Abstract
Abstract: Background
Depression and schizophrenia are debilitating mental illnesses with significant socio-economic impact. The high degree of comorbidity between the two disorders, and shared symptoms and risk factors, suggest partly common pathogenic mechanisms. Supported by human and animal studies, maternal immune activation (MIA) has been intimately associated with the development of schizophrenia. However, the link between MIA and depression has remained less clear, in part due to the lack of appropriate animal models.
Objective
Here we aim to summarize findings obtained from studies using MIA animal models and discuss their relevance for preclinical depression research.
Methods
Results on molecular, cellular and behavioral phenotypes in MIA animal models were collected by literature search (PubMed) and evaluated for their significance for depression.
Results
Several reports on offspring depression-related behavioral alterations indicate an involvement of MIA in the development of depression later in life. Depression-related behavioral phenotypes were frequently paralleled by neurogenic and neurotrophic deficits and modulated by several genetic and environmental factors.
Conclusion
Literature evidence analyzed in this review supports a relevance of MIA as animal model for a specific early life adversity, which may prime an individual for the development of distinct psychopathologies later life. MIA animal models may present a unique tool for the identification of additional exogenous and endogenous factors, which are required for the manifestation of a specific neuropsychiatric disorder, such as depression, later in life. Hereby, novel insights into the molecular mechanisms involved in the pathophysiology of depression may be obtained, supporting the identification of alternative therapeutic strategies.
Keywords: Animal model, depression, LPS, maternal immune activation, poly (I:C), schizophrenia
1. Introduction
Mental illnesses, including highly prevalent psychiatric disorders, such as depression and schizophrenia, pose a serious global health burden with the number of affected individuals increasing steadily [1]. Co-morbidity is high between these disorders, as there is an increased risk for developing depression in schizophrenia patients and likewise, people with depressive disorders are more likely to suffer from psychosis. Furthermore, depression and schizophrenia have common genetic risk factors and display some overlapping symptoms, which suggests partly shared underlying pathogenic principles [2-5]. However, the precise molecular mechanisms causing these complex brain disorders remain incompletely understood - partially challenged by the lack of appropriate experimental systems. Animal models relevant for both, depression and schizophrenia, may hence offer a unique opportunity to tackle the scientific questions pertaining to the pathophysiology and therapeutic management of these devastating illnesses of the human mind.
Epidemiological studies provide evidence that maternal immune activation (MIA) caused by gestational infection increases the risk for developing schizophrenia in adult life [6, 7], most prominently when the infection occurs during the late first and early second trimester of pregnancy [8, 9]. Based on these findings several animal models for MIA have been generated, either by the application of live viruses or immunogenic substances, in order to enable further research aiming at identifying the pathophysiological principles involved [10-13]. Numerous studies in these animal models demonstrated a link between MIA and the development of schizophrenia- related phenotypes in the adult offspring [14]. In the case of depression, epidemiological studies report conflicting results regarding the potential role of MIA in the development of the disorder [15-18]. This discrepancy may relate to the fact that - as opposed to more than 80% of schizophrenia cases being related to heritability- the genetic contribution to depression (40% of cases associated to hereditary basis) seems to be much smaller [19, 20]. Hence, the specific genetic dotation not only determines the individual susceptibility to adverse early life events (including gestational infection) but may also influence which specific psychopathology the individual may develop. Consequently, larger population cohorts with ascertained genetic and environmental risk factors may be needed in order to comprehensively investigate the role of MIA in the etiology of depression in people [21]. Thus, precisely because the association to other psychopathological entities is much stronger, preclinical investigations into a potential link between prenatal infection and depression and an examination of its biological underpinnings are relevant for enhancing our understanding of the pathogenesis of the disorder, a prerequisite for the development of alternative therapeutic strategies.
Indeed, increasing evidence from animal experiments suggests a possible involvement of MIA in the patho- physiology of depression-related abnormalities at the behavioral [22-27], morphological [22, 25, 28-30] and pharmacological level [25], hereby challenging the prevalent view of MIA being an animal model of schizophrenia exclusively. Alternatively, MIA may induce or contribute to a diverse set of neurodevelopmental aberrations potentially involved in the neurobehavioral abnormalities which are related to a spectrum of psychiatric illnesses, rather than constituting an environmental insult relevant to the development of a single disorder [13, 31]. Based on these considerations, animal models of MIA provide an optimal tool investigating shared as well as divergent mechanisms of depression and schizophrenia. Several excellent reviews focusing on the link between schizophrenia and MIA exist [11, 14, 32]. The emphasis of this review is on the role of MIA in the pathophysiology of depression with the aim to provide an extensive overview of the available literature and to discuss open questions and future directions.
2. Animal models of depression
For the examination of the neurobiological underpinnings of highly complex mental illnesses, as is the case for schizophrenia and depression, investigations in live animals are indispensable. Until now, examinations at the neural network and behavioral level cannot be carried out in in-silico simulations or in-vitro assays, highlighting the relevance of animal model systems that allow testing hypotheses derived from epidemiological studies, which cannot be investigated in human subjects in light of obvious ethical and practical limitations. However, despite the high genetic resemblance of men and mice some mental functions, such as abstract thoughts or executive planning, are considered uniquely human. Likewise, mimicking some of the symptoms of mental disorders such as hallucinations, feelings of worthlessness and suicidal thoughts/suicide currently remain challenging in experimental animals. Considering the complexity of the pathophysiology of mental disorders, animal models fall into place as most useful when attempting to reconstruct single aspects of these illnesses, rather than attempting to represent the entire spectrum of symptoms associated with a given psychiatric condition [31]. Similarly, various animal models of depression exist, with every individual one reflecting a slice of the pie of the entire range of the symptoms attributed to the heterogeneous clinical picture related to this complex disorder.
In general, animal models of human diseases are traditionally subjected to their adherence to the classical criteria of construct, face and predictive validity [33]. Construct validity is fulfilled when the disease and the model share the same genetic or environmental risk factors. Face validity is reflected in the degree by which symptoms of the disease are also represented in the model. Predictive validity depends on the reversal of the disease-related phenotype displayed in the animal model by established phar- macological treatments for the disease.
In humans, a variety of genetic and environmental risk factors for the development of depression have been identified to date [20, 34, 35]. Hence, the generally accepted notion assigns a multifactorial origin to the pathophysiology of depression, with a number of interactions between several genetic and environmental risk factors [36]. This consideration further highlights the need for animal models with a high degree of constructive validity. One of the most relevant environmental risk factors for the development of depression is exposure to chronic stress [37]. Accordingly, several rodent models of depression exist, which are based upon the exposure to various types of stressors at different time-points during the animal’s lifespan including stress exposure during the prenatal period [38, 39].
3. Animal models of prenatal psychosocial stress
Adverse early life events, including stress experience during the gestational period, are known to modify the sensitivity to the impact of stress in adulthood and influence an individual’s susceptibility for the development of depression [40]. In humans, various forms of prenatal stress comprising nutritional [41-43], psychological [44, 45] and infectious stress [15] have been associated to the development of mental disorders, including depression, later in life.
Epidemiological studies have provided evidence for a modulatory impact of prenatal stress on the fetal environment which may contribute to an increased susceptibility for the development of pathologies later in life, a process known as “fetal programming” [46]. As such, a role for prenatal stress as risk factor for psychiatric disorders, including depression, has been proposed [46, 47]. However, a definite confirmation for a direct and causal link between prenatal stress and an enhanced vulnerability for the development of depressive disorders is still elusive. One reason may be that epidemiological studies examining the relationship between prenatal stress and depression are constrained by the definition of stress, stress measurements, limited sample sizes and the lack of appropriate controls [48]. In contrast to human studies, preclinical research has provided a wealth of information supporting a role for prenatal psychosocial stress as contributing factor for the development of brain and behavioral abnormalities related to depression [49-51].
Several studies suggest that prenatal psychosocial stress acts to modify the hypothalamic-pituitary-adrenal (HPA) axis which results in either enhanced basal secretion of glucocorticoids (GC) or in increased secretion of GCs in response to a stressor (for review [50, 51]). In addition, it was found that the expression of corticotropin-releasing hormone (CRH) in the amygdala is increased in response to prenatal psychosocial stress hereby inducing anxiety-like behavior [52, 53]. Indeed, increased anxiety-like behavior in male offspring of prenatally stressed rat and mouse mothers has been repeatedly confirmed [50, 53-56]. In female offspring contradictory results were observed in tests for anxiety, possibly relating to the well-documented behavioral variance in the course of the female estrous cycle (for review see [49]). In addition to the abnormalities in behavior and stress response, prenatal psychosocially stressed animals have been also found to display cognitive impairments which potentially may relate to the observed reduction in adult hippocampal neurogenesis and brain-derived neurotrophic factor (BDNF) levels [57]. Interestingly, decreases in adult hippocampal neurogenesis and BDNF levels have also been observed in patients suffering from depression, further supporting the relevance of prenatal psychosocial stress as an animal model of depression [58].
The majority of studies investigating the effects of prenatal stress in the form of psychosocial stressors in experimental animal models have investigated its impact on depression- and anxiety-like behavior. In contrast, the impact of prenatal immunological stress has been mostly explored in light of the development of schizophrenia- and autism-related symptoms later in life [49]. Lately however, an increasing number of reports in animal models also suggest a relevance for MIA in the pathophysiology of depression [22-25, 27].
4. Animal models of MIA
Epidemiological studies from the influenza epidemic of 1957 provided the first link between gestational infection and the development of schizophrenia in adulthood [8, 59]. Based on these findings, Fatemi et al. established an animal model of prenatal influenza infection in mice [10]. The model is based on the intranasal delivery of a sublethal dose of a neurotropic human influenza strain to the pregnant dam [60-63]. Adult offspring show a multitude of alterations at the behavioral [64], morphological [61, 65] and gene expressional level [62, 66, 67] mainly associated to aberrations relevant for schizophrenia and autism. To date, the link between exposure to various infectious agents such as influenza, rubella, herpes simplex, toxoplasma gondii, measles, polio, and genital and/or reproductive infections during the prenatal period and the development of schizophrenia is well established [68]. In the case of depression, results of epidemiological studies have provided contradictory findings (for review see [31]) regarding the pathophysiological relevance of gestational infections. This fact further highlights the importance for the establishment of adequate animal models, which may enable the exploration of a potential causal relationship between MIA and depression and its molecular underpinnings.
Infectious agents can impact the developing fetus via activation of the maternal immune response or directly at the site of the placenta, the amniotic fluid or in the fetal organism itself [10]. Hence, other animal models of MIA, based on administration of immunogenic substances rather than of entire microorganisms have been developed. These models are exclusively based on the activation of maternal immune pathways, since the applied substances cannot penetrate the placenta. While the models using live microorganisms have the advantage that they most closely ressemble the human situation, the replacement of pathogens by immunogenic substances provides several other advantages. First, no specific laboratory safety requirements have to be fulfilled when working with immunogenic substances. Second, the immune response triggered by these substances typically lasts only 24-48 hours [69, 70]. Therefore, the gestational time-point of maternal immune stimulation can be controlled, which allows the assessment of sensitivity to MIA at different periods of pregnancy. Third, the intracellular pathways and effectors mediating the immune response are known and well defined.
The two most commonly used immunogenic substances for animal models of MIA are the bacterial endotoxin Lipopolysaccharide (LPS) and the synthetic double-stranded RNA analogue polyinosinic–polycytidilic acid (Poly(I:C)). LPS induces an innate immune response, which is usually triggered after bacterial infection while Poly(I:C) mimics the acute phase immune response to a viral infection, as double stranded RNA is exclusively present in viruses. Both substances stimulate the production of pro-inflammatory cytokines such as Interleukin-1beta (IL-1β), Interleukin-6 (IL-6) and tumor necrosis factors alpha (TNF-〈) through binding to toll-like receptors (TLRs). While LPS is recognized by TLR4, Poly(I:C) exerts its pro-inflammatory effects through activation of the TLR3 pathway, ultimately leading to nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) activation (reviewed in [31]). Poly(I:C) additionally induces the production of Interferon alpha (INF-〈) and Interferon beta (INF-β).
The effects of both types of immune stimulation in the context of animal models of MIA have been examined by the investigation of offspring behavioral, cognitive and pharmacological functions. The behavioral phenotypes have been mostly studied in the context of symptoms observed in schizophrenia and autism patients, including disturbances in sensorimotor gating [64, 71], selective attention [72-74], social interaction [75] and working memory [76] as well as sensitivity to psychostimulant drugs [76, 77].
As stimulation by Poly(I:C) or LPS is eliciting a time-wise limited immune response, specific windows of fetal development can subsequently be linked to effects of MIA [29, 78]. Therefore a starting point for deciphering the underlying pathomechanisms is to determine distinct gestational periods, which may represent a period of vulnerability of the developing fetus to MIA-induced brain and behavior alterations [13, 31]. Along these lines, mouse studies using Poly(I:C)-assisted MIA suggest a period between gestational day (GD) 9 and 12 as critical time frame for effects of maternal infection on depression-like behavior. MIA at both time points of pregnancy leads to behavioral phenotypes in the adult mouse offspring which are thought to reflect some of the deficits observed in depressed patients [13, 29, 31, 70, 74, 78]. The behavioral alterations, including augmented anhedonic states and an increase in behavioral despair, were also paralleled by neurogenic and neurotrophic deficits [22, 31]. Both neurogenic and neurotrophic deficits have been intimately linked to depression considering the reductions in hippocampal volumes in post-mortem brains of depressed patients and several findings in animal models linking adult neurogenesis and alterations in neurotrophic support to depression-like behavior (see for review [79]). Later gestational time points were shown to be relevant for other behavioral alterations, as offspring after Poly(I:C)-induced MIA at GD 16/17 displayed phenotypes related to epilepsy and cognitive decline [80, 81].
Collectively, there seem to be certain windows of susceptibility for the long-term effects of MIA on neuronal abnormalities in rodents resulting in functional disturbances related to the presentation of mental disorders, including depression. These gestational time-points in rodents correlate to the middle/ end of the first trimester in human pregnancy [10, 31, 82, 83]. Interestingly, recent epidemiological studies showed a correlation between maternal infection and an increased risk for the development of schizophrenia specifically when the infection occurred at the end of the first trimester [9, 68, 84, 85]. This vulnerability at a certain gestational period may depend on both maternal and fetal factors: First, the maternal immune response is changing during pregnancy with a gradual decrease of pro-inflammatory immune responses over time [86, 87]. Second, a specific set of maternal and fetal cytokines are activated at distinct time-points during gestation which may differentially impact the fetal brain [29], with the fetal immune system only reaching its final development during late gestation/ early postnatal period [31, 88]. Lastly, the impact and long-term consequences of adverse environments impinging on the fetal nervous system depends on the particular step of maturation during which the developing brain is impacted.
As described above, the relative paucity of epidemiological studies examining a possible link between MIA and depression rendered somewhat conflicting results [31]. Hence, on clinical grounds no definite causal relationship has been postulated so far, in part also due to the methodological limitations inherent to epidemiological research. It is here where animal models of MIA come into play allowing for a more direct approach to cause and effect in a controlled preclinical setting. Indeed, a series of reports on experiments in animal models provide evidence for an involvement of MIA in the development of distinct deficits related to depressive-disorders at the behavioral [22, 24-27, 63], cellular [22, 28] and molecular [12, 22, 25, 27] level.
5. From MIA to alterations in brain functions: Behavioral, cellular and molecular correlates in animal models
Results from animal models have shown that phenotypes related to various mental disorders can be induced by MIA, affecting shared as well as unique neurobiological mechanisms involved in distinct psychopathologies, including schizophrenia and depression. Due to the high degree of complexity of the various factors involved and interacting in the pathophysiology of these mental illnesses, the precise mechanisms which MIA is affecting remain elusive. Enhancing our understanding of how and by which means maternal infection during pregnancy alters the pre- and perinatal environment and induces long-lasting changes may provide novel insight into the pathophysiology of mental disorders. This knowledge may ultimately lead to the development of new therapeutic strategies [31]. Specifically, as MIA is a manipulation taking place during the prenatal phase no drug or other interactions with pharmacological compounds eventually tested in the adult animal are to be expected. Along these lines, MIA animal models constitute a suitable tool for the investigation and testing of preventive treatment regimens and compounds, which may inhibit the development of behavioral deficits in offspring after exposure to gestational infections. Albeit still controversial for depression, the role of preventive interventions in schizophrenia, which may halt the emergence of associated phenotypes is currently being intensively debated [89-91]. Additionally, investigating the molecular players involved in the observed depression-related alterations following MIA may lead to identification of novel targets for pharmacological compounds, allowing for the possibility of new symptomatic treatment approaches.
5.1. Cytokines as Modulators of the effects of MIA
Proinflamamtory cytokines have been tightly associated with the pathophysiology of depression. A series of preclinical and clinical studies have thus formed the basis for the “cytokine hypothesis of depression”. As such, increased levels of peripheral pro-inflammatory cytokines have been reported in depressed patients and proposed to influence pathophysiological processes implicated in depression such as neurotransmitter metabolism, neuroendocrine interactions and synaptic plasticity (reviewed in: [92]). This reflection of heightened immune activation may also result from chronic stress exposure, a precipitating factor for the development of depression, which has been shown to lead to activation of pro-inflammatory pathways via Nf-KB signaling [93]. Additionally, the systemic application of cytokines has been shown to induce symptoms of anxiety and depressed mood in somatically-ill patients [94]. These observations in people have been paralleled by similar findings in experimental animals [95].
Augmentation of a distinct set of cytokines in the maternal system resulting from MIA has been repeatedly demonstrated. The specific profile of pro- and anti-inflammatory factors depend on the particular immune activation protocol used [70, 96]. Among those cytokines prominently involved, are TNF-α, IL1-β and IL-6 [22, 97, 98] which are also highly implicated in the pathophysiology of depression [99]. While more specific literature on fetomaternal immune interactions is currently emerging [100] there is still no clear answer to the question if and which maternal cytokines are able to cross the placenta at certain gestational stages and under various (patho-) physiological conditions [31, 86]. However, evidence for the elicitation of inflammatory processes by MIA in the fetal system, including the brain, has been provided [13, 29, 69, 76, 101, 102]. The mechanisms of cytokine augmentation in the fetal compartment, being direct transport via the placenta or passively [103-107] are still incompletely understood and seem to be cytokine-specific [13, 104, 108].
Several studies in animal models have been carried out in order to elucidate a potential causal involvement of individual cytokines in the mechanisms underlying the association between MIA and depression later in life. As a result of these experiments, specific roles for IL-1 [109], IL-10 [76] and IL-6 [107] in the pathomechanisms linking MIA with the neurobiological and behavioral aberrations related to depression have been proposed. With its ability to cross the placental barrier, IL-6 [104] is likely to function as a key mediator linking maternal infection to offspring behavioral deficits [13, 23, 107, 110]. Indeed, it was found that administration of IL-6 on GD 12.5 generates behavioral disturbances phenocopying those observed after LPS or Poly(I:C)-induced MIA. By contrast, elimination of IL-6 by genetic or immunologic strategies prevented the effects of prenatally administered Poly(I:C) [107]. Interestingly, the effect seems to be specific for IL-6 since administration of other pro-inflammatory cytokines during pregnancy or deletion of those cytokines did not yield comparable results. Hereby, a central position of IL-6 in the molecular underpinnings linking MIA and mental illness is suggested. These data are further supported by other preclinical analyses which have linked prenatal IL-6 exposure to morphological and structural changes in the hippocampus [111] and suggested an involvement of IL-6 in the regulation of hippocampal neurogenesis [112, 113] and BDNF expression [107, 114]. Additionally, several clinical studies have demonstrated altered levels of IL-6 in patients suffering from depression [115]. While the precise mechanisms and signaling pathways mediating the involvement of IL-6 in MIA-associated development of depression remain to be comprehensively elucidated, evidence for a role of the STAT3-signaling pathway linking IL-6 and modulation of the serotonergic system through SERT has recently been provided [116].
However, individual cytokine responses as well as differences in the dam’s alterations in weight resulting from the immunogenic stimulation [117] and strain-dependent susceptibility [23] have to be considered as modulating factors influencing the phenotype of adult MIA offspring.
5.2. Behavioral Phenotypes Related to Depression Induced by MIA
In a variety of studies enhanced depression- and anxiety-like behavior has been reported in offspring after MIA. The specific results of studies analyzing behavioral phenotypes in animal models of MIA highly depend on the type and dose of the immunogenic substance used, the gestational time-point and the individual response of the mother to the immunological stimulation [29, 117] (see Table 1).
Table 1.
Summary of studies describing alterations in depression- and anxiety-like behavior in MIA offspring.
| Immunogen | Dose | Species | Sex | Gestational Day (GD) | Postnatal Age | Behavioral Test | Results | References |
|---|---|---|---|---|---|---|---|---|
| Poly(I:C) | 5 mg/kg | mice | nd | GD9 | 14-16 weeks |
OF | increased anxiety-like behavior | Meyer et al. [29] |
| GD17 | no change | |||||||
| Poly(I:C) | 5 mg/kg | mice | nd | GD12-GD17 | juvenile | OF | no change | Ozawa et al. [80] |
| adult | reduced anxiety-like behavior | |||||||
| Poly(I:C) | 4 mg/kg | rats | male | GD15 | PD 83-97 | SPT | increased depression-like behavior (only in mothers weight loss) |
Missault et al. [117] |
| Poly(I:C) | 20mg/kg | mice | nd | GD12.5 | 6-8 weeks | OF | increased anxiety-like behavior | Hsiao et al. [118] |
| Poly(I:C) | 20mg/kg | mice | male | GD12.5 | 8 weeks | SPT, FST | increased depression-like behavior | Khan et al. [22] |
| Poly(I:C) | 20mg/kg | mice | both | GD12 | PD 87-89 | LD box, EPM, FST and TST |
increased anxiety-like (females) and depression-like behavior (males) | Majidi-Zolbanin et al. [24] |
| Poly(I:C) | 5 mg/kg | mice | male | GD15 | 3 months | TST | increased depression-like behavior | Zhang et al. [26] |
| LPS | 25 ug/kg | mice | male | GD9 | 8-10 weeks | EPM, OF, LD box, FST, TST |
increased anxiety-like and depression-like behavior |
Depino et al. [27] |
| LPS | 66 ug/kg | rats | both | GD10.5 | PD 90 | FST, SPT | increased depression-like behavior | Lin & Wang et al. [25] |
| LPS | 0.12 ug/g | mice | both | GD17 | 8 months | EPM | increased anxiety-like behavior | Hava et al. [119] |
| LPS | 500 ug/kg | mice | male | GD17 | PD 80/90 | EPM, OF, EZM, LD box, TST, FST |
increased anxiety-like behavior and increased depression-like behavior | Babri et al. [23] |
nd – not defined; OF - open field, EPM - elevated plus maze, EZM - elevated zero maze, LD box- Light dark box, TST - tail suspension test, FST - forced swim test, SPT - sucrose preference test
A study by Meyer et al. [29] reports that mice exposed to Poly(I:C) prenatally on GD 9 but not on GD 17 show increased anxiety-like behavior in adulthood. In contrast, others describe a reduction in anxiety-like behavior in adult but not in juvenile offspring after Poly(I:C)-induced MIA [80]. After MIA induced by LPS on GD 17, increased offspring anxiety-like behavior has been reported [119]. Hence, it would be plausible to assume that the type and dose of immunogenic substance may determine the emotional phenotype of the adult offspring. Other studies have revealed that the offspring emotional behaviors, including anxiety-like and depression-like displays, relate to alterations in the stress response system, both in the immune stimulated pregnant females and in the adult offspring [23]. This observation is interesting and relevant for MIA as animal model of depression as it parallels descriptions in animal models based upon prenatal psychological stress [50, 51]. Moreover, these results further support the face validity of the MIA models considering the human situation where similar reports characterizing deficient HPA axis responsiveness in patients suffering from mood disorders are available [120]. Indeed, a model of the neural circuitry (Fig. 1) potentially underlying MIA-induced depression-like behavior in adult offspring comprises dysfunctional HPA axis activity as central element modulating altered stress sensitivity in MIA offspring, mirroring similar observations in other animal models of depression and human patients.
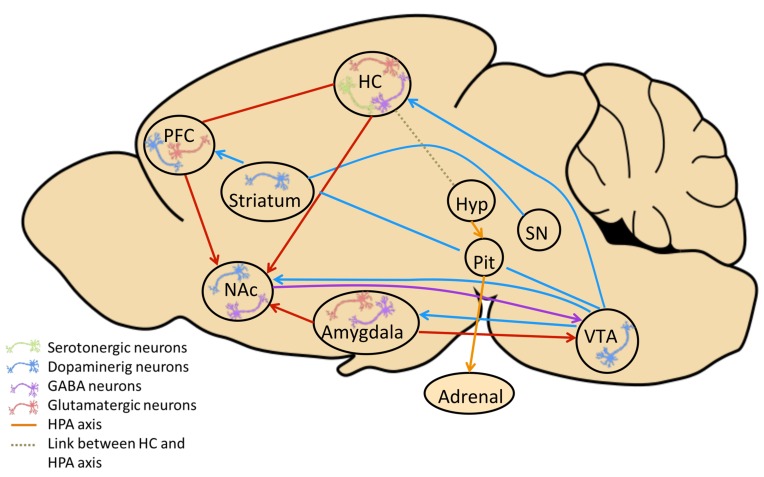
Fig. (1).
Model of the neural circuitry involved in depression-like behavior in adult MIA offspring. Depicted are dysfunctional brain structures and neural circuitries in adult MIA offspring, which relate to alterations described in depression. Aberrations in the HPA axis (orange connection) (from hypothalamus (Hyp) to pituitary gland (Pit) to adrenal gland (Adrenal)), have been reported after MIA [50, 51, 120] and in depressed patients and animal models of depression [23]. HPA abnormalities alter stress sensitivity and compromise the structural, molecular and functional integrity of brain regions with high density of glucocorticoid receptors, including the hippocampus (HC) (dotted line) as reported in MIA models [22, 28, 29], other animal models of depression and in depressed patients [192]. Altered HC - prefrontal cortex (PFC) connectivity has been shown in Poly(I:C) offspring [32, 193] paralleling deficient HC– PFC information transmission suggested to model dysfunctional anterior cingulate/orbitofrontal cortices reported in human depression [194]. Poly(I:C) induced MIA is documented to reduce the function of striatum and SN (substantia nigra) (blue connection) [195] and altered frontostriatal functional connectivity has been described in depressed patients [196]. Additionally, the mesolimbic dopaminergic pathway connecting the ventral tegmental area (VTA) and nucleus accumbens (Nac), further projecting to the amygdala, is modified in Poly (I:C) MIA offspring [72] and has been involved in the pathophysiology of depression (see for review: [197]). Further MIA-related aberrations of dopaminergic (blue neurons), serotonergic (green neurons) and GABAergic (purple neurons) neurotransmission are reported in offspring of Poly(I:C) MIA animal models in NAc, HC and PFC [11, 148-150, 198, 199].
In order to unravel the complex relationship between MIA and the emergence of depression-like behavior in later life, a study by Khan et al. [22] reports that Poly(I:C) administration on GD 12.5 induces behavioral despair, anhedonia and cognitive deficits in male adult offspring. Similar results are provided by Majidi et al. demonstrating increased depression-like behavior in male offspring following MIA at GD 12 [24]. Based upon the results described in several studies [22, 24, 75], GD 12 can be considered as particularly sensitive gestational time-point for effects of MIA on behavioral disturbances related to depression. However, increased depression-like behavior in adult offspring following MIA was also reported in mice after Poly(I:C) exposure on GD 15 [26]. Likewise, also prenatal LPS administration has been found to induce heightened depression-like behavior in adult mice [27] on GD 9.5 and rat [25] offspring on GD 10.5 which was reversible by chronic treatment with the antidepressant fluoxetine.
Interestingly, although in the human population the percentage of women among all depressed patients is much higher, with females having twice the lifetime rate of developing depression [121], few studies in MIA animal models have investigated potential sex differences in the effect of MIA on the offspring behavioral performance. Most of the few existing studies report no influence of sex on the resulting phenotypes following MIA with some exceptions (reviewed in: [10]). In a study by Majidi-Zolbanin et al. MIA induced by Poly(I:C) on GD 12 increased depression- and anxiety-like behavior in male offspring exclusively [24]. In contrast, in another study employing LPS on GD 10.5, MIA in rats caused increased depression-like behavior in male and female offspring [25]. Similarly, LPS exposure on GD 17 resulted in increased anxiety-like behavior in adult offspring with no significant effect of sex. Taken together, further differences are needed in order to conclusively determine a potential impact of the offspring sex on depression-like behavior and related structural, functional and molecular neuronal aberrations induced by MIA.
5.3. MIA-induced Cellular Abnormalities Related to Depression in the Adult Offspring Brain
The association of impaired hippocampal functions and depression originally stems from early post-mortem studies in depressed patients, where reduced hippocampal volumes were observed (see for review: [122]). Since then numerous studies in animal models have investigated the relationship between hippocampus, depression-like behavior and antidepressant treatment. Specifically adult neurogenesis in the hippocampal dentate gyrus has received immense attention in the field of depression research, as it is required for the therapeutic effects of antidepressants [123] and reversely pharmacological and non-pharmacological treatment induces progenitor cell proliferation and survival of newborn cells in the hippocampus [124].
In animal models of MIA, several studies have reported alterations in adult hippocampal neurogenesis. Poly(I:C)-induced MIA on GD 9 and GD 17 has been found to lead to a reduction in hippocampal neurogenesis on postnatal day (PD) 24 [29]. Similarly, Poly(I:C)-induced MIA at GD 12.5 was reported to not only result in a decrease in the number of proliferating cells but also to reduce the number of surviving newborn neurons and compromise neuronal cell maturation in the adult hippocampal dentate gyrus [22]. Additional evidence for an impairment of adult neurogenesis by MIA was provided by a study where Poly(I:C) at GD 17 reduced the number of newborn neurons in the hippocampal dentate gyrus of adult offspring [28]. A study by Piontkewitz reports reduced neurogenesis in the hippocampal dentate gyrus following MIA induced by Poly(I:C) at GD 15 in adolescent and young adult mice but not during later stages of adulthood [125].
Contradictory findings were reported for LPS-induced MIA; LPS administration every other day from GD 14-20 in rats decreased proliferating cells and newborn neurons in the adult hippocampal dentate gyrus [30]. LPS treatment on GD 10.5 decreased the number of newborn neurons in the rat adult hippocampus [25]. However, another study reports that LPS administration on GD 9 was not sufficient to affect adult hippocampal neurogenesis in mice [27]. Accordingly in rats, LPS exposure on GD 15/16 and GD 18/19 had no influence on hippocampal cell proliferation in adult offspring [126].
The majority of studies investigating offspring adult hippocampal neurogenesis after Poly(I:C)- assisted MIA reports decreases in the number of newborn neurons [22, 28, 29, 125]. Additionally a reduction in the survival of newborn neurons has been observed [22]. This observation parallels findings in several stress-based animal models of depression where decreased hippocampal neurogenesis has been reported [79]. These results have led to the assumption that reduced neurogenesis and structural changes may be linked to the hippocampal volumetric decreases and relate to the behavioral disturbances observed in depressed patients [127]. Since studies investigating the role of LPS-induced MIA in adult neurogenesis report contradictory findings, further examinations are needed in order to fully elucidate the relationship between MIA, adult neurogenesis and depression.
5.4. Molecular Alterations in the Adult Offspring Brain Following MIA
In search of the molecular mechanisms potentially mediating the neural deficits underlying the behavioral abnormalities in MIA offspring, alterations in neurotrophic support have been a focus of several investigations. Adult hippocampal neurogenesis is known to be modulated by growth factors, which themselves have been implicated in the pathophysiology of depression [128]. BDNF is one of the neurotrophic factors most tightly linked to both, neurogenic disturbances and depression in human patients and in respective animal models [129, 130]. Based on this observation it is plausible to assume a potential involvement of BDNF in MIA-induced depression-like behavior. This hypothesis is further supported by the fact that pro-inflammatory cytokines and the cytokine activated transcription factor NF-κB - which has been proposed to act as a mediator between cytokine action and neurogenesis in depression [131] - inhibit neurogenesis [132] and BDNF expression. Indeed, maternal immune stimulation by LPS early in pregnancy (GD 9-10) results in a long-lasting reduction of BDNF expression [25, 133]. However, when LPS is applied at GD 17 the expression of BDNF in the offspring brain is reduced on PD 21 but not in adulthood [134]. The critical role of immunogen type and timing in MIA is further highlighted by a study using Poly(I:C) induced MIA on GD 14 which reports upregulation of BDNF and its receptor tropomyosin receptor kinase B (TrkB) in various regions of the offspring brain at different postnatal ages [135]. Interestingly, the vascular endothelial growth factor (VEGF), another trophic molecule implicated in the pharmacological action of antidepressant drugs and related to adult hippocampal neurogenesis [136-141] has also been found to be differentially regulated after MIA. A decrease in hippocampal VEGFA and its receptor VEGFR2 expression was observed in adult male offspring after Poly(I:C) exposure on GD 12.5 [22]. While collectively these observations propose altered neurotrophic support in MIA-induced depression-like behavior and dysfunctional neurogenesis, the precise mechanisms and signaling cascades involved remain to be fully elucidated.
Another protein known to modulate neurogenesis - among other neurobiological processes - is the glycoprotein Reelin [142]. Reelin has received attention in several MIA studies as its expression is reduced in patients suffering from schizophrenia and bipolar disorder [143] and altered by antipsychotic, antidepressant and mood stabilizing phar- macotherapy [144]. Indeed, downregulated Reelin expression was described in offspring after prenatal LPS exposure on GD 15/16 during fetal development [145] and in the hippocampus of adult mice following LPS administration on GD 9 [27]. Furthermore, a decrease of Reelin positive cells in adult offspring was reported for Poly(I:C)-induced MIA on GD 9 in the hippocampus and on GD 9 and 17 in the prefrontal cortex [29, 76].
Aiming to characterize dysfunctional neurotransmission possibly involved in the psychopathological disturbances related to MIA, many excellent reviews highlight findings of abnormalities in the dopaminergic and the glutamatergic system, neurotransmitter systems prominently related to schizophrenia [11, 110, 146], as MIA was first considered as an animal model of schizophrenia exclusively. Regarding the serotonergic system, whose dysfunction is most commonly associated with depressive disorders [147], accumulating evidence for a modulation by MIA using different immuno- genic stimulations is becoming available. As such, it was found that MIA induced by LPS reduces serotonin (5-HT) and noradrenalin levels in the adult hippocampus and shifts the 5-HIAA/5-HT ratio towards the 5-HT metabolite 5-HIAA in the cerebellum [27]. In the dorsal raphe of adult offspring a decrease of 5-HT neurons and a reduction of 5-HT in several brain regions was found following prenatal LPS administration [148]. Moreover, prenatal administration of Poly(I:C) decreases 5-HT and its metabolite in the hippocampus, nucleus accumbens and the lateral globus pallidus [149] as does prenatal exposure to influenza in the adult cerebellum [67, 150].
As seen from the variety of individual neuromolecular and functional alterations described in adult MIA offspring, information on the various signaling cascades involved and their modulation by the precise timing of MIA together with the type and dosage of immunogen is still sketchy. Further investigations into the interaction of the different key players at the molecular, morphological and functional level will be needed to shed light onto the neural underpinnings of the multifaceted behavioral abnormalities induced by MIA.
6. Investigating the relevance of MIA for the pathophysiology of depression: pathomechanistic principles and modulatory influences
6.1. Genetic Foundations
Studies using various inbred and outbred rodent strains may allow for the determination of the impact of different genetic backgrounds on the susceptibility to the effects of MIA. Interstrain variability in the levels of baseline anxiety- and depression-like behavior as well as stress sensitivity is well documented [151-153]. Hence, it is likely that also the effects of MIA, which seem to involve alterations in the body´s stress response system [23] may depend on the specific genetic background of the mouse strain used. Indeed, LPS on GD 17 increased corticosterone levels and anxiety-like behavior exclusively in pregnant mice of the NMRI strain, but not in C57Bl/6 dams. Interestingly, only adult offspring from NMRI immune stimulated mothers showed increased anxiety- and depression-like behavior. In contrast, C57Bl/6 adult offspring whose mothers did not exhibit any differences in corticosterone levels and anxiety-like behavior following gestational LPS administration also did not show any significant differences in anxiety- and depression-like behavior, demonstrating that the strain-dependent reaction of the pregnant dam to the immune stimulation critically determines the offspring behavioral phenotype [23]. Strain-treatment effects were reported for autism relevant phenotypes when using the C57BL/6J and BTBR T+tf/J mouse strains following Poly (I:C)-induced MIA. In the same study, dysregulated cytokine responses in adult offspring of Poly(I:C) exposed mothers were exclusively reported for BTBR mice, which are known for their genetic susceptibility to the development of autism- like phenotypes [75]. These data point towards a critical involvement of the genetic background for the long-term consequences of MIA. Thus, it is suggested that the individual’s genetic dotation on which MIA is impinging, together with other environmental variables might determine the specific effects of MIA on offspring phenotype and its relevance for a given psychopathology.
6.2. Gene x Environment (G x E) Interactions
As indicated above, complex mental illnesses - like depression - are likely to be originating from an intricate interaction between various genetic determinants and a set of environmental stimuli. The specific components of this multifaceted “nature x nurture” interaction may also explain the high degree of variability of the symptomatology presented by different patients [154-157]. These observations invite proposing that rather than a distinct disorder, depression may in fact comprise a series of psychopathological entities with common etiological foundations potentially based upon a shared set of interacting genetic and environmental variables (G x E interactions). In order to gain greater insight into the role of G x E interactions in depression and other mental disorders, researchers have developed and investigated animal models representing individual identified risk factors. Among those, maternal infection during pregnancy has been postulated to be associated with a higher susceptibility for the development of depression later in life [15, 18]. It remains to be determined if also for depression, as is the case for schizophrenia, and suggested by the animal studies described above, MIA may only exert an effect in genetic predisposed subjects. This case would also explain why only a modest impact of early immune challenges per se has been revealed by epidemiological studies [13, 158, 159] suggesting that MIA could act as a priming factor, increasing offspring vulnerability to other adverse conditions within their lifetime. Within the framework of this “two” or “multiple hit” model certain genetic and environmental risk factors including maternal, psychological and immunological factors have been identified to contribute to some extent to an enhanced likelihood of suffering from depression later in life [25, 36, 160-162].
Exploring the role of MIA as single environmental risk factor, an increasing number of studies is using genetically modified mice to investigate the effects of the interaction of specific genetic predispositions and early life stress caused by infection [107, 163-166]. One of the genes explored in this context is encoding for the scaffolding protein disrupted in schizophrenia 1 (DISC1), mutations of which have been shown to be associated with a higher occurrence of psychiatric disorders including schizophrenia, depression and bipolar disorder [2]. Interestingly, when MIA was induced by Poly(I:C) treatment on GD 9 behavioral abnormalities related to schizophrenia and depression were observed and found to associate with modulation of the HPA axis/stress response, 5HT-signaling and neuroanatomical and transcriptional alterations only in DISC1 knockout mice. These results provide first experimental evidence for G x E interactions in the pathomechanisms associating MIA with depression as neither the genetic deletion of DISC1 nor the solely prenatal exposure to Poly(I:C) led to a depression-like phenotype in the adult offspring which was only observed when the environmental adversity of MIA acted on the DISC1-based genetic predisposition [163]. Furthermore, MIA causes reduced prepulse inhhibition (PPI) and impaired cognitive and social behavior in wildtype mice. However, in mice carrying a DISC1 mutation these effects were enhanced compared to wildtype mice [164]. Similarly, in Nurr1 knockout mice, a transcription factor essential for normal dopaminergic development, the genetic predisposition and MIA caused synergistic effects on locomotor hyperactivity, PPI deficits and on impaired attentional shifting and sustained attention. Furthermore, MIA in addition to the genetic manipulation caused altered development of prefrontal and ventral striatal dopamine systems [165].
In contrast, genetically induced IL-6 deficiency abolished the detrimental effects of MIA on PPI, social interaction and exploration of novel objects [107]. Interestingly, IL-6 knockout mice display reduced depression-like behavior in assays for behavioral despair and anhedonia [167] pointing once more to a key role of IL-6 in mediating the effects of MIA and in playing a crucial role in the pathophysiology of depression. Additionally, a study investigating a genetically modified mouse overexpressing the anti-inflammatory cytokine IL-10 demonstrated that in these mice MIA has no effect on PPI, attentional learning, exploration of novel environments and to the behavioral response to acute NMDA receptor blockade [166].
Current research approaches also examine the interactions between multiple adverse conditions for the development of depression-like behavior in the context of MIA, by combining MIA with other identified environmental risk factors, such as chronic stress exposure later in life. Using this strategy, it was found that in MIA offspring the experience of peripubertal stress synergistically induced behavioral and hippocampal neuronal deficiencies associated with psychiatric disorders in humans [168]. Based on these observations, MIA can be considered a priming condition, sensitizing an individual to the effects of additional adversities impinging on the organism later in life, hereby contributing to the development of psychopathologies. As the current state of knowledge indicates that MIA may not be considered as risk factor specific for a certain mental illness [12] it is plausible to assume that the interaction of MIA with additional secondary determinants such as other environmental conditions or genetic factors may be responsible for the later development of a particular disease-related phenotype.
6.3. The role of Maternal Care Behavior in the effects of MIA on Depression
In addition to disturbances caused by the direct response of the maternal and fetal system to the immunogen, indirectly induced modulations of the perinatal environment, may contribute to the behavioral and neural alterations observed in MIA offspring. Alterations in maternal care behavior of dams experiencing immune stimulation during pregnancy could constitute such a secondary effect of MIA, potentially relating to the psychopathological phenotype displayed in affected offspring. Indeed, maternal care behavior has been shown to be sensitive to adverse environmental conditions during pregnancy [169] and dysfunctional maternal care behavior is tightly linked to the development of mental illness later in life [170]. Here the effect of deficits in maternal care behavior has been suggested to be mediated through a modulatory impact on HPA axis function and its relevance for the regulation of emotional, cognitive and neuroendocrine responses to stress [171]. Interestingly, the level of maternal care behavior - mainly pup licking and grooming, the main source of palpable stimulation for the newborn [172] – in interaction with the animal´s genetic background, have been found to modulate adult hippocampal neurogenesis [173], which is tightly associated to depression-like behavior and antidepressant treatment response, as described above. Additionally, licking and grooming is known to affect offspring stress sensitivity through alterations in the HPA axis [174], which has also been found in MIA offspring [23].
Several studies have begun to explore the effects of MIA on maternal care behavior in order to determine the relevance of this indirect effect of MIA on offspring brain function and behavior. As such, gestational Poly(I:C) has been found to disturb maternal behavior, especially licking and grooming and caused higher nest-building behavior, with offspring displaying higher sensitivity to acquiring learned fear responses. Interestingly, this behavioral phenotype was also observed in pups adopted by Poly(I:C) exposed mothers and may therefore relate to the deficits in the maternal care rather than to the direct effect of the gestational immune stimulation on the developing fetus [175]. Similarly, another study also showed that adoption of control pups by Poly(I:C) exposed mothers was sufficient to induce behavioral alterations related to schizophrenia in the adult offspring [176]. However, adoption of pups from Poly(I:C) exposed mothers by control dams did not prevent the behavioral pathophenotype suggesting that although the postnatal environment cannot reverse MIA related alterations, the solely disruption of maternal care due to MIA itself may induce behavioral disturbance related to human psycho- pathologies in the offspring.
Looking for a biological mechanism, potentially mediating the effect of maternal care behavior on the behavioral outcome later in life, epigenetic processes are a likely candidate due to their long-lasting nature and dynamic modulation by environmental factors [177]. Indeed, the regulation of epigenetic processes – alterations in gene expression without effects on the DNA sequence itself - by maternal care behavior and its persistent impact on gene expression and related behavioral outcomes has been repeatedly demonstrated [178]. Searching for the molecular correlates of this effect, the glucocorticoid receptor gene - a key player of the endogenous stress response system [179] - represents a likely candidate since it has been found to be prone to epigenetic modifications, also in the context of maternal care behavior [180]. MIA-induced dysfunctions of the HPA axis, directly or indirectly through maternal care behavior - possibly mediated through epigenetic processes – could also constitute a link to depression in humans where a derangement of the stress response system is assumed to be of pathophysiological relevance [181].
6.4. The role of Epigenetics in the Impact of MIA on the Development of Depression
Seeking to unravel the molecular events possibly underlying the persistent nature of the impact of MIA on adult offspring brain structure, function and behavior, epigenetic processes can be considered a prime candidate mechanism, as indicated above. Indeed, alterations of the epigenetic profile, the so called epigenome, manifesting in modulations of DNA methylation patterns and histone modifications have been implicated in the pathophysiology of depression [182]. IL-6, which is thought to mediate some of the effects of MIA has been reported to promote global as well as gene specific epigenetic modifications [183-187].
The impact of MIA on the epigenome has only begun to be explored recently. Basil and colleagues have found that Poly(I:C) exposure on GD 9 induces significant changes in global DNA methylation and in methylation at the promoter of methyl-CpG-binding protein 2 (MeCP2), a protein implicated in neurodevelopmental disorders [188] in the offspring hypothalamus [189]. In contrast, no changes in histone methylation in the cortex of adult mice after Poly(I:C) exposure on GD 12.5 and GD 17.5 were observed [190], highlighting the critical relevance of the gestational time point of the immunogenic insult for the molecular, cellular and behavioral outcome in MIA offspring. Focusing on histone modifications, another study showed that Poly(I:C) administration on GD 9 induces histone hypomethylation in the cortex of juvenile but not adult offspring, which however presented with increased anxiety-like behavior. The results support the hypothesis that MIA may result in epigenetic modifications causing long-lasting changes in gene expression contributing to neurodevelopmental alterations in life-long modulations of neural function, ultimately leading to the observed behavioral abnormalities in adult life [191]. Collectively, these first studies indicate an involvement of epigenetic regulations in the impact of MIA on offspring brain and behavior. However, further investigations are needed in order to decipher the precise nature of these epigenetic processes and to demonstrate their causal involvement in MIA-induced pathophysiological mechanisms related to depression.
7. Conclusions and perspectives
Current evidence supports the notion that MIA acts as an early life adverse condition, which alters neurodevelopment and induces life-long modification in neural function in the offspring. Based on the concept of the “multiple-hit hypothesis” which proposes multi-level gene x environment interactions at the core of the pathomechanisms of complex mental illnesses, such as depression, MIA can be viewed as one of the contributing environmental factors (Fig. 2). The wide range of neural and behavioral abnormalities observed in animal models of gestational infection further highlight the relevance of the type and intensity of the immunogenic insult and the time-point in pregnancy as critical determinants for the specific phenotype observed in MIA offspring. Consequently, MIA might not constitute a risk factor for a specific disorder [12, 13] but rather present an environmental challenge determining the individuals susceptibility for the development of psychopathologies later in life. The precise nature of the resulting psychiatric condition may then depend on the specific genetic profile and other environmental factors impacting in the course of an organism´s lifetime and explain the discrepancy observed in epidemiological studies investigating the relevance of gestational infection for the development of depression. Hence, in order to study the specific relevance of MIA for the pathophysiology of depression, the use of animal models, which allow for controlled experimental conditions of type and dose of immunogenic stimulation, gestational timing and environmental settings, appears as highly relevant tool.
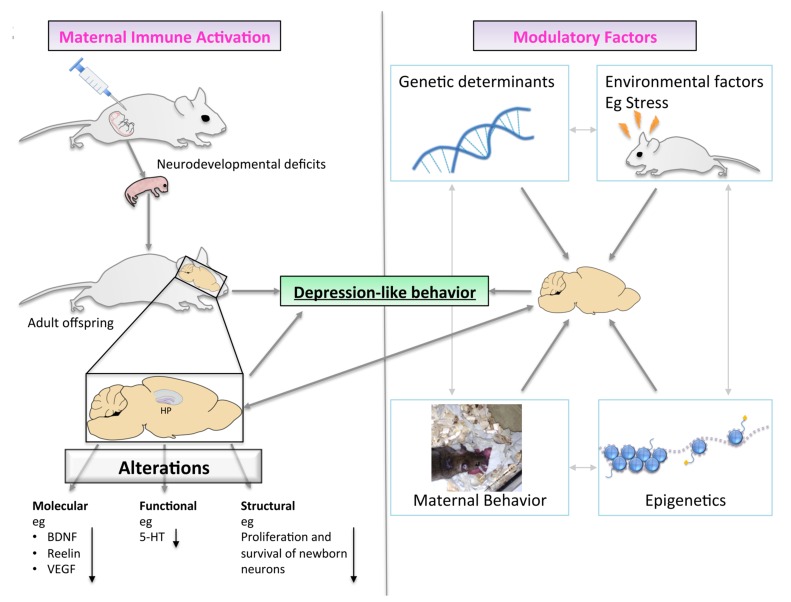
Fig. (2).
A proposed role for MIA in the development of offspring depression-like behavior. Adult MIA offspring present with neural deficits at the molecular, structural and functional level, which are associated with depression-like behavior. The underlying pathophysiological mechanisms involve a complex interplay between the neurodevelopmental deficits induced by MIA and modulatory factors including genetic and environmental variables.
Considering the role of MIA as model for depression research, some of the classical criteria describing the validity of animal models are being fulfilled. More and larger epidemiological studies are being needed in order to convincingly demonstrate a link between gestational infection and the development of depression later in life. Thus, it is yet too early to ascertain definite construct validity to MIA as animal model of depression. The display of anhedonia, a cardinal feature of depression in human patients, observed in MIA offspring [22] provides strong support for relevant face validity of the MIA model, albeit further studies investigating strain and sex-dependency are needed in order to confirm and extend these seminal findings. Regarding the aspect of predictive validity, it was reported that chronic antidepressant treatment ameliorates the observed phenotype in depression-like behavior following LPS-induced MIA in rats [25]. However, the reversibility of depression-related behavioral alterations in MIA offspring by various classes of antidepressant drugs is still pending.
MIA animal models may represent a suitable preclinical setting enabling scientists to examine the specific genetic and environmental risk factors, setting the course for the development of depression in the context of an early immunogenic insult. Moreover, the precise nature of the cellular and molecular events involved, including isolation of specific molecular key elements that may be amenable to pharmacological modulation, may be identified in future studies using MIA animal models. Hereby identified candidate mechanisms and molecules may then be examined in the human population in a translational approach ultimately contributing to an enhanced understanding of the pathophysiology of depression and development of novel therapeutic approaches.
ACKNOWLEDGEMENTS
MR collected data and wrote the manuscript. SB and BM conceived and contributed art work and illustrations. AB participated in the design and writing of the article. DDP wrote and edited the manuscript.
Daniela D. Pollak is supported by the Austrian Science Fund (FWF; P 27520). Marianne Ronovsky received a fellowship by “Verein zur Foerderung der Forschung auf dem Gebiet der Neonatologie und paediatrischen Intensivmedizin”.
LIST OF ABBREVIATIONS
- 5-HT
Serotonin
- BDNF
Brain-derived neurotrophic factor
- CRH
Corticotropin-releasing hormone
- DISC1
Disrupted in schizophrenia 1
- EPM
Elevated plus maze
- G x E
Gene x Environment
- GD
Gestational day
- GC
Glucocorticoid
- HPA
Hypothalamic-pituitary-adrenal
- INF-〈
Interferon alpha
- INF-β
Interferon beta
- IL-1β
Interleukin-1beta
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- MIA
Maternal immune activation
- MeCP2
Methyl-CpG-binding protein 2
- NF-κB
Nuclear factor 'kappa-light-chain-enhancer' of activated B-cells
- Poly(I:C)
Polyinosinic–polycytidilic acid
- PD
Postnatal day
- PPI
Prepulse inhibition
- TLR
Toll-like receptor
- TrkB
Tropomyosin receptor kinase B
- TNF-〈
Tumor necrosis factors alpha
- VEGF
Vascular endothelial growth factor
CONFLICT OF INTEREST
The authors declare no conflict of interest. The present manuscript has not been previously published and is not under consideration for publication elsewhere.
REFERENCES
- 1.WHO. The global burden of disease: update. 2004 [Google Scholar]
- 2.Samsom J.N., Wong A.H. Schizophrenia and Depression Co-Morbidity: What We have Learned from Animal Models. Front. Psychiatry. 2015;6:13. doi: 10.3389/fpsyt.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DSM-V-TR: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition Text Revision. American Psychiatric Association; 2013. [Google Scholar]
- 4.Buckley P.F., Miller B.J., Lehrer D.S., Castle D.J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 2009;35(2):383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty J.L., Owen M.J. Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med. 2014;6(4):29. doi: 10.1186/gm546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown A.S., Derkits E.J. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J.Psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown A.S., Susser E.S. In utero infection and adult schizophrenia. Ment. Retard. Dev. Disabil. Res. Rev. 2002;8(1):51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 8.Mednick S.A., Machon R.A., Huttunen M.O., Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1988;45(2):189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 9.Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M., Babulas V.P., Susser E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61(8):774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 10.Meyer U., Feldon J., Fatemi S.H. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci. Biobehav.Rev. 2009;33(7):1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Eyles D., Feldon J., Meyer U. Schizophrenia Do all roads lead to dopamine or is this where they start? Evidence from two epidemiologically informed developmental rodent models. Transl. Psychiatry. 2012;2:e81. doi: 10.1038/tp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey L., Boksa P. Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev. Neurobiol. 2012;72(10):1335–1348. doi: 10.1002/dneu.22043. [DOI] [PubMed] [Google Scholar]
- 13.Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol. Psychiatry. 2014;75(4):307–315. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Meyer U., Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog. Neurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Machon R.A., Mednick S.A., Huttunen M.O. Adult major affective disorder after prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1997;54(4):322–328. doi: 10.1001/archpsyc.1997.01830160040006. [DOI] [PubMed] [Google Scholar]
- 16.Mino Y., Oshima I., Okagami K. Mood disorders and influenza epidemics in Japan. Psychiatry Clin. Neurosci. 2000;54(1):59–65. doi: 10.1046/j.1440-1819.2000.00638.x. [DOI] [PubMed] [Google Scholar]
- 17.Pang D., Syed S., Fine P., Jones P.B. No association between prenatal viral infection and depression in later life--a long-term cohort study of 6152 subjects. Can. J. Psychiatry. 2009;54(8):565–570. doi: 10.1177/070674370905400809. [DOI] [PubMed] [Google Scholar]
- 18.Cannon M., Cotter D., Coffey V.P., Sham P.C., Takei N., Larkin C., Murray R.M., O'Callaghan E. Prenatal exposure to the 1957 influenza epidemic and adult schizophrenia: a follow-up study. Br. J. Psychiatry. 1996;168(3):368–71. doi: 10.1192/bjp.168.3.368. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan P.F., Kendler K.S., Neale M.C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry. 2003;60(12):1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan P.F., Neale M.C., Kendler K.S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 21.Wray N.R., Pergadia M.L., Blackwood D.H., Penninx B.W., Gordon S.D., Nyholt D.R., Ripke S., MacIntyre D.J., McGhee K.A., Maclean A.W., Smit J.H., Hottenga J.J., Willemsen G., Middeldorp C.M., de Geus E.J., Lewis C.M., McGuffin P., Hickie I.B., van den Oord E.J., Liu J.Z., Macgregor S., McEvoy B.P., Byrne E.M., Medland S.E., Statham D.J., Henders A.K., Heath A.C., Montgomery G.W., Martin N.G., Boomsma D.I., Madden P.A., Sullivan P.F. Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol. Psychiatry. 2012;17(1):36–48. doi: 10.1038/mp.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan D., Fernando P., Cicvaric A., Berger A., Pollak A.F., Monje J., Pollak D.D. Long-term effects of maternal immune activation on depression-like behavior in the mouse. Transl. Psychiatry. 2014;4:e363. doi: 10.1038/tp.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babri S., Doosti M.H., Salari A.A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav. Immun. 2014;37:164–176. doi: 10.1016/j.bbi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Majidi-Zolbanin J., Doosti M.H., Kosari-Nasab M., Salari A.A. Prenatal maternal immune activation increases anxiety- and depressive-like behaviors in offspring with experimental autoimmune encephalomyelitis. Neuroscience. 2015;294:69–81. doi: 10.1016/j.neuroscience.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y.L., Wang S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behav. Brain Res. 2014;259:24–34. doi: 10.1016/j.bbr.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z., van Praag H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav. Immun. 2015;45:60–70. doi: 10.1016/j.bbi.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Depino A.M. Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience. 2015;299:56–65. doi: 10.1016/j.neuroscience.2015.04.065. [DOI] [PubMed] [Google Scholar]
- 28.Meyer U., Knuesel I., Nyffeler M., Feldon J. Chronic clozapine treatment improves prenatal infection-induced working memory deficits without influencing adult hippocampal neurogenesis. Psychopharmacology (Berl.) 2010;208(4):531–543. doi: 10.1007/s00213-009-1754-6. [DOI] [PubMed] [Google Scholar]
- 29.Meyer U., Nyffeler M., Engler A., Urwyler A., Schedlowski M., Knuesel I., Yee B.K., Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006;26(18):4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graciarena M., Depino A.M., Pitossi F.J. Prenatal inflammation impairs adult neurogenesis and memory related behavior through persistent hippocampal TGFbeta1 downregulation. Brain Behav.Immun. 2010;24(8):1301–9. doi: 10.1016/j.bbi.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Reisinger S., Khan D., Kong E., Berger A., Pollak A., Pollak D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol.Ther. 2015;149:213–26. doi: 10.1016/j.pharmthera.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Dickerson D.D., Bilkey D.K. Aberrant neural synchrony in the maternal immune activation model: using translatable measures to explore targeted interventions. Front. Behav. Neurosci. 2013;7:217. doi: 10.3389/fnbeh.2013.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollak D.D., Rey C.E., Monje F.J. Rodent models in depression research: classical strategies and new directions. Ann. Med. 2010;42(4):252–264. doi: 10.3109/07853891003769957. [DOI] [PubMed] [Google Scholar]
- 34.Kendler K.S., Karkowski-Shuman L. Stressful life events and genetic liability to major depression: genetic control of exposure to the environment? Psychol. Med. 1997;27(3):539–547. doi: 10.1017/S0033291797004716. [DOI] [PubMed] [Google Scholar]
- 35.Kendler K.S., Walters E.E., Neale M.C., Kessler R.C., Heath A.C., Eaves L.J. The structure of the genetic and environmental risk factors for six major psychiatric disorders in women.Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Arch. Gen. Psychiatry. 1995;52(5):374–83. doi: 10.1001/archpsyc.1995.03950170048007. [DOI] [PubMed] [Google Scholar]
- 36.Lopizzo N., Bocchio C.L., Cattane N., Plazzotta G., Tarazi F.I., Pariante C.M., Riva M.A., Cattaneo A. Gene-environment interaction in major depression: focus on experience-dependent biological systems. Front. Psychiatry. 2015;6:68. doi: 10.3389/fpsyt.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittenger C., Duman R.S. Stress, depression, and neuro- plasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 38.Stepanichev M., Dygalo N.N., Grigoryan G., Shishkina G.T., Gulyaeva N. Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. BioMed Res. Int. 2014;932757 doi: 10.1155/2014/932757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13(10):1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEwen B.S. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- 41.Brown A.S., Susser E.S., Lin S.P., Neugebauer R., Gorman J.M. Increased risk of affective disorders in males after second trimester prenatal exposure to the Dutch hunger winter of 1944-45. Br. J. Psychiatry. 1995;166(5):601–606. doi: 10.1192/bjp.166.5.601. [DOI] [PubMed] [Google Scholar]
- 42.Brown A.S., van Os J., Driessens C., Hoek H.W., Susser E.S. Further evidence of relation between prenatal famine and major affective disorder. Am. J. Psychiatry. 2000;157(2):190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- 43.Stein A.D., Pierik F.H., Verrips G.H., Susser E.S., Lumey L.H. Maternal exposure to the Dutch famine before conception and during pregnancy: quality of life and depressive symptoms in adult offspring. Epidemiology. 2009;20(6):909–915. doi: 10.1097/EDE.0b013e3181b5f227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice F., Jones I., Thapar A. The impact of gestational stress and prenatal growth on emotional problems in offs. Acta Psychiatr.Scand. 2007;115(3):171–83. doi: 10.1111/j.1600-0447.2006.00895.x. [DOI] [PubMed] [Google Scholar]
- 45.Rice F., Harold G.T., Boivin J., van den Bree M., Hay D.F., Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol. Med. 2010;40(2):335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunkel S.C., Tanner L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr. Opin. Psychiatry. 2012;25(2):141–148. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babenko O., Kovalchuk I., Metz G.A. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 2015;48:70–91. doi: 10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 48.Graignic-Philippe R., Dayan J., Chokron S., Jacquet A.Y., Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci. Biobehav. Rev. 2014;43:137–162. doi: 10.1016/j.neubiorev.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Markham J.A., Koenig J.I. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacology (Berl.) 2011;214(1):89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav.Immun. 2005;19(4):296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci. Biobehav. Rev. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Brunton P.J., Russell J.A. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J. Neuroendocrinol. 2010;22(4):258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- 53.Cratty M.S., Ward H.E., Johnson E.A., Azzaro A.J., Birkle D.L. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675(1-2):297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- 54.Zuena A.R., Mairesse J., Casolini P., Cinque C., Alema G.S., Morley-Fletcher S., Chiodi V., Spagnoli L.G., Gradini R., Catalani A., Nicoletti F., Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One. 2008;3(5):e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung S., Son G.H., Park S.H., Park E., Lee K.H., Geum D., Kim K. Differential adaptive responses to chronic stress of maternally stressed male mice offspring. Endocrinology. 2005;146(7):3202–3210. doi: 10.1210/en.2004-1458. [DOI] [PubMed] [Google Scholar]
- 56.Walf A.A., Frye C.A. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10(3):251–60.. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- 57.Weinstock M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem. Res. 2007;32(10):1730–1740. doi: 10.1007/s11064-007-9339-4. [DOI] [PubMed] [Google Scholar]
- 58.Lee M.M., Reif A., Schmitt A.G. Major depression: a role for hippocampal neurogenesis? Curr. Top. Behav. Neurosci. 2013;14:153–179. doi: 10.1007/7854_2012_226. [DOI] [PubMed] [Google Scholar]
- 59.Mednick S.A., Huttunen M.O., Machon R.A. Prenatal influenza infections and adult schizophrenia. Schizophr. Bull. 1994;20(2):263–267. doi: 10.1093/schbul/20.2.263. [DOI] [PubMed] [Google Scholar]
- 60.Fatemi S.H., Sidwell R., Akhter P., Sedgewick J., Thuras P., Bailey K., Kist D. Human influenza viral infection in utero increases nNOS expression in hippocampi of neonatal mice. Synapse. 1998;29(1):84–88. doi: 10.1002/(SICI)1098-2396(199805)29:1<84:AID-SYN8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 61.Fatemi S.H., Emamian E.S., Kist D., Sidwell R.W., Nakajima K., Akhter P., Shier A., Sheikh S., Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol.Psychiatry. 1999;4(2):145–54. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- 62.Fatemi S.H., Cuadra A.E., El-Fakahany E.E., Sidwell R.W. andThuras, P., Prenatal viral infection causes alterations in nNOS expression in developing mouse brains. Neuroreport. 2000;11(7):1493–1496. doi: 10.1097/00001756-200005150-00026. [DOI] [PubMed] [Google Scholar]
- 63.Fatemi S.H., Emamian E.S., Sidwell R.W., Kist D.A., Stary J.M., Earle J.A., Thuras P. Human influenza viral infection in utero alters glial fibrillary acidic protein immunoreactivity in the developing brains of neonatal mice. Mol. Psychiatry. 2002;7(6):633–640. doi: 10.1038/sj.mp.4001046. [DOI] [PubMed] [Google Scholar]
- 64.Shi L., Fatemi S.H., Sidwell R.W., Patterson P.H. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fatemi S.H., Earle J., Kanodia R., Kist D., Emamian E.S., Patterson P.H., Shi L., Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol. Neurobiol. 2002;22(1):25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fatemi S.H., Araghi-Niknam M., Laurence J.A., Stary J.M., Sidwell R.W., Lee S. Glial fibrillary acidic protein and glutamic acid decarboxylase 65 and 67 kDa proteins are increased in brains of neonatal BALB/c mice following viral infection in utero. Schizophr. Res. 2004;69(1):121–123. doi: 10.1016/S0920-9964(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 67.Fatemi S.H., Reutiman T.J., Folsom T.D., Huang H., Oishi K., Mori S., Smee D.F., Pearce D.A., Winter C., Sohr R., Juckel G. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr. Res. 2008;99(1-3):56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer U., Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav. Brain. 2009;204(2):322–34. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 69.Cunningham C., Campion S., Teeling J., Felton L., Perry V.H. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav. Immun. 2007;21(4):490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Meyer U., Feldon J., Schedlowski M., Yee B.K. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci. Biobehav. Rev. 2005;29(6):913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 71.Borrell J., Vela J.M., Arevalo-Martin A., Molina-Holgado E., Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26(2):204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 72.Meyer U., Feldon J., Schedlowski M., Yee B.K. Immunological stress at the maternal-foetal interface: a link between neuro- development and adult psychopathology. Brain Behav. Immun. 2006;20(4):378–388. doi: 10.1016/j.bbi.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Zuckerman L., Rehavi M., Nachman R. andWeiner, I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28(10):1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- 74.Zuckerman L., Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169(3-4):308–18. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- 75.Schwartzer J.J., Careaga M., Onore C.E., Rushakoff J.A., Berman R.F., Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl. Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer U., Nyffeler M., Yee B.K., Knuesel I., Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav. Immun. 2008;22(4):469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Fortier M.E., Joober R., Luheshi G.N., Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J. Psychiatr. Res. 2004;38(3):335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Meyer U., Yee B.K., Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13(3):241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt H.D., Duman R.S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 2007;18(5-6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 80.Ozawa K., Hashimoto K., Kishimoto T., Shimizu E., Ishikura H., Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol. Psychiatry. 2006;59(6):546–54. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 81.Pineda E., Shin D., You S.J., Auvin S., Sankar R., Mazarati A. Maternal immune activation promotes hippocampal kindling epileptogenesis in mice. Ann. Neurol. 2013;74(1):11–19. doi: 10.1002/ana.23898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Atladottir H.O., Thorsen P., Ostergaard L., Schendel D.E., Lemcke S., Abdallah M., Parner E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010;40(12):1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 83.Bowen J.M., Chamley L., Keelan J.A., Mitchell M.D. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23(4):257–273. doi: 10.1053/plac.2001.0782. [DOI] [PubMed] [Google Scholar]
- 84.Babulas V., Factor-Litvak P., Goetz R., Schaefer C.A., Brown A.S. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am. J. Psychiatry. 2006;163(5):927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 85.Li Q., Cheung C., Wei R., Hui E.S., Feldon J., Meyer U., Chung S., Chua S.E., Sham P.C., Wu E.X., McAlonan G.M. Prenatal immune challenge is an environmental risk factor for brain and behavior change relevant to schizophrenia: evidence from MRI in a mouse model. PLoS One. 2009;4(7):e6354. doi: 10.1371/journal.pone.0006354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bilbo S.D., Schwarz J.M. The immune system and developmental programming of brain and behavior. Front. Neuroendocrinol. 2012;33(3):267–286. doi: 10.1016/j.yfrne.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robinson D.P., Klein S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holsapple M.P., West L.J., Landreth K.S. Species comparison of anatomical and functional immune system development. Birth Defects Res. B Dev. Reprod. Toxicol. 2003;68(4):321–334. doi: 10.1002/bdrb.10035. [DOI] [PubMed] [Google Scholar]
- 89.Klosterkotter J., Schultze-Lutter F., Bechdolf A., Ruhrmann S. Prediction and prevention of schizophrenia: what has been achieved and where to go next? World Psychiatry. 2011;10(3):165–174. doi: 10.1002/j.2051-5545.2011.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tandon R., Nasrallah H.A., Keshavan M.S. Schizophrenia, "just the facts" 5. Treatment and prevention. Past, present, and future. Schizophr. Res. 2010;122(1-3):1–23. doi: 10.1016/j.schres.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 91.Bechdolf A., Muller H., Stutzer H., Wagner M., Maier W., Lautenschlager A., Heinz W., de Millas B., Janssen W., Gaebel T.M., Michel M., Schneider F., Lambert M., Naber D., Brune M., Kruger-Ozgurdal S., Wobrock T., Riedel M., Klosterkotter J. Rationale and baseline characteristics of PREVENT: a second-generation intervention trial in subjects at-risk (prodromal) of developing first-episode psychosis evaluating cognitive behavior therapy, aripiprazole, and placebo for the prevention of psychosis. Schizophr. Bull. 2011;37(Suppl. 2):S111–S121. doi: 10.1093/schbul/sbr083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raison C.L., Capuron L., Miller A.H. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miller G.E., Chen E., Sze J., Marin T., Arevalo J.M., Doll R., Ma R., Cole S.W. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol. Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Capuron L., Fornwalt F.B., Knight B.T., Harvey P.D., Ninan P.T., Miller A.H. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J. Affect. Disord. 2009;119(1-3):181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dantzer R., O'Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reimer T., Brcic M., Schweizer M., Jungi T.W. poly(I:C) and LPS induce distinct IRF3 and NF-kappaB signaling during type-I IFN and TNF responses in human macrophages. J. Leukoc. Biol. 2008;83(5):1249–1257. doi: 10.1189/jlb.0607412. [DOI] [PubMed] [Google Scholar]
- 97.Dammann O., Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr. Res. 1997;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Howerton C.L., Bale T.L. Prenatal programing: at the intersection of maternal stress and immune activation. Horm.Behav. 2012;62(3):237–42. doi: 10.1016/j.yhbeh.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arck P.C., Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat. Med. 2013;19(5):548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 101.Urakubo A., Jarskog L.F., Lieberman J.A., Gilmore J.H. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr.Res. 2001;47(1):27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 102.Ning H., Wang H., Zhao L., Zhang C., Li X.Y., Chen Y.H., Xu D.X. Maternally-administered lipopolysaccharide (LPS) increases tumor necrosis factor alpha in fetal liver and fetal brain: its suppression by low-dose LPS pretreatment. Toxicol. Lett. 2008;176(1):13–19. doi: 10.1016/j.toxlet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Ashdown H., Dumont Y., Ng M., Poole S., Boksa P., Luheshi G.N. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol.Psychiatry. 2006;11(1):47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 104.Dahlgren J., Samuelsson A.M., Jansson T., Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 2006;60(2):147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- 105.Gayle D.A., Beloosesky R., Desai M., Amidi F., Nunez S.E., Ross M.G. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286(6):R1024–R1029. doi: 10.1152/ajpregu.00664.2003. [DOI] [PubMed] [Google Scholar]
- 106.Gilmore J.H., Jarskog L.F. andVadlamudi, M S. aternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J. Neuroimmunol. 2005;159(1-2):106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 107.Smith S.E., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zaretsky M.V., Alexander J.M., Byrd W., Bawdon R.E. Transfer of inflammatory cytokines across the placenta. Obstet. Gynecol. 2004;103(3):546–50.. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- 109.Girard S., Tremblay L. Lepage, M.and Sebire, G. IL-1 receptor antagonist protects against placental and neurodevelopmental defects induced by maternal inflammation. J. Immunol. 2010;184(7):3997–4005. doi: 10.4049/jimmunol.0903349. [DOI] [PubMed] [Google Scholar]
- 110.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav.Immun. 2010;24(6):881–97. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Samuelsson A.M., Jennische E., Hansson H.A., Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neuro- degeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290(5):R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 112.Vallieres L., Campbell I.L. Gage, F.H. andSawchenko, P.E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 2002;22(2):486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 114.Murphy P.G., Borthwick L.A., Altares M., Gauldie J., Kaplan D., Richardson P.M. Reciprocal actions of interleukin-6 and brain-derived neurotrophic factor on rat and mouse primary sensory neurons. Eur. J. Neurosci. 2000;12(6):1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- 115.Hiles S.A., Baker A.L. de Malmanche, T.and Attia, J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav. Immun. 2012;26(7):1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Kong E., Sucic S., Monje F.J., Reisinger S.N., Savalli G., Diao W., Khan D., Ronovsky M., Cabatic M., Koban F., Freissmuth M., Pollak D.D. Corrigendum: STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behaviour. Sci. Rep. 2015;5:11965. doi: 10.1038/srep11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Missault S., Van den Eynde K., Vanden B.W., Fransen E., Weeren A., Timmermans J.P., Kumar-Singh S., Dedeurwaerdere S. The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neuro- developmental model. Brain Behav. Immun. 2014;42:138–146. doi: 10.1016/j.bbi.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 118.Hsiao E.Y., McBride S.W., Chow J., Mazmanian S.K., Patterson P.H. Modeling an autism risk factor in mice leads to permanent immune dysregulation. Proc. Natl. Acad. Sci. USA. 2012;109(31):12776–12781. doi: 10.1073/pnas.1202556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hava G., Vered L., Yael M., Mordechai H., Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev. Psychobiol. 2006;48(2):162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- 120.Jacobson L. Hypothalamic-pituitary-adrenocortical axis: neuro- psychiatric aspects. Compr. Physiol. 2014;4(2):715–738. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- 121.Altemus M., Sarvaiya N., Neill E.C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 2014;35(3):320–330. doi: 10.1016/j.yfrne.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duman R.S., Monteggia L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 123.Sahay A., Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 124.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 125.Piontkewitz Y., Bernstein HG., Bernstein HG., Dobrowolny H.G., Bogerts H.B., Weiner I. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain Behav. Immun. 2012;26(2):353–63. doi: 10.1016/j.bbi.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 126.Cui K., Ashdown H., Luheshi G.N., Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr. Res. 2009;113(2-3):288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 127.Warner-Schmidt J.L., Duman R.S. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- 128.Castren E., Voikar V., Rantamaki T. Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 129.Karege F., Perret G., Bondolfi G., Schwald M., Bertschy G., Aubry J.M. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 130.Shimizu E., Hashimoto K., Okamura N., Koike K., Komatsu N., Kumakiri C., Nakazato M., Watanabe H., Shinoda N., Okada S., Lyo M. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry. 2003;54(1):70–75. doi: 10.1016/S0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 131.Calabrese F., Rossetti A.C., Racagni G., Gass P., Riva M.A., Molteni R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell. Neurosci. 2014;8:430. doi: 10.3389/fncel.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bortolotto V., Cuccurazzu B. NF-kappaB mediated regulation of adult hippocampal neurogenesis: relevance to mood disorders and antidepressant activity. 2014 doi: 10.1155/2014/612798. 612798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kirsten T.B., Queiroz-Hazarbassanov N., Bernardi M.M., Felicio L.F. Prenatal zinc prevents communication impairments and BDNF disturbance in a rat model of autism induced by prenatal lipopolysaccharide exposure. Life Sci. 2015;130:12–17. doi: 10.1016/j.lfs.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 134.Golan H.M., Lev V., Hallak M., Sorokin Y., Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. 2005. [DOI] [PubMed]
- 135.Hemmerle A.M., Ahlbrand R., Bronson S.L., Lundgren K.H., Richtand N.M., Seroogy K.B. Modulation of schizophrenia-related genes in the forebrain of adolescent and adult rats exposed to maternal immune activation. Schizophr. Res. 2015;168(1-2):411–420. doi: 10.1016/j.schres.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Warner-Schmidt J.L., Duman R.S. VEGF is an essential mediator of the neurogenic and behavioral actions of anti- depressants. Proc. Natl. Acad. Sci. USA. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boku S., Hisaoka-Nakashima K., Nakagawa S., Kato A., Kajitani N., Inoue T., Kusumi I., Takebayashi M. Tricyclic antidepressant amitriptyline indirectly increases the proliferation of adult dentate gyrus-derived neural precursors: an involvement of astrocytes. PLoS One. 2013;8(11):e79371. doi: 10.1371/journal.pone.0079371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Greene J., Banasr M., Lee B., Warner-Schmidt J., Duman R.S. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34(11):2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fournier N.M., Lee M., Banasr B., Elsayed M., Duman R.S. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology. 2012;63(4):642–652. doi: 10.1016/j.neuropharm.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Licht T., Goshen I., Avital A., Kreisel T., Zubedat S., Eavri R., Segal M., Yirmiya R., Keshet E. Reversible modulations of neuronal plasticity by VEGF. Proc. Natl. Acad. Sci. USA. 2011;108(12):5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cao L., Jiao X., Zuzga D.S., Liu Y., Fong D.M., Young D., During M.J. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat. Genet. 2004;36(8):827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- 142.Pujadas L., Gruart A., Bosch C., Delgado L., Teixeira C.M., Rossi D., de Lecea L., Martinez A., Delgado-Garcia J.M., Soriano E. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. 2010;30(13):4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fatemi S.H., Earle J.A., McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol. Psychiatry. 2000;5(6):654–63. doi: 10.1038/sj.mp.4000783. 571. [DOI] [PubMed] [Google Scholar]
- 144.Fatemi S.H., Reutiman T.J., Folsom T.D. Chronic psychotropic drug treatment causes differential expression of Reelin signaling system in frontal cortex of rats. Schizophr.Res. 2009;111(1-3):138–138. doi: 10.1016/j.schres.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Ghiani C.A., Mattan N.S., Nobuta H., Malvar J.S., Boles J., Ross M.G., Waschek J.A., Carpenter E.M., Fisher R.S., de Vellis J. Early effects of lipopolysaccharide-induced inflammation on foetal brain development in rat. ASN Neuro. 2011;3(4) doi: 10.1042/AN20110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meyer U., Feldon J. To poly(I:C) or not to poly(I:C): advancing preclinical schizophrenia research through the use of prenatal immune activation models. Neuropharmacology. 2012;62(3):1308–1321. doi: 10.1016/j.neuropharm.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 147.Owens M.J., Nemeroff C.B. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 1994;40(2):288–295. [PubMed] [Google Scholar]
- 148.Wang S., Yan J.Y., Lo Y.K., Carvey P.M., Ling Z. Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res. 2009;1265:196–204. doi: 10.1016/j.brainres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 149.Winter C., Djodari-Irani A., Sohr R., Morgenstern R.J., Feldon G. Juckel, and Meyer, U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 2009;12(4):513–524. doi: 10.1017/S1461145708009206. [DOI] [PubMed] [Google Scholar]
- 150.Winter C., Reutiman T.J., Folsom T.D., Sohr R., Wolf R.J., Juckel G., Fatemi S.H. Dopamine and serotonin levels following prenatal viral infection in mouse--implications for psychiatric disorders such as schizophrenia and autism. Eur. Neuropsychopharmacol. 2008;18(10):712–716. doi: 10.1016/j.euroneuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Crawley J.N., Belknap J.K., Collins A., Crabbe J.C., Frankel W., Henderson N., Hitzemann R.J., Maxson S.C., Miner L.L., Silva A.J., Wehner J.M., Wynshaw-Boris A., Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl.) 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 152.Millstein R.A., Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007;31(1):3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 153.Mineur Y.S., Belzung C., Crusio W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175(1):43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 154.Caspi A., Moffitt T.E. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 2006;7(7):583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 155.Taylor S.E., Way B.M., Welch W.T., Hilmert C.J., Lehman B.J., Eisenberger N.I. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol. Psychiatry. 2006;60(7):671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 156.Aguilera M., Arias B., Wichers M., Barrantes-Vidal N., Moya J., Villa H., van Os J., Ibanez M.I., Ruiperez M.A., Ortet G., Fananas L. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol. Med. 2009;39(9):1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- 157.Caspi A., Sugden K., Moffitt T.E., Taylor I.A., Craig W., Harrington H., McClay J., Mill J., Martin J., Braithwaite A., Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 158.Clarke M.C., Tanskanen A., Huttunen M., Whittaker J.C., Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am. J. Psychiatry. 2009;166(9):1025–30. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 159.Selten J.P., Frissen A., Lensvelt-Mulders G., Morgan V.A. Schizophrenia and 1957 pandemic of influenza: meta-analysis. Schizophr. Bull. 2010;36(2):219–228. doi: 10.1093/schbul/sbp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feingold D., Weiser M., Rehm J., Lev-Ran S. The association between cannabis use and mood disorders: A longitudinal study. J. Affect. Disord. 2015;172:211–218. doi: 10.1016/j.jad.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 161.Lopez-Leon S., Janssens A.C., Gonzalez-Zuloeta L., A.M., Del-Favero J., Claes S.J., Oostra B.A., van Duijn C.M. Meta-analyses of genetic studies on major depressive disorder. Mol.Psychiatry. 2008;13(8):772–85. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 162.Uher R. Gene-environment interactions in common mental disorders: an update and strategy for a genome-wide search. Soc. Psychiatry Psychiatr. Epidemiol. 2014;49(1):3–14. doi: 10.1007/s00127-013-0801-0. [DOI] [PubMed] [Google Scholar]
- 163.Abazyan B., Nomura J., Kannan G., Ishizuka K., Tamashiro K.L., Nucifora F., Pogorelov V., Ladenheim B., Yang C., Krasnova I.N., Cadet J.L., Pardo C., Mori S., Kamiya A., Vogel M.W., Sawa A., Ross C.A., Pletnikov M.V. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biol. Psychiatry. 2010;68(12):1172–1181. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lipina T.V., Zai C.D., Hlousek J., Roder C., Wong A.H. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. J. Neurosci. 2013;33(18):7654–7666. doi: 10.1523/JNEUROSCI.0091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vuillermot S., Joodmardi E., Perlmann T., Ogren S.O., Feldon J., Meyer U. Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. J. Neurosci. 2012;32(2):436–451. doi: 10.1523/JNEUROSCI.4831-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Meyer U., Murray P.J., Urwyler A., Yee B.K., Schedlowski M., Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol. Psychiatry. 2008;13(2):208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 167.Chourbaji S., Urani A., Inta I., Sanchis-Segura C., Brandwein C., Zink M., Schwaninger M. andGass, P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 2006;23(3):587–594. doi: 10.1016/j.nbd.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Giovanoli S., Engler H., Engler A., Richetto J., Voget M., Willi R., Winter C., Riva M.A., Mortensen P.B., Feldon J., Schedlowski M., Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 169.Meek L.R., Dittel P.L., Sheehan M.C., Chan J.Y., Kjolhaug S.R. Effects of stress during pregnancy on maternal behavior in mice. Physiol. Behav. 2001;72(4):473–9. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- 170.Meaney M.J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 171.Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., de Kloet E.R., Joels M., Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stern J.M. Offspring-induced nurturance: animal-human parallels. Dev. Psychobiol. 1997;31(1):19–37. doi: 10.1002/(SICI)1098-2302(199707)31:1<19:AID-DEV3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 173.Koehl M., van der Veen R., Gonzales D., Piazza P.V., Abrous D.N. Interplay of maternal care and genetic influences in programming adult hippocampal neurogenesis. Biol. Psychiatry. 2012;72(4):282–289. doi: 10.1016/j.biopsych.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 174.Francis D.D., Meaney M.J. Maternal care and the development of stress responses. Curr. Opin. Neurobiol. 1999;9(1):128–134. doi: 10.1016/S0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- 175.Schwendener S., Meyer U., Feldon J. Deficient maternal care resulting from immunological stress during pregnancy is associated with a sex-dependent enhancement of conditioned fear in the offspring. J. Neurodev. Disord. 2009;1(1):15–32. doi: 10.1007/s11689-008-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meyer U., Schwendener S., Feldon J., Yee B.K. Prenatal and postnatal maternal contributions in the infection model of schizophrenia. Exp. Brain Res. 2006;173(2):243–257. doi: 10.1007/s00221-006-0419-5. [DOI] [PubMed] [Google Scholar]
- 177.Feil R., Fraga M.F. Epigenetics and the environment: emerging patterns and implications. Nat. Rev. Genet. 2011;13(2):97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- 178.Champagne F.A. Early environments, glucocorticoid receptors, and behavioral epigenetics. Behav. Neurosci. 2013;127(5):628–636. doi: 10.1037/a0034186. [DOI] [PubMed] [Google Scholar]
- 179.Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/S0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 180.Weaver I.C., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 181.Baumeister D., Lightman S.L., Pariante C.M. The Interface of Stress and the HPA Axis in Behavioural Phenotypes of Mental Illness. Curr. Top. Behav. Neurosci. 2014;18:13–24. doi: 10.1007/7854_2014_304. [DOI] [PubMed] [Google Scholar]
- 182.Nestler E.J. Epigenetic mechanisms of depression. JAMA Psychiatry. 2014;71(4):454–456. doi: 10.1001/jamapsychiatry.2013.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Foran E., Garrity-Park M.M., Mureau C., Newell J., Smyrk T.C., Limburg P.J., Egan L.J. Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol. Cancer Res. 2010;8(4):471–481. doi: 10.1158/1541-7786.MCR-09-0496. [DOI] [PubMed] [Google Scholar]
- 184.Fukushima N., Sato N., Sahin F., Su G.H., Hruban R.H., Goggins M. Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br.J. Cancer. 2003;89(2):338–43. doi: 10.1038/sj.bjc.6601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wehbe H., Henson R., Meng F., Mize-Berge J., Patel T. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res. 2006;66(21):10517–24. doi: 10.1158/0008-5472.CAN-06-2130. [DOI] [PubMed] [Google Scholar]
- 186.Gasche J.A., Hoffmann J., Boland C.R., Goel A. Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancer cells. Int. J. Cancer. 2011;129(5):1053–1063. doi: 10.1002/ijc.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kong E., Sucic S., Monje F.J., Savalli G., Diao W., Khan D., Ronovsky M., Cabatic M., Koban F., Freissmuth M., Pollak D.D. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci. Rep. 2015;5:9009. doi: 10.1038/srep09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Na E.S., Monteggia L.M. The role of MeCP2 in CNS development and function. Horm. Behav. 2011;59(3):364–368. doi: 10.1016/j.yhbeh.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Basil P., Li Q., Dempster E.L., Mill J., Sham P.C., Wong C.C., McAlonan G.M. Prenatal maternal immune activation causes epigenetic differences in adolescent mouse brain. Transl. Psychiatry. 2014;4:e434. doi: 10.1038/tp.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Connor C.M., Dincer A., Straubhaar J., Galler J.R., Houston I.B., Akbarian S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr. Res. 2012;140(1-3):175–184. doi: 10.1016/j.schres.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tang B., Jia H., Kast R.J., Thomas E.A. Epigenetic changes at gene promoters in response to immune activation in utero. Brain Behav. Immun. 2013;30:168–175. doi: 10.1016/j.bbi.2013.01.086. [DOI] [PubMed] [Google Scholar]
- 192.Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front. Physiol. 2015;6:230. doi: 10.3389/fphys.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhang Y., Cazakoff B.N., Thai C.A., Howland J.G. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2012;62(3):1299–1307. doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rocher C., Spedding M., Munoz C., Jay T.M. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb. Cortex. 2004;14(2):224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- 195.Deleidi M., Hallett P.J., Koprich J.B., Chung C.Y., Isacson O. The Toll-like receptor-3 agonist polyinosinic:polycytidylic acid triggers nigrostriatal dopaminergic degeneration. J. Neurosci. 2010;30(48):16091–101. doi: 10.1523/JNEUROSCI.2400-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Furman D.J., Hamilton J.P., Gotlib I.H. Frontostriatal functional connectivity in major depressive disorder. Biol. Mood Anxiety Disord. 2011;1(1):11. doi: 10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dailly E., Chenu F., Renard C.E., Bourin M. Dopamine, depression and antidepressants. Fundam. Clin. Pharmacol. 2004;18(6):601–607. doi: 10.1111/j.1472-8206.2004.00287.x. [DOI] [PubMed] [Google Scholar]
- 198.Bitanihirwe B.K., Peleg-Raibstein D., Mouttet F., Feldon J., Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010;35(12):2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nyffeler M., Meyer U., Yee B.K., Feldon J., Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143(1):51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]