Abstract
Depression is the most prevalent and among the most debilitating of psychiatric disorders. The precise neurobiology of this illness is unknown. Several lines of evidence suggest that peripheral and central inflammation plays a role in depressive symptoms, and that anti-inflammatory drugs can improve depressive symptoms in patients with inflammation-related depression. Signaling via brain-derived neurotrophic factor (BDNF) and its receptor, tropomycin receptor kinase B (TrkB) plays a key role in the pathophysiology of depression and in the therapeutic mechanisms of antidepressants. A recent paper showed that lipopolysaccharide (LPS)-induced inflammation gave rise to depression-like phenotype by altering BDNF-TrkB signaling in the prefrontal cortex, hippocampus, and nucleus accumbens, areas thought to be involved in the antidepressant effects of TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF) and TrkB antagonist, ANA-12. Here we provide an overview of the tryptophan-kynurenine pathway and BDNF-TrkB signaling in the pathophysiology of inflammation-induced depression, and propose mechanistic actions for potential therapeutic agents. Additionally, the authors discuss the putative role of TrkB agonists and antagonists as novel therapeutic drugs for inflammation-related depression.
Keywords: Brain-derived neurotrophic factor (BDNF), Depression, Hippocampus, Inflammation, Nucleus accumbens, Prefrontal cortex, TrkB
INTRODUCTION
Depression is a serious illness affecting approximately 17 percent of the population at some point in their lifetime, resulting in highly unfavorable social and economic outcomes [1]. Despite the current plethora of antidepressant therapies, their benefits are limited. Only one third of depressed patients show significant improvement in response to first-line treatment [2]. In addition, there is a time lag spanning several weeks to months before a therapeutic effect is observed, reflected in the high suicide rate amongst depressed patients [3-5]. These factors point to a critical, unmet need for rapid onset antidepressants, particularly in depressed patients with an increased risk of suicide.
The N-methyl-D-aspartate receptor antagonist, ketamine, is an effective antidepressant agent for treatment-resistant depression [6-14]. A single subanesthetic dose (0.5 mg/kg) of ketamine produces robust and rapid antidepressant effects in two-thirds of patients with treatment resistant depression, and this response can last for over one week [9, 10, 15, 16]. Interestingly, a single infusion of ketamine greatly improved suicidal thinking in depressed patients [17-19]. However, the clinical use of ketamine is limited by the potential to elicit psychotomimetic and dissociative side effects as well as abuse liability [11, 20]. Therefore, it is a great clinical imperative to develop rapid-onset antidepressants without these side effects.
Current evidence points to the role of inflammatory processes in the pathophysiology of depression [6, 21-23]. In this review article, we will evaluate the role of inflammation in the pathophysiology of depression. In turn, we will assess the roles of the tryptophan-kynurenine pathway and brain-derived neurotrophic factor (BDNF) and its receptor TrkB signaling in inflammation-related depression. Finally, we will propose TrkB agonists and antagonists as potential rapid-onset antidepressants for inflammation-related depression.
INFLAMMATION IN DEPRESION
Altered Pro-inflammatory Cytokines in Patients with Depression
Patients with depression show increased blood concentrations of pro-inflammatory cytokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and other acute phase proteins, C-reactive protein (CRP), haptoglobin and neopterin [24, 25]. Meta-analysis showed higher serum levels of TNF-α in drug-free depressed patients relative to healthy controls [26]. Furthermore, a recent meta-analysis showed decreased blood IL-6 levels after antidepressant treatment, regardless of clinical outcome, whereas persistently elevated TNF-α was associated with prospectively determined treatment resistance [27]. In a postmortem study, the pro-inflammatory cytokines genes are increased in the frontal cortex of subjects with a history of depression compared with healthy controls [28].
Antidepressant treatment can attenuate the expression of inflammatory biomarkers in depression [29]. Sluzewska et al. [30] reported on a reduction of serum IL-6 after fluoxetine treatment in depressed patients. Antidepressants such as imipramine, clomipramine, venlafaxine, fluoxetine, sertraline and trazodone, reduce the interferon-γ (INF-γ)/ IL-10 ratio of in vitro human blood samples (a ratio of pro-inflammatory/anti-inflammatory drive), consistent with an anti-inflammatory action [31-33]. These findings suggest that peripheral and central inflammation may play a key role in depression, and highlights a putative role for anti-inflammatory drugs in their treatment.
Pro-inflammatory Cytokines, Sickness Behavior and Depressive Behavior
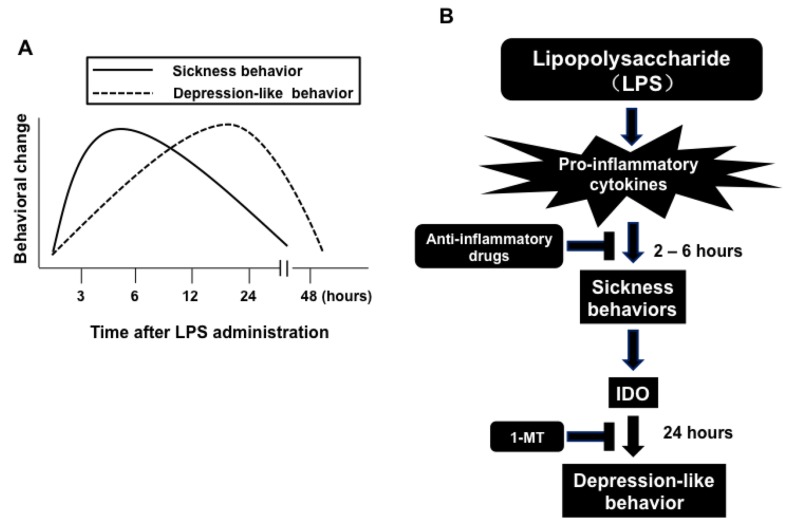
The bacterial endotoxin, lipopolysaccharide (LPS) is widely used to create an inflammation-related model of depression [34-39]. Systemic administration of LPS to rodents causes brain expression of pro-inflammatory cytokines, such as IL-1β and TNF-α. These cytokines are capable of inducing sickness behavior (Fig. 1) [34]. Furthermore, systemic or central administration of IL-1β or TNF-α to rodents induces sickness behaviors [34]. Sickness behavior includes hypomotility, hyperthermia, hypophagia, hyperalgesia, decreased interest in exploration, decreased sexual activity and increased sleep [40-43]. Pretreatment with imipramine attenuated LPS-induced sickness behavior [44]. Peripheral administration of LPS resulted in sickness behavior that peaked two to six hours after dosing and then gradually waned (Fig. 1A). Depression-like behavior, as measured by increased immobility in the forced swimming test (FST) or the tail suspension test (TST), and decreased sucrose preference, emerged on this background (Fig. 1A) [45]. Pro-inflammatory cytokines were activated in the brain in response to peripheral administration of LPS, and depression-like behavior peaked at 24 hours post-LPS administration (Fig. 1B) [45]. Pro-inflammatory cytokines can also enhance activity of ubiquitous indoleamine 2,3-dioxygenase (IDO), which peaks at 24 hours after LPS administration. IDO activation results in decreased tryptophan levels and increased production of kynurenine and other tryptophan-derived metabolites [36, 45]. Taken together, these data suggest that IDO may be a key mediator of inflammation-induced depressive-like phenotype, through catabolism via the tryptophan-kynurenine pathway [36].
Fig. (1).
The relationship between sickness behavior and depression-like behavior. (A): The sickness behavior that peaks 2 to 6 hours later and gradually wanes by peripheral administration of lipopolysaccharide (LPS). Pro-inflammatory cytokine signaling is activated in the brain as a response to peripheral LPS injection, with depression-like behavior peaking 24 hours after LPS injection. (B): The sickness behavior was induced by pro-inflammatory cytokines which also can enhance activity of the ubiquitous indoleamine 2,3-dioxygenase (IDO), peaking 24 hours after LPS administration. Activation of IDO results in decreased tryptophan (TRP) levels and increased production of kynurenine (KYN) and other tryptophan-derived metabolites. Anti-inflammatory drugs (e.g. minocycline) can block both LPS-induced sickness behavior and depression-like behavior. By contrast, administration of 1-methyl tryptophan (1-MT), a competitive inhibitor of IDO, blocks LPS induced depression-like behavior without altering LPS-induced sickness behavior. This figure is a modified version from a previous report [45].
Several lines of evidence demonstrate that the antibiotic drug minocycline, a second-generation tetracycline, promotes potent anti-inflammatory and neuroprotective effects in several animal models of neuropsychiatric disorders, including schizophrenia [46-50], depression [51] and methamphetamine (or amphetamine)-related disorders [52-56]. Furthermore, current antidepressants, including SSRIs and SNRIs, are able to prevent depression-like behavior and alterations in serum pro-inflammatory cytokines, such as TNF-α, induced by a single dose of LPS [35]. Pretreatment with minocycline blocked both LPS-induced sickness and depression-like behaviors in mice [36]. LPS-induced depression-like behavior could be prevented by blocking the release of pro-inflammatory cytokines, two to six hours after LPS administration. Interestingly, pretreatment with 1-methyl tryptophan (1-MT), an inhibitor of IDO, could block LPS-induced depression-like phenotype without altering LPS-induced sickness behavior (Fig. 1B) [36, 45].
Chronic inflammation also can induce depression-like behavior in rodent [57, 58]. Repeated administration of low dose of LPS (0.1 mg/kg/day for 7 days) showed depression-like phenotype in rodents, and LPS-induced depression-like phenotype is attenuated by anti-inflammatory compounds [57]. Thus, it is likely that depression-like behavior by chronic inflammation may be similar to depression-like behavior by acute inflammation [58].
Peripheral Interlukin-6 (IL-6) in Depression
Peripheral IL-6 is known to be secreted by macrophages and monocytes to stimulate differentiation and proliferation of B cells. A recent paper showed elevated serum IL-6 levels in depressed patients, but obtained mixed results (no change or decreases) for IL-6 levels in cerebrospinal fluid (CSF) [59]. A meta-analysis showed that patients with suicidality showed high blood levels of IL-6 compared with control subjects, implying that peripheral IL-6 may be associated with suicidal ideation, a core symptom of depression [60]. Very recently, we reported that serum IL-6 may represent a predictable biomarker for ketamine’s antidepressant action in patients with treatment-resistant depression [61].
Hodes et al. [62] reported that the highest levels of blood IL-6 were found only in mice that went on to develop a susceptible behavioral phenotype (depression-like behavior), following chronic stress, and that blood IL-6 levels remained elevated for a least one month. Furthermore, serum IL-6 levels negatively correlated with social interaction behavior following repeated social defeat stress. Data from stress-susceptible bone marrow chimeras revealed increased social avoidance behavior after exposure to either sub-threshold repeated social defeat stress, or a purely emotional stressor, termed witness defeat. Therefore, depleting the cytokine IL-6 from the whole body or just from leukocytes promotes resilience in a social defeat stress model [62]. From our research, we found that alterations in peripheral but not brain IL-6, probably contributed to resilience versus susceptibility to inescapable stress in the rat learned helpless model [63]. This makes it unlikely that brain-derived IL-6 might be involved in the pathogenesis of depression [63, 64].
A recent review concluded that novel investigational drugs targeting IL-6 signaling could be beneficial in the treatment of depression [65]. At present, commercially available IL-6 antagonists, such as tocilizumab (ACTEMRAⓇ) have been widely used to treat patients with rheumatoid arthritis, a disease characterized by an excessive expression of pro-inflammatory cytokines in peripheral tissues. Taken together, these results suggest that novel drugs targeting peripheral IL-6 could be beneficial in patients with depression [63, 64].
Tryptophan, Serotonin and the Kynurenine Pathway in Inflammation-related Depression
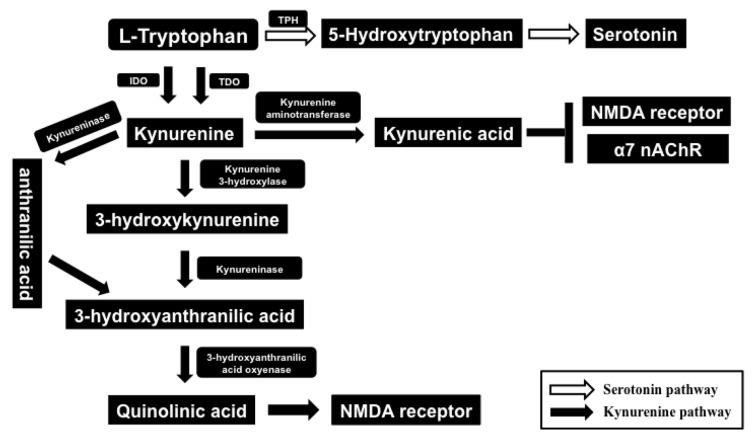
Tryptophan is an essential amino acid required for protein synthesis and serves as a precursor for serotonin (5-HT; 5-hydroxytryptamine). Ninety five percent of tryptophan is degraded in the liver through the kynurenine pathway, and the remaining tryptophan is used for the synthesis of 5-HT [45]. Tryptophan oxidation occurs via tryptophan dioxygenase (TDO) and indoleamine 2,3-dioxygenase (IDO) (Fig. 2). In general, tryptophan degradation by IDO is negligible. However, IDO is highly inducible by pro-inflammatory cytokines, including interferon-γ (IFN-γ) and TNF-α, [36, 45, 66]. Abnormalities in the tryptophan-kynurenine pathway are implicated in the pathophysiology of a number of neuropsychiatric diseases [36, 45, 66]. Tryptophan is a precursor of 5-HT and its bioavailability regulates the synthesis of 5-HT. Therefore, it seems that decreased levels of tryptophan may have the potential to influence serotonergic neurotransmission in the brain. In addition, IDO is expressed in the brain so that fluctuations in its enzymatic activity could also impact on 5-HT biosynthesis (Fig. 2) [36, 45, 66, 67].
Fig. (2).
Tryptophan-kynurenine pathway. The essential amino acid L-tryptophan is converted to 5-hydroxytryptophan by tryptophan hydroxylase (TPH), and this is metabolized to serotonin (5-HT) by 5-hydroxytryptophan decarboxylase. The kynurenine pathway is initiated by the conversion of tryptophan to kynurenine by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). Kynurenine is mostly hydroxylated (kynurenine hydroxylase) to 3-hydroxykynurenine. Kynureninase acts upon both 3-hydroxykynurenine and kynurenine; on 3-hydroxykynurenine to form 3-hydroxyanthranilic acid; and on kynurenine to form anthranilic acid (this latter conversion accounts for only a minority of kynureninase activity). Three-hydroxyanthranilic acid is converted into quinolinic acid, an NMDA receptor agonist by 3-hydroxyanthranilic acid oxygenase. Kynurenine can also be converted into kynurenic acid by kynurenine aminotransferase. By contrast, kynurenic acid is an antagonist at NMDA and α7 nicotinic acetylcholine receptors (nAChRs), both of which play roles in the pathophysiology of psychiatric disease.
Kynurenine is a major metabolite of tryptophan and is readily transported across the blood–brain-barrier (Fig. 2). In the brain it is metabolized further in perivascular macrophages, microglia and astrocytes, to generate neuroactive compounds [66, 68, 69]. Kynurenine has two catabolic branches, leading to the formation of either 3-hydroxykynurenine (3-HK) and quinolinic acid (QA) or kynurenic acid (KA) (Fig. 2) [69, 71]. The compound 3-HK generates free-radical species able to induce oxidative stress and lipid peroxidation, whereas QA is an NMDA receptor agonist [45, 66, 70, 72]. By contrast, KA is an NMDA receptor antagonist with neuroprotective effects [45, 66, 71, 72]. In addition, KA is a known endogenous antagonist at α7 nicotinic acetylcholine receptors (nAChR) which are implicated in the pathophysiology of neuro- psychiatric disorders [73-79]. Thus, it is reasonable to propose that imbalanced levels of metabolites from the kynurenine pathway could underlie pathogenic processes in a number of neuropsychiatric diseases, including inflammation-associated depression (Fig. 2) [36, 43, 66, 72].
BDNF-TRKB SIGNALING IN DEPRESSION
BDNF-TrkB Signaling
Brain-derived neurotrophic factor (BDNF), one of the major neurotrophic factors, is a 27-kDa polypeptide important for the survival, differentiation and outgrowth of peripheral and central neurons during development and in adulthood [80-82] (Fig. 3). It is well known that BDNF is integral to use-dependent plasticity mechanisms, such as long-term potentiation, learning and memory [80-85].
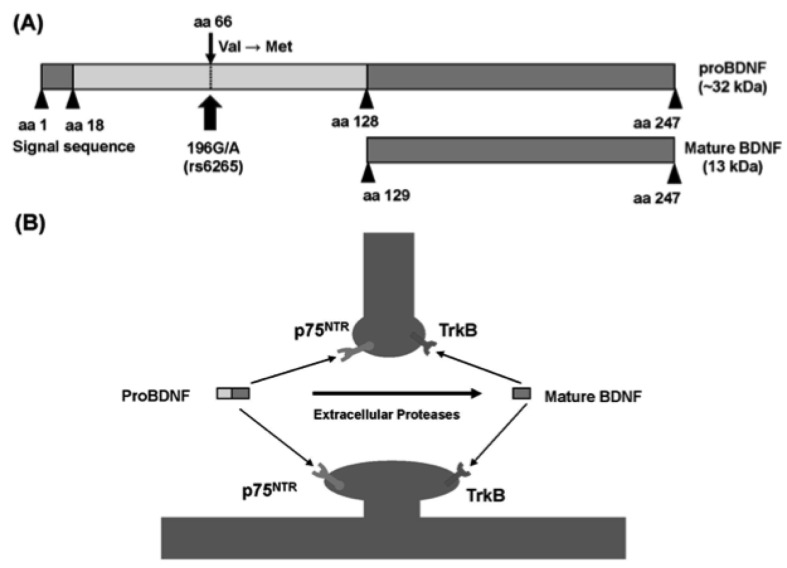
Fig. (3).
Structure of proBDNF and mature BDNF. (A): Arrowheads indicate known protease cleavage sites involved in the processing to mature BDNF. The position of the single nucleotide polymorphism (rs6265, Val66Met) in human BDNF is indicated by arrows. (B): Extrasynaptic cleavage of proBDNF to mature BDNF. ProBDNF preferentially binds p75NTR. ProBDNF is cleaved by extracellular proteases at synapses and converted to mature BDNF. Mature BDNF preferentially binds the TrkB receptor. This figure is a modified version taken from a previous report (88).
The BDNF gene encodes a precursor peptide, proBDNF. Mature BDNF is initially synthesized as a precursor protein (preproBDNF) in the endoplasmic reticulum. Following cleavage of the signal peptide, proBDNF is transported to the Golgi for sorting into either constitutive or regulated secretory vesicles. The proBDNF is converted to mature BDNF by intracellular and extracellular proteases, and this mature form binds to TrkB [86]. It was originally thought that only secreted, mature BDNF possessed biological activity, and that proBDNF, localized exclusively in intracellular vesicles, served as an inactive precursor. However, accumulating evidence suggest that proBDNF may also be biologically active. ProBDNF might induce neuronal apoptosis, via activation of the p75NTR receptor. Taken together, these findings suggest that proBDNF and BDNF elicit opposing effects via p75NTR and TrkB receptors, respectively. Thus, both proBDNF and BDNF are instrumental in several key physiological functions [86-91] (Fig. 3).
Impact of Pro-inflammatory Cytokines on BDNF
Several in vivo studies demonstrated the effect of inflammation on the expression of BDNF in the brain. Administration of LPS or pro-inflammatory cytokines results in a significant reduction of BDNF gene and protein expression [38, 92]. Intraperitoneal injection of IL-1β or LPS significantly decreases BDNF mRNA levels in the rat hippocampus [93], with similar reductions in several cortical regions [94, 95]. Recently, we reported that LPS significantly decreased BDNF protein levels in the prefrontal cortex (PFC) and hippocampus, whereas LPS significantly increased these levels in the nucleus accumbens (NAc) [38]. In rats, expression of Bdnf gene exons I, II, and IV was reduced in CA1 and DG of the hippocampus after acute treatment with E. coli [96], suggesting that inflammation may affect specific isoforms of the Bdnf gene. However, there is no detail information about the effects of inflammation on the expression of specific BDNF transcripts. Further studies are needed to study the precise mechanisms underlying the modulation of BDNF by immune and inflammatory processes.
BDNF-TrkB Signaling in Prefrontal Cortex and Hippocampus
Multiple lines of evidence suggest that BDNF is integral to both the pathophysiology of depression and the therapeutic mechanisms of antidepressants [97-104]. Reports have documented antidepressant-like effects for BDNF in animal models of depression. First, infusion of BDNF into the midbrain and a single bilateral infusion of BDNF into the DG and CA3 pyramidal cell layers of the hippocampus produced antidepressant effects in the rat model of learned helplessness [105, 106]. The antidepressant effects were observed as early as three days after a single BDNF infusion, and lasted for at least 10 days [106]. In addition, infusion of the broad-spectrum Trk inhibitor, K252a, blocked these antidepressant effects, suggesting that BDNF-TrkB signaling was pivotal to the therapeutic action of antidepressants [106].
Loss of BDNF in the forebrain attenuated the actions of antidepressants [107], while responses typically elicited by antidepressants were lost in mice with either reduced brain BDNF levels, or inhibited TrkB signaling [108, 109]. A viral-mediated gene transfer approach found that BDNF in the DG was most likely the prime candidate for mediating the therapeutic effect of antidepressants [110]. Studies using postmortem brain samples showed reduced BDNF protein in the hippocampus and PFC of psychiatric disorder patients who had committed suicide, compared with non-psychiatric controls [111, 112]. Recently, we reported that LPS-induced inflammation reduced BDNF in CA3 and DG of the hippocampus and PFC, resulting in depression-like behavior in mice [38]. In the learned helplessness model of rats, BDNF levels in the PFC, and CA3 and DG of the hippocampus were significantly lower in depressed rats compared with control and resilient rats, implying that in the brain, regional differences in BDNF levels may promote resilience to inescapable stress [113]. This provides more evidence that decreased levels of BDNF in forebrain regions, such as hippocampus and PFC may contribute to the pathophysiology of depression.
BDNF-TrkB Signaling in the Ventral Tegmental Area (VTA)-nucleus Accumbens (NAc)
Nestler’s group showed that increased BDNF in the ventral tegmental area (VTA) - nucleus accumbens (NAc) pathway plays a role in the onset of depression [114, 115]. Intra-VTA infusions of BDNF resulted in a 57 percent shorter latency to immobility, relative to controls (a depression-like phenotype) [116]. Additionally, rats received intra-NAc injections of a virus expressing a truncated version of the BDNF receptor had an almost fivefold longer latency to immobility, compared to rats that had received a vehicle injection, or a virus expressing the full-length BDNF receptor (an antidepressant-like effect) [116]. Using a viral-mediated, mesolimbic dopamine pathway-specific BDNF knockdown, Berton et al. [116] identified an essential role for BDNF in this pathway, in the depression-like behavior seen after social defeat stress. These findings highlight BDNF activity in the VTA-NAc pathway as necessary for the development of a depressive-like phenotype [114, 116]. A study using postmortem brain samples showed that the levels of BDNF protein in the NAc of patients with depression are increased, relative to controls (about 40 percent) [117]. Recently, we reported that LPS-induced inflammation increased BDNF levels in the NAc, resulting in depression-like phenotype in mice [38]. In the rat learned helplessness model, we found that BDNF levels in the NAc were significantly higher in depressed rats compared with control and resilient rats, suggesting again that regional differences in BDNF levels may promote resilience to inescapable stress [113].
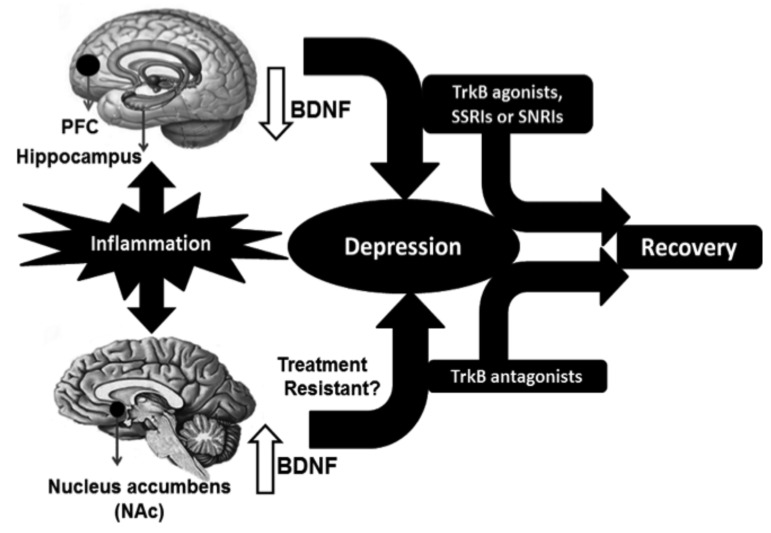
Taken together, these findings suggest that BDNF acts within the VTA-NAc pathway to induce a depression-like phenotype, whereas in the hippocampus and PFC, it produces antidepressant-like effects [99, 100, 38, 115, 118] (Fig. 4).
Fig. (4).
Schematic outline for the proposed use of TrkB ligands as novel therapeutic drugs in depression. In preclinical studies, inflammation promotes reduced BDNF in the PFC and hippocampus, as well as increased BDNF in the NAc of the brain, resulting in a depression-like phenotype in rodents. From preclinical data, we propose that TrkB agonists and current antidepressants, such as SSRIs and SNRIs could be effective therapeutic drugs for depressed patients with decreased levels of BDNF in the PFC and hippocampus. In contrast, TrkB antagonists confer potential therapeutic benefits for patients with treatment-resistant depression showing increased BDNF levels in the VTA-NAc pathway.
BDNF-TrkB Signaling as a Novel Therapeutic Target in Depression
The aforementioned findings imply that reduced levels of BDNF in DG and CA3 of the hippocampus and PFC, as well as increased levels of BDNF in the NAc could promote depression-like phenotype in rodents. In a recent study, we reported that the TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF) [119-121] and TrkB antagonist, ANA-12 [120-122], show antidepressant effects on depression-like phenotype and morphological changes in mice, after LPS administration [38]. Dosing with 7,8-DHF promoted antidepressant effects in LPS-induced depression-like phenotype, while pretreatment with ANA-12 blocked this activity. Surprisingly, we found that ANA-12 alone elicited antidepressant-like effects on LPS-induced depression-like phenotype, and that bilateral infusion of ANA-12 into the NAc produced antidepressant activity in this model [38]. Interestingly, 7,8-DHF significantly improved the reduced phosphorylation of TrkB in the PFC, CA3, and DG seen in LPS-treated mice, whereas ANA-12 significantly improved the increased phosphorylation of TrkB in the NAc of these mice [38]. Together these results point to the possibility that LPS-induced inflammation causes depression-like phenotype by altering BDNF levels and spine density in CA3, DG, PFC, and NAc; areas most likely targeted in the antidepressant action of both 7,8-DHF and ANA-12 [38]. Very recently, we found that direct bilateral infusion of 7,8-DHF (but not ANA-12) into the hippocampus (CA3 and DG) and PFC, and direct bilateral infusions of ANA-12 (but not 7,8-DHF) into the NAc promoted antidepressant activity in the rat learned helplessness model [123], implying that stimulation at TrkB in CA3 and DG of the hippocampus and PFC, and blockade of TrkB in the NAc confer antidepressant effects. Interestingly, 7,8-DHF significantly improved decreased phosphorylation of TrkB in the PFC, CA3, and DG whereas ANA-12 significantly improved increased phosphorylation of TrkB in the NAc [123].
In summary, it is likely that decreased levels of BDNF in DG and CA3 of the hippocampus and PFC, and conversely, increased levels of BDNF in the NAc may promote depression-like phenotype (Fig. 4). We found that similar to ketamine, a single administration of TrkB ligands (7,8-DHF and ANA-12) conferred a rapid antidepressant effect in the social defeat stress model of depression [124]. However, unlike ketamine, the antidepressant effects of 7,8-DHF and ANA-12 in this model were not detectable seven days after a single dosing [124], implying that ketamine is a longer-lasting antidepressant than TrkB ligands. The rapid-onset and sustained antidepressant effects of ketamine will be highly interesting characteristics in the clinical setting.
From this perspective, we would like to propose the use of TrkB ligands as potential therapeutic antidepressant drugs (Fig. 4). Post-mortem data from depressed patients identified an association between depression and decreased BDNF levels in the hippocampus and PFC, and increased in BDNF in the NAc [111, 112, 117, 118]. From our results, TrkB agonists could represent effective therapeutic drugs for depressed patients with decreased BDNF levels in the hippocampus and PFC. In addition, these findings add weight to the theory that TrkB antagonists can act as therapeutic agents for treatment-resistant depression in patients who show increased BDNF-TrkB signaling in the VTA-NAc pathway (Fig. 4) [125].
CONCLUSION
As described above, peripheral and central inflammation might be associated with onset of depressive symptoms in rodents and patients with depression. It is, therefore, highly plausible that anti-inflammatory drugs could ameliorate these symptoms in depression patients. Accumulating evidence suggest that decreased BDNF-TrkB signaling in PFC and CA3 and DG of the hippocampus, as well as increased BDNF-TrkB signaling in the VTA-NAc pathway plays a role in the pathophysiology of depression. Finally, TrkB agonists and TrkB antagonists have the potential to act as therapeutic drugs for patients with lowered BDNF in the hippocampus and PFC, and those with raised BDNF in the NAc, respectively.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Grant-in-Aid for Scientific Research on Innovative Areas of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to K.H. 24116006).
LIST OF ABBREVIATIONS
- ANA-12
N2-(2-{[(2-oxoazepan-3-yl) amino] carbonyl}phenyl) benzo[b]thiophene-2-carboxamide
- BDNF
Brain-derived neurotrophic factor
- DG
Dentate gyrus
- 7,8-DHF
7,8-Dihydroxyflavone
- INF-γ
Interferon-γ
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-10
Interlukin-10
- LPS
Lipopolysaccharide
- NAc
Nucleus accumbens
- PFC
Prefrontal cortex
- TrkB
tropomycin receptor kinase B
- VTA
Ventral tegmental area
CONFLICT OF INTEREST
Dr. Hashimoto has served as a scientific consultant to Astellas, Dainippon-Sumitomo and Taisho, and he has also received research support from Abbvie, Dainippon-Sumitomo, Mochida, Otsuka, and Taisho. The other authors declare no conflict of interest.
REFERENCES
- 1.Kessler R.C., McGonagle K.A., Zhao S., Nelson C.B., Hughes M., Eshleman S., Wittchen H.U., Kendler K.S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch. Gen. Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [http://dx.doi.org/10.1001/ archpsyc.1994.03950010008002]. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi M.H., Rush A.J., Wisniewski S.R., Nierenberg A.A., Warden D., Ritz L., Norquist G., Howland R.H., Lebowitz B., McGrath P.J., Shores-Wilson K., Biggs M.M., Balasubramani G.K., Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [http://dx.doi.org/10.1176/appi.ajp.163.1.28]. [PMID: 16390886]. [DOI] [PubMed] [Google Scholar]
- 3.Jick H., Kaye J.A., Jick S.S. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292(3):338–343. doi: 10.1001/jama.292.3.338. [http://dx.doi. org/10.1001/jama.292.3.338]. [PMID: 15265848]. [DOI] [PubMed] [Google Scholar]
- 4.Simon G.E., Savarino J., Operskalski B., Wang P.S. Suicide risk during antidepressant treatment. Am. J. Psychiatry. 2006;163(1):41–47. doi: 10.1176/appi.ajp.163.1.41. [http://dx.doi.org/10.1176/appi.ajp.163.1.41]. [PMID: 16390887]. [DOI] [PubMed] [Google Scholar]
- 5.Duman R.S., Li N., Liu R.J., Duric V., Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41. doi: 10.1016/j.neuropharm.2011.08.044. [http://dx.doi.org/10. 1016/j.neuropharm.2011.08.044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res. Brain Res. Rev. 2009;61(2):105–123. doi: 10.1016/j.brainresrev.2009.05.005. [http://dx.doi.org/10.1016/j.brainresrev.2009.05.005]. [PMID: 19481572]. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev. Neurother. 2011;11(1):33–36. doi: 10.1586/ern.10.176. [http://dx.doi.org/10.1586/ern.10.176]. [PMID: 21158553]. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K., Malchow B., Falkai P., Schmitt A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(5):367–377. doi: 10.1007/s00406-013-0399-y. [http://dx.doi.org/10.1007/s00406-013-0399-y]. [PMID: 23455590]. [DOI] [PubMed] [Google Scholar]
- 9.Aan Het Rot M., Zarate C.A., Jr, Charney D.S., Mathew S.J. Ketamine for depression: where do we go from here? Biol. Psychiatry. 2012;72(7):537–547. doi: 10.1016/j.biopsych.2012.05.003. [http://dx.doi.org/10.1016/ j.biopsych.2012.05.003]. [PMID: 22705040]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarate C.A., Jr, Mathews D.C., Furey M.L. Human biomarkers of rapid antidepressant effects. Biol. Psychiatry. 2013;73(12):1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [http://dx.doi.org/10.1016/j.biopsych.2012.11.031]. [PMID: 23374639]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashimoto K. The R-stereoisomer of ketamine as an alternative for ketamine for treatment-resistant major depression. Clin. Psychopharmacol. Neurosci. 2014;12(1):72–73. doi: 10.9758/cpn.2014.12.1.72. [http://dx.doi. org/10.9758/cpn.2014.12.1.72]. [PMID: 24851126]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang C., Hashimoto K. Rapid antidepressant effects and abuse liability of ketamine. Psychopharmacology (Berl.) 2014;231(9):2041–2042. doi: 10.1007/s00213-014-3543-0. [http://dx.doi.org/10.1007/s00213-014-3543-0]. [PMID: 24668037]. [DOI] [PubMed] [Google Scholar]
- 13.Hillhouse T.M., Porter J.H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015;23(1):1–21. doi: 10.1037/a0038550. [http://dx.doi.org/10.1037/ a0038550]. [PMID: 25643025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteggia L.M., Zarate C., Jr Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr. Opin. Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [http://dx.doi.org/10.1016/j.conb. 2014.12.004]. [PMID: 25562451]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [http://dx.doi.org/10.1016/S0006-3223(99)00230-9]. [PMID: 10686270]. [DOI] [PubMed] [Google Scholar]
- 16.Zarate C.A., Jr, Singh J.B., Carlson P.J., Brutsche N.E., Ameli R., Luckenbaugh D.A., Charney D.S., Manji H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [http://dx.doi.org/10.1001/archpsyc.63.8.856]. [PMID: 16894061]. [DOI] [PubMed] [Google Scholar]
- 17.DiazGranados N.; Ibrahim, L.A.; Brutsche, N.E.; Ameli, R.; Henter, I.D.; Luckenbaugh, D.A.; Machado-Vieira, R.; Zarate, C.A., Jr Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J. Clin. Psychiatry. 2010;71(12):1605–1611. doi: 10.4088/JCP.09m05327blu. [http://dx.doi.org/10.4088/JCP.09m05327blu]. [PMID: 20673547]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinstatler L., Youssef N.A. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D. 2015;15(1):37–43. doi: 10.1007/s40268-015-0081-0. [http://dx.doi.org/10.1007/s40268-015-0081-0]. [PMID: 25773961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar R., Fam J., Yeo E.Y., Dawe G.S. Ketamine and suicidal ideation in depression: Jumping the gun? Pharmacol. Res. 2015;99:23–35. doi: 10.1016/j.phrs.2015.05.003. [http://dx.doi.org/10.1016/j.phrs.2015.05.003]. [PMID: 25982932]. [DOI] [PubMed] [Google Scholar]
- 20.Domino E.F. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113(3):678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [PMID: 20693870]. [DOI] [PubMed] [Google Scholar]
- 21.Miller A.H., Maletic V., Raison C.L. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [http://dx.doi. org/10.1016/j.biopsych.2008.11.029]. [PMID: 19150053]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raison C.L., Lowry C.A., Rook G.A. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry. 2010;67(12):1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [http://dx.doi.org/10.1001/archgenpsychiatry.2010.161]. [PMID: 21135322]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto K. Inflammatory biomarkers as differential predictors of antidepressant response. Int. J. Mol. Sci. 2015;16(4):7796–7801. doi: 10.3390/ijms16047796. [http://dx.doi.org/10.3390/ijms16047796]. [PMID: 25856677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howren M.B., Lamkin D.M., Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [http://dx.doi.org/10.1097/PSY. 0b013e3181907c1b]. [PMID: 19188531]. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.K., Na K.S., Shin K.H., Jung H.Y., Choi S.H., Kim J.B. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(5):1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [http://dx.doi.org/10.1016/j.pnpbp.2007.03.004]. [PMID: 17433516]. [DOI] [PubMed] [Google Scholar]
- 26.Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E.K., Lanctôt K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [http://dx.doi. org/10.1016/j.biopsych.2009.09.033]. [PMID: 20015486]. [DOI] [PubMed] [Google Scholar]
- 27.Strawbridge R., Arnone D., Danese A., Papadopoulos A. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015 doi: 10.1016/j.euroneuro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Shelton R.C., Claiborne J., Sidoryk-Wegrzynowicz M., Reddy R., Aschner M., Lewis D.A., Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol. Psychiatry. 2011;16(7):751–762. doi: 10.1038/mp.2010.52. [http:// dx.doi.org/10.1038/mp.2010.52]. [PMID: 20479761]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 2009;24(1):27–53. doi: 10.1007/s11011-008-9118-1. [http://dx.doi.org/10.1007/s11011-008-9118-1]. [PMID: 19085093]. [DOI] [PubMed] [Google Scholar]
- 30.Sluzewska A., Rybakowski J.K., Laciak M., Mackiewicz A., Sobieska M., Wiktorowicz K. Interleukin-6 serum levels in depressed patients before and after treatment with fluoxetine. Ann. N. Y. Acad. Sci. 1995;762:474–476. doi: 10.1111/j.1749-6632.1995.tb32372.x. [http://dx.doi.org/10.1111/ j.1749-6632.1995.tb32372.x]. [DOI] [PubMed] [Google Scholar]
- 31.Lin A., Song C., Kenis G., Bosmans E., De Jongh R., Scharpé S., Maes M. The in vitro immunosuppressive effects of moclobemide in healthy volunteers. J. Affect. Disord. 2000;58(1):69–74. doi: 10.1016/s0165-0327(99)00076-2. [http://dx.doi.org/10.1016/S0165-0327(99)00076-2]. [PMID: 10760560]. [DOI] [PubMed] [Google Scholar]
- 32.Maes M., Song C., Lin A.H., Bonaccorso S., Kenis G., De Jongh R., Bosmans E., Scharpé S. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20(4):370–379. doi: 10.1016/S0893-133X(98)00088-8. [http://dx.doi.org/10.1016/S0893-133X(98) 00088-8]. [PMID: 10088138]. [DOI] [PubMed] [Google Scholar]
- 33.Kubera M., Lin A.H., Kenis G., Bosmans E., van Bockstaele D., Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J. Clin. Psychopharmacol. 2001;21(2):199–206. doi: 10.1097/00004714-200104000-00012. [http://dx. doi.org/10.1097/00004714-200104000-00012]. [PMID: 11270917]. [DOI] [PubMed] [Google Scholar]
- 34.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [http://dx.doi.org/ 10.1006/brbi.2000.0613]. [DOI] [PubMed] [Google Scholar]
- 35.Ohgi Y., Futamura T., Kikuchi T., Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2013;103(4):853–859. doi: 10.1016/j.pbb.2012.12.003. [http://dx. doi.org/10.1016/j.pbb.2012.12.003]. [PMID: 23262300]. [DOI] [PubMed] [Google Scholar]
- 36.O’Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., Kelley K.W., Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [http://dx.doi.org/10.1038/sj.mp.4002148]. [PMID: 18195714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma M., Ren Q., Zhang J.C., Hashimoto K. Effects of brilliant blue G on serum tumor necrosis factor-α levels and depression-like behavior in mice after lipopolysaccharide administration. Clin. Psychopharmacol. Neurosci. 2014;12(1):31–36. doi: 10.9758/cpn.2014.12.1.31. [http://dx. doi.org/10.9758/cpn.2014.12.1.31]. [PMID: 24851118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J.C., Wu J., Fujita Y., Yao W., Ren Q., Yang C., Li S.X., Shirayama Y., Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2015;18(4):pyu077. doi: 10.1093/ijnp/pyu077. [http://dx.doi.org/10.1093/ijnp/pyu077]. [PMID: 25628381]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao W., Zhang J.C., Dong C., Zhuang C., Hirota S., Inanaga K., Hashimoto K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015;136:7–12. doi: 10.1016/j.pbb.2015.06.012. [http://dx.doi.org/10.1016/j.pbb.2015. 06.012]. [PMID: 26150007]. [DOI] [PubMed] [Google Scholar]
- 40.Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12(2):123–137. doi: 10.1016/s0149-7634(88)80004-6. [http://dx.doi.org/ 10.1016/S0149-7634(88)80004-6]. [PMID: 3050629]. [DOI] [PubMed] [Google Scholar]
- 41.Smith R.S. The macrophage theory of depression. Med. Hypotheses. 1991;35(4):298–306. doi: 10.1016/0306-9877(91)90272-z. [http://dx.doi.org/10.1016/0306-9877(91)90272-Z]. [PMID: 1943879]. [DOI] [PubMed] [Google Scholar]
- 42.Kent S., Bluthé R.M., Kelley K.W., Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 1992;13(1):24–28. doi: 10.1016/0165-6147(92)90012-u. [http://dx.doi.org/10.1016/0165-6147(92) 90012-U]. [PMID: 1542935]. [DOI] [PubMed] [Google Scholar]
- 43.Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann. N. Y. Acad. Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [b]. [DOI] [PubMed] [Google Scholar]
- 44.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711(1-2):163–174. doi: 10.1016/0006-8993(95)01415-2. [http://dx.doi.org/10.1016/ 0006-8993(95)01415-2]. [DOI] [PubMed] [Google Scholar]
- 45.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [http://dx.doi.org/10.1038/nrn2297]. [PMID: 18073775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L., Shirayama Y., Iyo M., Hashimoto K. Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology. 2007;32(9):2004–2010. doi: 10.1038/sj.npp.1301313. [http://dx.doi.org/10.1038/sj.npp.1301313]. [PMID: 17228338]. [DOI] [PubMed] [Google Scholar]
- 47.Fujita Y., Ishima T., Kunitachi S., Hagiwara H., Zhang L., Iyo M., Hashimoto K. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antibiotic drug minocycline. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(2):336–339. doi: 10.1016/j.pnpbp.2007.08.031. [http://dx.doi.org/10.1016/j.pnpbp.2007.08.031].[PMID: 17884273]. [DOI] [PubMed] [Google Scholar]
- 48.Levkovitz Y., Mendlovich S., Riwkes S., Braw Y., Levkovitch-Verbin H., Gal G., Fennig S., Treves I., Kron S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry. 2010;71(2):138–149. doi: 10.4088/JCP.08m04666yel. [http://dx.doi.org/10.4088/JCP.08m04666yel]. [PMID: 19895780]. [DOI] [PubMed] [Google Scholar]
- 49.Chaudhry I.B., Hallak J., Husain N., Minhas F., Stirling J., Richardson P., Dursun S., Dunn G., Deakin B. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J. Psychopharmacol. (Oxford) 2012;26(9):1185–1193. doi: 10.1177/0269881112444941. [http://dx.doi.org/10.1177/0269881112444941].[PMID: 22526685]. [DOI] [PubMed] [Google Scholar]
- 50.Chaves C., Marque C.R., Maia-de-Oliveira J.P., Wichert-Ana L., Ferrari T.B., Santos A.C., Araújo D., Machado-de-Sousa J.P., Bressan R.A., Elkis H., Crippa J.A., Guimarães F.S., Zuardi A.W., Baker G.B., Dursun S.M., Hallak J.E. Effects of minocycline add-on treatment on brain morphometry and cerebral perfusion in recent-onset schizophrenia. Schizophr. Res. 2015;161(2-3):439–445. doi: 10.1016/j.schres.2014.11.031. [http://dx.doi.org/10.1016/j.schres.2014.11.031]. [PMID: 25497439]. [DOI] [PubMed] [Google Scholar]
- 51.Arakawa S., Shirayama Y., Fujita Y., Ishima T., Horio M., Muneoka K., Iyo M., Hashimoto K. Minocycline produced antidepressant-like effects on the learned helplessness rats with alterations in levels of monoamine in the amygdala and no changes in BDNF levels in the hippocampus at baseline. Pharmacol. Biochem. Behav. 2012;100(3):601–606. doi: 10.1016/j.pbb.2011.09.008. [http://dx.doi.org/ 10.1016/j.pbb.2011.09.008]. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L., Kitaichi K., Fujimoto Y., Nakayama H., Shimizu E., Iyo M., Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(8):1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [http://dx.doi.org/10.1016/j.pnpbp.2006.05.015]. [PMID: 16839653]. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L., Shirayama Y., Shimizu E., Iyo M., Hashimoto K. Protective effects of minocycline on 3,4-methylenedioxy- methamphetamine-induced neurotoxicity in serotonergic and dopaminergic neurons of mouse brain. Eur. J. Pharmacol. 2006;544(1-3):1–9. doi: 10.1016/j.ejphar.2006.05.047. [http://dx.doi.org/10.1016/j.ejphar.2006.05.047]. [PMID: 16859675]. [DOI] [PubMed] [Google Scholar]
- 54.Hashimoto K., Tsukada H., Nishiyama S., Fukumoto D., Kakiuchi T., Iyo M. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol. Psychiatry. 2007;61(5):577–581. doi: 10.1016/j.biopsych.2006.03.019. [http://dx.doi.org/10.1016/j.biopsych.2006.03.019]. [PMID: 16712806]. [DOI] [PubMed] [Google Scholar]
- 55.Fujita Y., Kunitachi S., Iyo M., Hashimoto K. The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol. Biochem. Behav. 2012;101(2):303–306. doi: 10.1016/j.pbb.2012.01.005. [http://dx.doi.org/10.1016/j.pbb.2012.01.005]. [PMID: 22260872]. [DOI] [PubMed] [Google Scholar]
- 56.Sofuoglu M., Mooney M., Kosten T., Waters A., Hashimoto K. Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology (Berl.) 2011;213(1):61–68. doi: 10.1007/s00213-010-2014-5. [http://dx.doi.org/10.1007/s00213-010-2014-5]. [PMID: 20838775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martín-de-Saavedra M.D., Budni J., Cunha M.P., Gómez-Rangel V., Lorrio S., Del Barrio L., Lastres-Becker I., Parada E., Tordera R.M., Rodrigues A.L., Cuadrado A., López M.G. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. 2013;38(10):2010–2022. doi: 10.1016/j.psyneuen.2013.03.020. [http://dx.doi.org/10.1016/j.psyneuen.2013.03.020]. [PMID: 23623252]. [DOI] [PubMed] [Google Scholar]
- 58.Guo J., Lin P., Zhao X., Zhang J., Wei X., Wang Q., Wang C. Etazolate abrogates the lipopolysaccharide (LPS)-induced downregulation of the cAMP/pCREB/BDNF signaling, neuroinflammatory response and depressive-like behavior in mice. Neuroscience. 2014;263:1–14. doi: 10.1016/j.neuroscience.2014.01.008. [http://dx.doi.org/10.1016/j.neuroscience.2014.01.008]. [PMID: 24434771]. [DOI] [PubMed] [Google Scholar]
- 59.Young J.J., Bruno D., Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [http://dx.doi.org/10.1016/j.jad.2014.07.032]. [PMID: 25128861]. [DOI] [PubMed] [Google Scholar]
- 60.Black C., Miller B.J. Meta-analysis of cytokines and chemokines in suicidality: distinguishing suicidal versus nonsuicidal patients. Biol. Psychiatry. 2015;78(1):28–37. doi: 10.1016/j.biopsych.2014.10.014. [http://dx.doi.org/10.1016/j.biopsych.2014.10.014]. [DOI] [PubMed] [Google Scholar]
- 61.Yang J.J., Wang N., Yang C., Shi J.Y., Yu H.Y., Hashimoto K. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol. Psychiatry. 2015;77(3):e19–e20. doi: 10.1016/j.biopsych.2014.06.021. [http://dx.doi. org/10.1016/j.biopsych.2014.06.021]. [PMID: 25104172]. [DOI] [PubMed] [Google Scholar]
- 62.Hodes G.E., Pfau M.L., Leboeuf M., Golden S.A., Christoffel D.J., Bregman D., Rebusi N., Heshmati M., Aleyasin H., Warren B.L., Lebonté B., Horn S., Lapidus K.A., Stelzhammer V., Wong E.H., Bahn S., Krishnan V., Bolaños-Guzman C.A., Murrough J.W., Merad M., Russo S.J. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl. Acad. Sci. USA. 2014;111(45):16136–16141. doi: 10.1073/pnas.1415191111. [http://dx.doi.org/10.1073/pnas.1415191111]. [PMID: 25331895]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang C., Shirayama Y., Zhang J.C., Ren Q., Hashimoto K. Peripheral interleukin-6 promotes resilience versus susceptibility to inescapable electric stress. Acta Neuropsychiatr. 2015;27(5):312–316. doi: 10.1017/neu.2015.36. [http://dx.doi.org/10.1017/neu.2015.36]. [PMID: 26017899]. [DOI] [PubMed] [Google Scholar]
- 64.Fonseka T.M., McIntyre R.S., Soczynska J.K., Kennedy S.H. Novel investigational drugs targeting IL-6 signaling for the treatment of depression. Expert Opin. Investig. Drugs. 2015;24(4):459–475. doi: 10.1517/13543784.2014.998334. [http://dx.doi.org/10.1517/13543784.2014.998334]. [PMID: 25585966]. [DOI] [PubMed] [Google Scholar]
- 65.Yang C., Hashimoto K. Peripheral IL-6 signaling: a promising therapeutic target for depression? Expert Opin. Investig. Drugs. 2015;24(7):989–990. doi: 10.1517/13543784.2015.1055669. [http://dx.doi.org/10.1517/13543784.2015. 1055669]. [PMID: 26044205]. [DOI] [PubMed] [Google Scholar]
- 66.Müller N., Schwarz M.J. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol. Psychiatry. 2007;12(11):988–1000. doi: 10.1038/sj.mp.4002006. [http://dx.doi.org/ 10.1038/sj.mp.4002006]. [DOI] [PubMed] [Google Scholar]
- 67.Wichers M.C., Koek G.H., Robaeys G., Verkerk R., Scharpe´ S., Maes M. IDO and interferon-a-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol. Psychiatry. 2005;10(6):538–544. doi: 10.1038/sj.mp.4001600. [http://dx.doi.org/ 10.1038/sj.mp.4001600]. [DOI] [PubMed] [Google Scholar]
- 68.Stone T.W. Endogenous neurotoxins from tryptophan. Toxicon. 2001;39(1):61–73. doi: 10.1016/s0041-0101(00)00156-2. [http://dx.doi.org/10.1016/S0041-0101(00) 00156-2]. [PMID: 10936623]. [DOI] [PubMed] [Google Scholar]
- 69.Okuda S., Nishiyama N., Saito H., Katsuki H. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [http://dx.doi.org/ 10.1046/j.1471-4159.1998.70010299.x]. [PMID: 9422375]. [DOI] [PubMed] [Google Scholar]
- 70.Santamaría A., Galván-Arzate S., Lisý V., Ali S.F., Duhart H.M., Osorio-Rico L., Ríos C., St’astný F. Quinolinic acid induces oxidative stress in rat brain synaptosomes. Neuroreport. 2001;12(4):871–874. doi: 10.1097/00001756-200103260-00049. [http://dx.doi.org/10.1097/00001756-200103260-00049]. [PMID: 11277599]. [DOI] [PubMed] [Google Scholar]
- 71.Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002;1(8):609–620. doi: 10.1038/nrd870. [http://dx.doi.org/10.1038/nrd870]. [PMID: 12402501]. [DOI] [PubMed] [Google Scholar]
- 72.Walker A.K., Budac D.P., Bisulco S., Lee A.W., Smith R.A., Beenders B., Kelley K.W., Dantzer R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology. 2013;38(9):1609–1616. doi: 10.1038/npp.2013.71. [http://dx.doi.org/10.1038/npp.2013.71]. [PMID: 23511700]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hashimoto K., Koike K., Shimizu E., Iyo M. α7 Nicotinic receptor agonists as potential therapeutic drugs for schizophrenia. Curr. Med. Chem. -. CNS Agents. 2005;5:171–184. [Google Scholar]
- 74.Toyohara J., Hashimoto K. α7 Nicotinic receptor agonists: potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and Alzheimer's disease. Open Med. Chem. J. 2010;4:37–56. doi: 10.2174/1874104501004010037. [PMID: 21249164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Erhardt S., Olsson S.K., Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23(2):91–101. doi: 10.2165/00023210-200923020-00001. [http://dx. doi.org/10.2165/00023210-200923020-00001]. [PMID: 19173370]. [DOI] [PubMed] [Google Scholar]
- 76.Ishikawa M., Hashimoto K. α7 nicotinic acetylcholine receptor as a potential therapeutic target for schizophrenia. Curr. Pharm. Des. 2011;17(2):121–129. doi: 10.2174/138161211795049561. [http://dx.doi.org/10.2174/138161211795049561]. [PMID: 21355839]. [DOI] [PubMed] [Google Scholar]
- 77.Hashimoto K. Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin. Ther. Targets. 2014;18(9):1049–1063. doi: 10.1517/14728222.2014.934225. [http://dx.doi.org/10.1517/14728222.2014.934225]. [PMID: 24965576]. [DOI] [PubMed] [Google Scholar]
- 78.Hashimoto K. Tropisetron and its targets in Alzheimer’s disease. Expert Opin. Ther. Targets. 2015;19(1):1–5. doi: 10.1517/14728222.2014.983901. [http://dx.doi.org/ 10.1517/14728222.2014.983901]. [PMID: 25399811]. [DOI] [PubMed] [Google Scholar]
- 79.Hashimoto K. Targeting of α7 nicotinic acetylcholine receptors in the treatment of schizophrenia and the use of auditory sensory gating as a translational biomarker. Curr. Pharm. Des. 2015;21(26):3797–3806. doi: 10.2174/1381612821666150605111345. [http://dx.doi.org/10.2174/1381612821666150605111345]. [PMID: 26044974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barde Y.A., Davies A.M., Johnson J.E., Lindsay R.M., Thoenen H. Brain derived neurotrophic factor. Prog. Brain Res. 1987;71:185–189. doi: 10.1016/s0079-6123(08)61823-3. [http://dx.doi.org/10.1016/S0079-6123(08)61823-3]. [PMID: 3588941]. [DOI] [PubMed] [Google Scholar]
- 81.Schinder A.F., Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23(12):639–645. doi: 10.1016/s0166-2236(00)01672-6. [http://dx.doi. org/10.1016/S0166-2236(00)01672-6]. [PMID: 11137155]. [DOI] [PubMed] [Google Scholar]
- 82.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [http://dx.doi.org/10.1146/annurev.neuro.24.1.677]. [PMID: 11520916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu B., Pang P.T., Woo N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005;6(8):603–614. doi: 10.1038/nrn1726. [http://dx.doi.org/ 10.1038/nrn1726]. [PMID: 16062169]. [DOI] [PubMed] [Google Scholar]
- 84.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [http://dx.doi.org/10.1098/rstb.2006.1894]. [PMID: 16939974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malcangio M., Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends Pharmacol. Sci. 2003;24(3):116–121. doi: 10.1016/S0165-6147(03)00025-7. [http://dx. doi.org/10.1016/S0165-6147(03)00025-7]. [PMID: 12628355]. [DOI] [PubMed] [Google Scholar]
- 86.Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron. 2003;39(5):735–738. doi: 10.1016/s0896-6273(03)00538-5. [http://dx.doi.org/10.1016/S0896-6273(03)00538-5]. [PMID: 12948441]. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto K. BDNF variant linked to anxiety-related behaviors. BioEssays. 2007;29(2):116–119. doi: 10.1002/bies.20534. [http://dx.doi.org/10.1002/bies. 20534]. [PMID: 17226799]. [DOI] [PubMed] [Google Scholar]
- 88.Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin. Neurosci. 2010;64(4):341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [http://dx.doi. org/10.1111/j.1440-1819.2010.02113.x]. [PMID: 20653908]. [DOI] [PubMed] [Google Scholar]
- 89.Barker P.A. Whither proBDNF? Nat. Neurosci. 2009;12(2):105–106. doi: 10.1038/nn0209-105. [http://dx.doi.org/10.1038/nn0209-105]. [PMID: 19172162]. [DOI] [PubMed] [Google Scholar]
- 90.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [http://dx.doi.org/10.1016/j.pneurobio.2012.09.001]. [DOI] [PubMed] [Google Scholar]
- 91.Deinhardt K., Chao M.V. Shaping neurons: Long and short range effects of mature and proBDNF signalling upon neuronal structure. Neuropharmacology. 2014;76:603–609. doi: 10.1016/j.neuropharm.2013.04.054. [http://dx.doi.org/ 10.1016/j.neuropharm.2013.04.054]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raetz C.R., Whitfield C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [http://dx.doi.org/10.1146/ annurev.biochem.71.110601.135414]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lapchak P.A., Araujo D.M., Hefti F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience. 1993;53(2):297–301. doi: 10.1016/0306-4522(93)90196-m. [http://dx.doi.org/10.1016/0306-4522(93)90196-M]. [PMID: 8492907]. [DOI] [PubMed] [Google Scholar]
- 94.Guan Z., Fang J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav. Immun. 2006;20(1):64–71. doi: 10.1016/j.bbi.2005.04.005. [http://dx.doi.org/10.1016/j.bbi.2005.04.005]. [PMID: 15922558]. [DOI] [PubMed] [Google Scholar]
- 95.Schnydrig S., Korner L., Landweer S., Ernst B., Walker G., Otten U., Kunz D. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [http://dx.doi.org/ 10.1016/j.neulet.2007.09.067]. [DOI] [PubMed] [Google Scholar]
- 96.Chapman T.R., Barrientos R.M., Ahrendsen J.T., Hoover J.M., Maier S.F., Patterson S.L. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol. Aging. 2012;33(4):832.e1–832.e14. doi: 10.1016/j.neurobiolaging.2011.07.015. [http://dx.doi. org/10.1016/j.neurobiolaging.2011.07.015]. [PMID: 21907460]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duman R.S., Heninger G.R., Nestler E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [http://dx.doi.org/10.1001/archpsyc.1997.01830190015002]. [PMID: 9236543]. [DOI] [PubMed] [Google Scholar]
- 98.Altar C.A. Neurotrophins and depression. Trends Pharmacol. Sci. 1999;20(2):59–61. doi: 10.1016/s0165-6147(99)01309-7. [http://dx.doi.org/10.1016/S0165-6147(99) 01309-7]. [PMID: 10101965]. [DOI] [PubMed] [Google Scholar]
- 99.Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [http://dx.doi.org/10.1016/S0896-6273(02)00653-0]. [PMID: 11931738]. [DOI] [PubMed] [Google Scholar]
- 100.Hashimoto K., Shimizu E., Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res. Brain Res. Rev. 2004;45(2):104–114. doi: 10.1016/j.brainresrev.2004.02.003. [http://dx.doi.org/10.1016/j.brainresrev. 2004.02.003]. [PMID: 15145621]. [DOI] [PubMed] [Google Scholar]
- 101.Hashimoto K. Understanding depression: linking brain-derived neurotrophic factor, transglutaminase 2 and serotonin. Expert Rev. Neurother. 2013;13(1):5–7. doi: 10.1586/ern.12.140. [http://dx.doi.org/10.1586/ern.12.140]. [PMID: 23253384]. [DOI] [PubMed] [Google Scholar]
- 102.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [pneurobio.].[http://dx.doi.org/10.1016/j.pneurobio.2012.09.001]. [PMID: 23044468]. [DOI] [PubMed] [Google Scholar]
- 103.Martinowich K., Manji H., Lu B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [http://dx.doi.org/10.1038/nn1971].[PMID: 17726474]. [DOI] [PubMed] [Google Scholar]
- 104.Castrén E. Neurotrophins and psychiatric disorders. HandbookExp. Pharmacol. 2014;220:461–461. doi: 10.1007/978-3-642-45106-5_17. [DOI] [PubMed] [Google Scholar]
- 105.Siuciak J.A., Lewis D.R., Wiegand S.J., Lindsay R.M. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [http:// dx.doi.org/10.1016/S0091-3057(96)00169-4]. [PMID: 8981620]. [DOI] [PubMed] [Google Scholar]
- 106.Shirayama Y., Chen A.C., Nakagawa S., Russell D.S., Duman R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [PMID: 11943826]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monteggia L.M., Barrot M., Powell C.M., Berton O., Galanis V., Gemelli T., Meuth S., Nagy A., Greene R.W., Nestler E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [http://dx.doi.org/10.1073/pnas.0402141101]. [PMID: 15249684]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saarelainen T., Hendolin P., Lucas G., Koponen E., Sairanen M., MacDonald E., Agerman K., Haapasalo A., Nawa H., Aloyz R., Ernfors P., Castrén E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [PMID: 12514234]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Monteggia L.M., Luikart B., Barrot M., Theobold D., Malkovska I., Nef S., Parada L.F., Nestler E.J. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry. 2007;61(2):187–197. doi: 10.1016/j.biopsych.2006.03.021. [http://dx.doi.org/10.1016/j.biopsych.2006.03.021]. [PMID: 16697351]. [DOI] [PubMed] [Google Scholar]
- 110.Adachi M., Barrot M., Autry A.E., Theobald D., Monteggia L.M. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry. 2008;63(7):642–649. doi: 10.1016/j.biopsych.2007.09.019. [http://dx.doi.org/10.1016/j.biopsych. 2007.09.019]. [PMID: 17981266]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dwivedi Y., Rizavi H.S., Conley R.R., Roberts R.C., Tamminga C.A., Pandey G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [http://dx.doi.org/10.1001/archpsyc.60.8.804]. [PMID: 12912764]. [DOI] [PubMed] [Google Scholar]
- 112.Karege F., Vaudan G., Schwald M., Perroud N., La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005;136(1-2):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [http://dx.doi.org/ 10.1016/j.molbrainres.2004.12.020]. [PMID: 15893584]. [DOI] [PubMed] [Google Scholar]
- 113.Yang C., Shirayama Y., Zhang J.C., Ren Q., Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int. J. Neuropsychopharmacol. 2015;18(7):pyu121. doi: 10.1093/ijnp/pyu121. [http://dx.doi. org/10.1093/ijnp/pyu121]. [PMID: 25568287]. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Eisch A.J., Bolaños C.A., de Wit J., Simonak R.D., Pudiak C.M., Barrot M., Verhaagen J., Nestler E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry. 2003;54(10):994–1005. doi: 10.1016/j.biopsych.2003.08.003. [http://dx.doi.org/10.1016/j.biopsych.2003.08.003]. [PMID: 14625141]. [DOI] [PubMed] [Google Scholar]
- 115.Nestler E.J., Carlezon W.A., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [http://dx.doi.org/10.1016/j.biopsych.2005.09.018]. [PMID: 16566899]. [DOI] [PubMed] [Google Scholar]
- 116.Berton O., McClung C.A., Dileone R.J., Krishnan V., Renthal W., Russo S.J., Graham D., Tsankova N.M., Bolanos C.A., Rios M., Monteggia L.M., Self D.W., Nestler E.J. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [http://dx.doi.org/10.1126/science.1120972]. [PMID: 16469931]. [DOI] [PubMed] [Google Scholar]
- 117.Krishnan V., Han M.H., Graham D.L., Berton O., Renthal W., Russo S.J., Laplant Q., Graham A., Lutter M., Lagace D.C., Ghose S., Reister R., Tannous P., Green T.A., Neve R.L., Chakravarty S., Kumar A., Eisch A.J., Self D.W., Lee F.S., Tamminga C.A., Cooper D.C., Gershenfeld H.K., Nestler E.J. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [http://dx.doi.org/10.1016/j.cell.2007.09.018]. [PMID: 17956738]. [DOI] [PubMed] [Google Scholar]
- 118.Sun H., Kennedy P.J., Nestler E.J. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38(1):124–137. doi: 10.1038/npp.2012.73. [http://dx.doi.org/ 10.1038/npp.2012.73]. [PMID: 22692567]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jang S.W., Liu X., Yepes M., Shepherd K.R., Miller G.W., Liu Y., Wilson W.D., Xiao G., Blanchi B., Sun Y.E., Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA. 2010;107(6):2687–2692. doi: 10.1073/pnas.0913572107. [http://dx.doi.org/10.1073/pnas.0913572107]. [PMID: 20133810]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ren Q., Zhang J.C., Fujita Y., Ma M., Wu J., Hashimoto K. Effects of TrkB agonist 7,8-dihydroxyflavone on sensory gating deficits in mice after administration of methamphetamine. Pharmacol. Biochem. Behav. 2013;106:124–127. doi: 10.1016/j.pbb.2013.03.016. [http://dx.doi. org/10.1016/j.pbb.2013.03.016]. [DOI] [PubMed] [Google Scholar]
- 121.Ren Q., Zhang J.C., Ma M., Fujita Y., Wu J., Hashimoto K. Protective effects of TrkB agonist 7,8-dihydroxyflavone on the behavioral changes and neurotoxicity in mice after administration of methamphetamine. Psychopharmacology (Berl.) 2014;231(1):159–166. doi: 10.1007/s00213-013-3221-7. [http://dx.doi.org/10.1007/s00213-013-3221-7]. [PMID: 23934209]. [DOI] [PubMed] [Google Scholar]
- 122.Cazorla M., Prémont J., Mann A., Girard N., Kellendonk C., Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Invest. 2011;121(5):1846–1857. doi: 10.1172/JCI43992. [http://dx.doi.org/10.1172/JCI43992]. [PMID: 21505263]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shirayama Y., Yang C., Zhang J.C., Ren Q., Yao W., Hashimoto K. Alterations in brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in the brain regions of a learned helplessness rat model and the antidepressant effects of a TrkB agonist and antagonist. Eur. Neuropsychopharmacol. 2015;25(12):2449–2458. doi: 10.1016/j.euroneuro.2015.09.002. [http://dx.doi.org/10.1016/j.euroneuro.2015. 09.002]. [PMID: 26419294]. [DOI] [PubMed] [Google Scholar]
- 124.Zhang J.C., Yao W., Dong C., Yang C., Ren Q., Ma M., Han M., Hashimoto K. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl.) 2015;232(23):4325–4335. doi: 10.1007/s00213-015-4062-3. [http://dx.doi.org/10.1007/s00213-015-4062-3]. [PMID: 26337614]. [DOI] [PubMed] [Google Scholar]
- 125.Hashimoto K. Brain-derived neurotrophic factor (BDNF) - TrkB signaling in depression: Biomarker and novel therapeutic target. In: Srinivasan V., editor. Melatonin and Neuroprotective Agents in Neuropsychiatric Disorders. [Google Scholar]