] [Google Scholar]
- 148.Grassi-Oliveira R., Brieztke E., Teixeira A., Pezzi J.C., Zanini M., Lopes R.P., Bauer M.E. Peripheral chemokine levels in women with recurrent major depression with suicidal ideation. Rev. Bras. Psiquiatr. 2012;34(1):71–75. doi: 10.1590/s1516-44462012000100013. [http://dx.doi.org/10.1590/S1516-44462012000100013]. [PMID: 22392392]. [DOI] [PubMed] [Google Scholar]
- 149.Isung J., Mobarrez F., Nordström P., Åsberg M., Jokinen J. Low plasma vascular endothelial growth factor (VEGF) associated with completed suicide. World J. Biol. Psychiatry. 2012;13(6):468–473. doi: 10.3109/15622975.2011.624549. [http://dx.doi.org/10.3109/15622975.2011.624549].[PMID: 22098148]. [DOI] [PubMed] [Google Scholar]
- 150.Drexhage R.C., Hoogenboezem T.H., Versnel M.A., Berghout A., Nolen W.A., Drexhage H.A. The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav. Immun. 2011;25(6):1206–1213. doi: 10.1016/j.bbi.2011.03.013. [http://dx.doi.org/10.1016/j.bbi.2011.03.013]. [PMID: 21443944]. [DOI] [PubMed] [Google Scholar]
- 151.Altamura A.C., Mundo E., Cattaneo E., Pozzoli S., Dell’osso B., Gennarelli M., Vergani C., Trabattoni D., Arosio B., Clerici M. The MCP-1 gene (SCYA2) and mood disorders: preliminary results of a case-control association study. Neuroimmunomodulation. 2010;17(2):126–131. doi: 10.1159/000258696. [http://dx.doi.org/10.1159/000258696].[PMID: 19923858]. [DOI] [PubMed] [Google Scholar]
- 152.Pae C.U., Kim J.J., Yu H.S., Lee C.U., Lee S.J., Jun T.Y., Lee C., Paik I.H. Monocyte chemoattractant protein-1 promoter -2518 polymorphism may have an influence on clinical heterogeneity of bipolar I disorder in the Korean population. Neuropsychobiology. 2004;49(3):111–114. doi: 10.1159/000076718. [http://dx.doi.org/10.1159/000076718].[PMID: 15034225]. [DOI] [PubMed] [Google Scholar]
- 153.Roh M.S., Lee K.Y., Joo E.J., Lee N., Kim Y.S. No association of the MCP-1 promoter A-2518G polymorphism with bipolar disorder in the Korean population. Neurosci. Lett. 2007;427(1):1–5. doi: 10.1016/j.neulet.2007.04.038. [http://dx.doi.org/10.1016/j.neulet.2007.04.038].[PMID: 17928143]. [DOI] [PubMed] [Google Scholar]
- 154.Padmos R.C., Hillegers M.H., Knijff E.M., Vonk R., Bouvy A., Staal F.J., de Ridder D., Kupka R.W., Nolen W.A., Drexhage H.A. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch. Gen. Psychiatry. 2008;65(4):395–407. doi: 10.1001/archpsyc.65.4.395. [http://dx.doi.org/10.1001/archpsyc.65.4.395].[PMID: 18391128]. [DOI] [PubMed] [Google Scholar]
- 155.Young J.J., Bruno D., Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [http://dx.doi.org/10.1016/j.jad.2014.07.032]. [PMID: 25128861]. [DOI] [PubMed] [Google Scholar]
- 156.Merendino R.A., Di Pasquale G., De Luca F., Di Pasquale L., Ferlazzo E., Martino G., Palumbo M.C., Morabito F., Gangemi S. Involvement of fractalkine and macrophage inflammatory protein-1 alpha in moderate-severe depression. Mediators Inflamm. 2004;13(3):205–207. doi: 10.1080/09511920410001713484. [http://dx.doi.org/10.1080/09511920410001713484]. [PMID: 15223613]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakatani N., Hattori E., Ohnishi T., Dean B., Iwayama Y., Matsumoto I., Kato T., Osumi N., Higuchi T., Niwa S., Yoshikawa T. Genome-wide expression analysis detects eight genes with robust alterations specific to bipolar I disorder: relevance to neuronal network perturbation. Hum. Mol. Genet. 2006;15(12):1949–1962. doi: 10.1093/hmg/ddl118. [http://dx.doi.org/10.1093/hmg/ddl118].[PMID: 16687443]. [DOI] [PubMed] [Google Scholar]
- 158.Fontenelle L.F., Barbosa I.G., Luna J.V., de Sousa L.P., Abreu M.N., Teixeira A.L. A cytokine study of adult patients with obsessive-compulsive disorder. Compr. Psychiatry. 2012;53(6):797–804. doi: 10.1016/j.comppsych.2011.12.007. [http://dx.doi.org/10.1016/j.comppsych.2011.12.007].[PMID: 22300901]. [DOI] [PubMed] [Google Scholar]
- 159.Janelidze S., Ventorp F., Erhardt S., Hansson O., Minthon L., Flax J., Samuelsson M., Traskman-Bendz L., Brundin L. Altered chemokine levels in the cerebrospinal fluid and plasma of suicide attempters. Psychoneuroendocrinology. 2013;38(6):853–862. doi: 10.1016/j.psyneuen.2012.09.010. [http://dx.doi.org/10.1016/j.psyneuen.2012.09.010].[PMID: 23062672]. [DOI] [PubMed] [Google Scholar]
- 160.Barbosa I.G., Rocha N.P., Bauer M.E., de Miranda A.S., Huguet R.B., Reis H.J., Zunszain P.A., Horowitz M.A., Pariante C.M., Teixeira A.L. Chemokines in bipolar disorder: trait or state? Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(2):159–165. doi: 10.1007/s00406-012-0327-6. [http://dx.doi.org/10.1007/s00406-012-0327-6].[PMID: 22584806]. [DOI] [PubMed] [Google Scholar]
- 161.Lindqvist D., Janelidze S., Hagell P., Erhardt S., Samuelsson M., Minthon L., Hansson O., Björkqvist M., Träskman-Bendz L., Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol. Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [http://dx.doi.org/10.1016/j.biopsych.2009.01.030]. [PMID: 19268915]. [DOI] [PubMed] [Google Scholar]
- 162.Eller T., Vasar V., Shlik J., Maron E.J. Effects of bupropion augmentation on pro-inflammatory cytokines in escitalopram-resistant patients with major depressive disorder. Psycho-pharmacol. 2009;23(7):854–858. doi: 10.1177/0269881108091077. [DOI] [PubMed] [Google Scholar]
- 163.Glassman A.H., Shapiro P.A. Depression and the course of coronary artery disease. Am. J. Psychiatry. 1998;155(1):4–11. doi: 10.1176/ajp.155.1.4. [http://dx.doi.org/10.1176/ajp.155.1.4].[PMID: 9433332]. [DOI] [PubMed] [Google Scholar]
- 164.Wong M.L., Dong C., Maestre-Mesa J., Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol. Psychiatry. 2008;13(8):800–812. doi: 10.1038/mp.2008.59. [http://dx.doi.org/10.1038/ mp.2008.59].[PMID: 18504423]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.van Zuiden M., Heijnen C.J., van de Schoot R., Amarouchi K., Maas M., Vermetten E., Geuze E., Kavelaars A. Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS One. 2011;6(12):e29142. doi: 10.1371/journal.pone.0029142. [http://dx.doi. org/10.1371/journal.pone.0029142].[PMID: 22195009]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ogłodek E.A., Szota A., Just M.J., Moś D., Araszkiewicz A. Comparison of chemokines (CCL-5 and SDF-1), chemokine receptors (CCR-5 and CXCR-4) and IL-6 levels in patients with different severities of depression. Pharmacol. Rep. 2014;66(5):920–926. doi: 10.1016/j.pharep.2014.06.001. [http://dx.doi.org/10.1016/j.pharep.2014.06.001].[PMID: 25150002]. [DOI] [PubMed] [Google Scholar]