Abstract
The autonomic nervous system is one of the major neural pathways activated by stress. In situations that are often associated with chronic stress, such as major depressive disorder, the sympathetic nervous system can be continuously activated without the normal counteraction of the parasympathetic nervous system. As a result, the immune system can be activated with increased levels of pro-inflammatory cytokines. These inflammatory conditions have been repeatedly observed in depression. In the search for the mechanism by which the immune system might contribute to depression, the enhanced activity of indoleamine 2,3-dioxygenase by pro-inflammatory cytokines has been suggested to play an important role. Indoleamine 2,3-dioxygenase is the first enzyme in the kynurenine pathway that converts tryptophan to kynurenine. Elevated activity of this enzyme can cause imbalances in downstream kynurenine metabolites. This imbalance can induce neurotoxic changes in the brain and create a vulnerable glial-neuronal network, which may render the brain susceptible to depression. This review focuses on the interaction between stress, the autonomic nervous system and the immune system which can cause imbalances in the kynurenine pathway, which may ultimately lead to major depressive disorder.
Keywords: Major depressive disorder, stress, autonomic nervous system, immune system, kynurenine pathway
INTRODUCTION
Major depressive disorder (MDD) is a chronic illness which results in functional impairment. It is characterized by frequent relapses, incomplete recovery and residual symptoms [1]. MDD is projected to become the leading cause of disease burden worldwide by 2030 [2]. As illnesses such as heart disease, type 2 diabetes, autoimmune diseases and cancer, are often associated with depression [3], various possibilities have been posited to explain the link between such illnesses and depression. The disruption of the immune system is viewed as the most plausible explanation [1]. For the past few decades, the immune system has been repeatedly associated with depression in numerous ways [4-7]. The inflammatory response system has been shown to be activated in MDD, but the levels of different inflammatory markers vary across studies [8]. Speculations have been made to explain how the immune system is activated in depression, with stress and its effects on the body receiving much of the attention.
Stress is defined as a state of threatened homeostasis following exposure to extrinsic or intrinsic adverse forces [9]. Acute stress refers to stress that occurs for minutes or hours, whereas chronic stress persists for days, weeks, or months [10]. The major pathways activated by stressors are the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS) [11]. Stress has repeatedly been associated with depression, and is reported to precipitate depressive episodes and to influence the severity, duration and natural course of the disorder [12]. Acute and chronic stressors have also been reported to affect the immune system, and an increase in various inflammatory markers has been reported in states of acute stress [13] and chronic stress [14]. A potential interaction between chronic stress and inflammatory cytokine responses to acute stress has also been reported [15].
In the search for the mechanism by which the immune system might contribute to depression, decreased serum tryptophan concentrations were noted in depressed patients who had inflammatory disorders, or who had received cytokine immunotherapy [16, 17]. Tryptophan functions as a biochemical precursor for serotonin, which in turn has been strongly implicated in the pathogenesis of depression [18]. In 1969, Lapin and Oxenkrug proposed that in depression the metabolism of tryptophan is shunted away from serotonin production towards kynurenine production, resulting in tryptophan depletion [19]. Cytokines produced in inflammatory states were considered to induce tryptophan depletion by enhancing the activity of indoleamine 2,3-dioxygenase (IDO), the first enzyme in the kynurenine (KYN) pathway that converts tryptophan to KYN [16, 20]. Based on these findings, the “neurodegeneration hypothesis of depression” was proposed [21] which hypothesized that imbalances in downstream KYN metabolites induce neurotoxic changes that create a vulnerable glial-neuronal network, which may contribute to the development of depression.
This review focuses on the interaction between stress, the ANS and the immune system, which can cause imbalances in the KYN pathway that may ultimately lead to depression. Although stress initially activates both the ANS and the HPA axis, this review will focus on the role of the ANS, which has received much less attention than the HPA axis [22].
STRESS, THE AUTONOMIC NERVOUS SYSTEM AND THE IMMUNE SYSTEM
Brief laboratory stressors, such as mental arithmetic and public speaking tasks, have been reported to induce increases in natural killer cell activity [23]. These increases were potentiated in individuals who were more cardiovascularly reactive to stress [24]. This was interpreted as individuals who showed the greatest sympathetic nervous system (SNS) and endocrine response to brief psychological stressors, also showed increased immune system alterations. Thus, the effect of stress on the neuroendocrine system and how this effect influences the immune system, has become a subject of interest in recent years [25].
Stress, the Sympathetic Nervous System and the Parasympathetic Nervous System
The ANS has two divisions, the SNS and the parasympathetic nervous system (PNS). In response to stressors, the hypothalamus secretes corticotropin-releasing hormone (CRH) and arginine vasopressin. From the paraventricular nucleus of the hypothalamus, CRH-containing neurons have projections to noradrenergic centers in the brainstem and spinal cord. The locus coeruleus of the brainstem sends direct projections to the sympathetic preganglionic neurons in the spinal cord and to the parasympathetic preganglionic neurons in the brainstem and the spinal cord [26]. In general, the locus coeruleus increases sympathetic activity through the activation of α1-adrenoceptors on preganglionic sympathetic neurons [27] and reduces parasympathetic activity through the activation of α2-adrenoceptors on preganglionic parasympathetic neurons [28]. The activation of the SNS in turn stimulates the release of CRH by the hypothalamus, creating a positive bidirectional feedback loop [29].
The principal neurotransmitters of the ANS are norepinephrine (NE), epinephrine (E) and acetylcholine (ACh) [30]. The sympathetic system controls catecholamine biosynthesis and secretion from the adrenal medulla, which is innervated by preganglionic sympathetic fibers of the splanchnic nerve [31]. Medullary cells mainly release E, and to a lesser extent NE, into the blood rather quickly when stimulated by the SNS in response to stressors [31]. Therefore, the principal end products of the SNS are NE and E. The PNS mainly uses ACh as its neurotransmitter [32]. Under normal conditions, the PNS is activated when the stressful situation is alleviated because the SNS and the PNS are highly coordinated to maintain physiological homeostasis [33]. However, in abnormal conditions in which the stressful situations persist, the SNS continues to be activated without the normal counteraction of the PNS. As a result, in chronically stressful situations, peripheral levels of catecholamine can increase and levels of acetylcholine can decrease [9] Fig. (1).
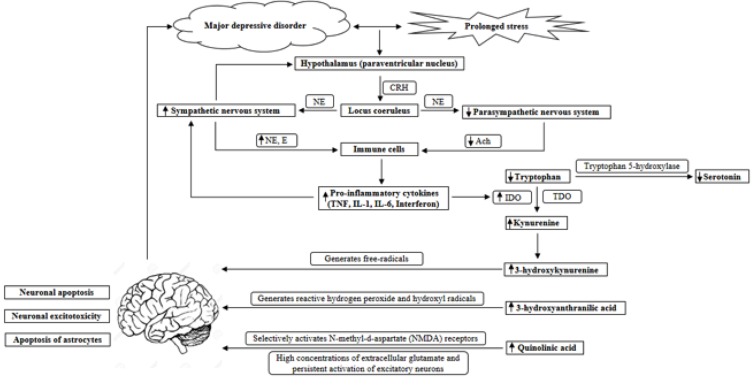
Fig. (1).
The interaction between stress, the autonomic nervous system, the immune system and the kynurenine pathway in the etiology of depression. The hypothalamus secretes CRH in response to stress, and from the paraventricular nucleus of the hypothalamus, CRH-containing neurons have projections to the locus coeruleus. The locus coeruleus sends direct projections to the sympathetic and parasympathetic preganglionic neurons, increasing sympathetic activity and decreasing parasympathetic activity through the activation of adrenoceptors. In turn, the activation of the sympathetic nervous system stimulates the release of CRH. When stress is prolonged, as in major depressive disorder, the sympathetic nervous system continues to be activated, with a lack of parasympathetic counter activity. As a result, NE and E levels are increased and ACh levels are decreased, which lead to an increased release of pro-inflammatory cytokines from immune cells. Pro-inflammatory cytokines, such as TNF, IL-1, IL-6 and interferons can induce IDO activity, which increases the KYN/tryptophan ratio. As a result, downstream metabolites, such as 3-hydroxykynurenine, 3-hydroxyanthranilic acid and quinolinic acid are increased, which all have neurotoxic effects on the brain. 3-hydroxykynurenine generates free-radicals and causes neuronal apoptosis. 3-hydroxyanthranilic acid generates highly reactive hydrogen peroxide and hydroxyl radicals. Quinolinic acid selectively activates N-methyl-d-aspartate (NMDA) receptors, and high concentrations of extracellular glutamate and persistent activation of excitatory neurons cause excitotoxicity. Therefore, the accumulation of quinolinic acid can result in neuronal excitotoxicity and the selective apoptosis of astrocytes. This can ultimately lead to neurodegenerative changes, which may render the brain susceptible to depression.
The Autonomic Nervous System and the Immune System
Inflammatory responses are characterized by a complex interaction between pro- and anti-inflammatory cytokines [34]. E and NE modulate the release of cytokines and inflammation through α- and β-adrenoceptors on immune cells [35]. Results of in vitro and in vivo studies have suggested that NE enhances tumor necrosis factor (TNF) production [36, 37]. Both catecholamines have been reported to stimulate interleukin (IL)-6 release by immune cells and other peripheral cells [38]. NE has also been shown to augment macrophage phagocytosis and tumoricidal activity [39]. In contrast, ACh has been reported to dose-dependently inhibit the release of TNF, along with other pro-inflammatory cytokines such as IL-1, IL-6, and IL-18 [40]. However, the production of IL-10, which is an anti-inflammatory cytokine, was reported to be unaffected by ACh. The inhibition of acetyl-cholinesterase activity, which increases ACh levels in the central nervous system, resulted in the suppression of the immune response, indicating that ACh has an immunoinhibitory role in the brain [41]. When stressful situations are prolonged, adrenergic agents can increase and ACh can decrease, because of the continuous activation of the SNS and the lack of counter activation of the PNS. As a result, pro-inflammatory cytokines, such as TNF, IL-1, and IL-6, can increase in prolonged stressful situations such as depression.
THE IMMUNE SYSTEM AND DEPRESSION
Sickness behavior, which refers to the depression-like symptoms that accompany the response to infection, was reported to be induced by cytokines such as IL-1 [42]. Sickness behavior due to peripheral immune activation was also reported to be reversed by the administration of the IL-1 receptor antagonist, which suggested a link between immune activation and depressive-like behavior. Thereafter, theories on how immune activation and depression were related were proposed. In the macrophage theory of depression, excessive secretion of macrophage monokines, such as IL-1, was proposed as the cause of depression [43]. Subsequent studies on depression in cancer patients receiving cytokine therapy supported the assumption that immune activation can cause depression [44-46]. Improvement in depressive symptoms in patients with moderate to severe psoriasis receiving TNF antagonist treatment was also reported, with improvement being independent of the effect of the treatment on joint pain [47]. Studies of medically healthy subjects reported an association between depressed mood and increased production of pro-inflammatory cytokines such as TNF, IL-1, and IL-6 [48-50]. TNF and IL-6 have also been reported to be elevated in MDD patients [51], and meta-analyses have confirmed IL-6 levels to be elevated in depressed patients [8]. Anti-inflammatory cytokines, such as IL-10, have been reported to be reduced in MDD patients [52].
Based on the evidence stated above, it is clear that a relationship exists between inflammation and depression. However, it is not yet evident how states of chronic inflammation may lead to depression. This could be explained by increased tryptophan degradation due to the enhanced IDO activity of the KYN pathway in inflammatory conditions.
CRH = corticotropin-releasing hormone; NE = norepinephrine; E = epinephrine; ACh = acetylcholine; TNF = tumor necrosis factor; IL-1 = interleukin-1; IL-6 = interleukin-6; IDO = indoleamine 2,3-dioxygenase; KYN = kynurenine.
The Tryptophan Breakdown Metabolic Pathway in Normal Conditions
When two atoms of oxygen are inserted into tryptophan, N-formylkynurenine is formed, which is the first step in the KYN pathway. Approximately only 1% of dietary tryptophan is converted to serotonin, the remaining 99% of tryptophan is metabolized through the KYN pathway [53]. Tryptophan is catalyzed into KYN by three different enzymes, tryptophan 2,3-dioxygenase (TDO), IDO1 and IDO2. However, the biological function of IDO2 is still unclear and needs clarification [54]. TDO is highly substrate-specific and dioxygenates only L- tryptophan and some tryptophan derivatives. The expression of TDO is normally restricted to mammalian liver cells where it is believed to regulate systemic tryptophan concentrations [55]. In normal conditions, the activity of TDO is generally stable and is mainly controlled by the tryptophan level itself [56]. IDO activity in nonpathological conditions is minimal [53]. The KYN pathway is initiated by the oxidative cleavage of the indole-ring of tryptophan by TDO and IDO, forming N-formylkynurenine [57]. KYN is then synthesized and metabolized through one of the three mechanisms. Kynurenine amino-transferase enzymes deaminate KYN, resulting in kynurenic acid. KYN is also degraded by kynureninase resulting in anthranilic acid. Hydroxylation of KYN by kynurenine monooxygenase produces 3-hydroxykynurenine. This 3-hydroxykynurenine is in turn converted into 3-hydroxyanthranilic acid by kynureninase, and then oxidized by 3-hydroxyanthranilic acid oxidase into 2-amino-3-carboxymuconic 6-semialdehyde, which reassembles to form quinolinic acid. Quinolinate phosphoribosyl transferase transaminates quinolinic acid to nicotinic acid and ultimately to NAD+. 2-amino-3-carboxymuconic 6-semialdehyde can also be metabolized and produces picolinic acid through the activity of 2-amino-3-carboxymuconic-6-semialdehyde decarboxylase [54, 56, 57].
TDO and IDO1 levels are much lower in the brain than in the periphery [58], and therefore up to 60% of KYN pathway metabolism in the brain is initiated by the brain penetrant KYN synthesized in the periphery [59]. KYN is further degraded in the brain into different metabolites, depending on the cell type in which KYN is produced or transported [60, 61]. The KYN pathway takes place predominantly in microglia and astrocytes in the central nervous system [57], however the KYN metabolism is separated in the brain, as kynurenine monooxygenase is expressed in microglia but not in astrocytes [62], and kynurenine amino-transferase enzymes are expressed in astrocytes but not in microglia [63]. Therefore, the 3-hydroxykynurenine arm of the pathway leading to quinolinic acid production takes place in microglia, and kynurenic acid production takes place in astrocytes. Also, intact neurons can degrade KYN into picolinic acid [64].
IDO = indoleamine 2,3-dioxygenase; KYN = kynurenine.
The Tryptophan Breakdown Metabolic Pathway in Inflammatory Conditions
IDO1 is expressed in a broad variety of mammalian cells related to immune function, such as activated macrophages and dendritic cells [54]. The first studies comparing the effect of interferons on IDO induction in immunocompetent cells were published by Werner-Felmayer G, et al. [65, 66]. The results of these studies demonstrated that induction of IDO is a common feature of interferon-gamma action, and the extent of this induction is influenced by extracellular L-tryptophan concentrations, and IDO is the only enzyme in the formation of 3-hydroxyanthranilic acid from tryptophan which is regulated by interferon-gamma. In states of inflammation and oxidative stress, proinflammatory cytokines, such as TNF [67] and reactive oxygen species, can induce IDO activity in extrahepatic tissues, such as the lung, placenta, kidney, spleen, blood and brain [68, 69]. The extrahepatic tryptophan metabolism then shifts the tryptophan metabolism away from the liver [70], and a large amount of tryptophan degradation takes place in the blood and lymphoid tissues through the KYN pathway [71]. Therefore, activation of IDO1 increases the KYN/tryptophan ratio [72]. Enhanced IDO activity during inflammation is initially a reflex mechanism that suppresses inflammatory reactions [56]. Tryptophan depletion through IDO activation induces a balance between pro-inflammatory T helper 17 cells and anti-inflammatory T-regulatory cells by suppressing effector T-cell activity and expanding T-regulatory cells [73], influencing the pro-inflammatory response [74]. As a result, immune tolerance is achieved and the KYN pathway homeostasis is maintained. However, if inflammatory conditions exist chronically, causing disturbances in the tryptophan metabolism, this may result in excessive tryptophan depletion and an accumulation of downstream metabolites of the KYN pathway.
Since kynurenine monooxygenase activity is enhanced by pro-inflammatory cytokines [68], 3-hydroxykynurenine formation is increased in inflammatory states, compared to kynurenic acid formation. The balance between the formation of 3-hydroxykynurenine and of kynurenic acid is shifted to the side of 3-hydroxykynurenine, which finally forms quinolinic acid [56]. The KYN pathway in the astrocytes and microglia in the brain can be enhanced in inflammatory conditions through the body–brain cross talk of the pro-inflammatory molecules, such as IL-1 [56]. Immune responses can also activate microglia and cause an influx of proinflammatory cytokines and macrophages into the brain [57]. Because macrophages have a much higher capacity for producing quinolinic acid than microglia, macrophage and cytokine influx can cause significant changes to the levels of KYN metabolites in the CNS [62]. Also, KYN metabolites, such as picolinic acid, activate the inflammatory reaction and further prolong the inflammatory process, which leads to increased quinolinic acid synthesis [75] Fig. (2).
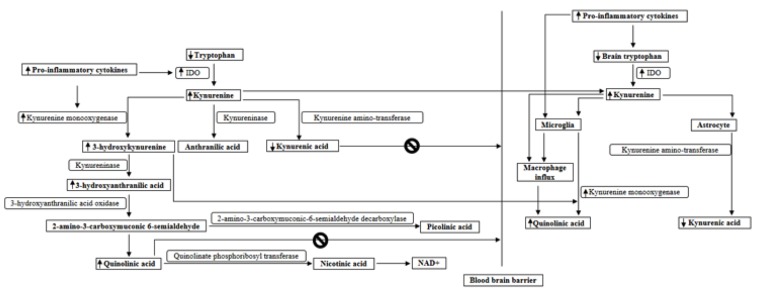
Fig. (2).
IDO and kynurenine monooxygenase activity are enhanced by pro-inflammatory cytokines, and as a result the balance between the formation of 3-hydroxykynurenine and of kynurenic acid is shifted to the side of 3-hydroxykynurenine, which forms 3-hydroxyanthranilic acid and finally quinolinic acid. As KYN synthesized in the periphery can also penetrate the brain, KYN pathway metabolism in the brain is also initiated by KYN that crosses the blood brain barrier. KYN and 3-hydroxykynurenine easily cross the BBB, whereas quinolinic acid and kynurenic acid crosses the BBB poorly. The 3-hydroxykynurenine arm of the pathway leading to quinolinic acid production takes place in microglia, and kynurenic acid production takes place in astrocytes. The KYN pathway in the microglia can be enhanced in inflammatory conditions, as kynurenine monooxygenase activity is enhanced by pro-inflammatory cytokines. Immune responses can also activate microglia and cause an influx of macrophages into the brain, which causes a significant increase in quinolinic acid synthesis.
Neurotoxic effects of the Kynurenine Pathway Metabolites in Depression
Initial theories on how the immune-mediated activation of the tryptophan degradation pathway was related to depression, suggested that disturbed metabolism of tryptophan affects the biosynthesis of serotonin, and this appeared to be associated with an increased susceptibility for depression. Also, it was suggested that activation of IDO could represent an important link between the immunological network and the pathogenesis of depression [76]. Studies on tryptophan depletion in healthy controls reported that lowering plasma tryptophan accompanied a decline in central serotonin [77]. Tryptophan depletion was reported to have mood-lowering effects in subgroups of recovered depressed patients and in vulnerable healthy subjects with a family history of depression [78]. However, subsequent studies reported that brain tryptophan did not decrease in inflammatory conditions, but rather it increased, accompanied by an increase in brain serotonin turnover [79]. The brain seemed to compensate for the decrease in circulating tryptophan induced by acute or chronic inflammation by increasing brain tryptophan and stimulating brain serotonin metabolism [80]. Also, a study on interferon α-treated patients reported stable cerebrospinal fluid tryptophan levels, despite the fact that the patients had decreased blood tryptophan levels [81]. Therefore due to the numerous conflicting results stated above, the hypothesis that decreased serotoninergic neurotransmission due to a decreased amount of tryptophan being responsible for inflammation-induced depression, was no longer tenable [64]. Thereafter, theories were suggested on how the immune-mediated activation of the tryptophan degradation pathway plays a role in depression. As KYN pathway metabolites contribute directly to neuroprotective and neurodegenerative changes in the brain through the network with several neurotransmitter systems, an imbalanced KYN pathway that increases neurotoxic KYN metabolites, which in turn induces an impaired glial–neuronal network, was viewed as the most plausible explanation [56].
The increase in tryptophan degradation in chronic inflammatory states results in extra amounts of peripheral KYN, which cross the BBB and become available for the downstream KYN pathway in the brain [56]. KYN pathway metabolites, such as 3-hydroxykynurenine, 3-hydroxyanthranilic acid and quinolinic acid, are increased in inflammatory states and are all neurotoxic [57]. Also, KYN and 3-hydroxykynurenine easily cross the BBB, whereas quinolinic acid crosses the BBB poorly [82]. 3-hydroxykynurenine generates free-radicals by oxidizing interacting molecules [83]. It has been shown to induce reduced viability, shrunken somata and reduced neuritic outgrowths in cultured striatal and cortical neurons, causing neuronal apoptosis [84]. 3-hydroxyanthranilic acid also auto-oxidizes and generates highly reactive hydrogen peroxide and hydroxyl radicals [85]. Quinolinic acid selectively activates N-methyl-d-aspartate (NMDA) receptors [86], stimulates neuronal release of glutamate, inhibits astroglial reuptake of glutamate [87] and reduces the activity of glutamine synthetase which facilitates glutamine production from glutamate and ammonia [88]. In turn, high concentrations of extracellular glutamate and persistent activation of excitatory neurons cause excitotoxicity [89]. Therefore, the accumulation of quinolinic acid can result in neuronal excitotoxicity and the selective apoptosis of astrocytes [90]. On the other hand, kynurenic acid, which is decreased in inflammatory states, scavenges free radicals, such as hydroxyls and superoxide anions, and possesses antioxidant properties [91]. Kynurenic acid can act as non-selective antagonists of NMDA receptors at high concentrations [92]. Through this modulation of glutamate signaling and antioxidant activity, kynurenic acid counteracts neurotoxicity [57]. However peripheral kynurenic acid crosses the BBB poorly [82]. If an imbalance between neurotoxic and neuroprotective KYN metabolites chronically persists, damage to the glial–neuronal network might be progressive, priming the brain to be vulnerable to pathologic conditions such as depression [56].
Previous studies have reported an imbalance between the neuroprotective and the neurotoxic KYN pathways in MDD. The mean plasma kynurenic acid values and the mean kynurenic acid/KYN ratio were reported to be significantly lower in MDD patients compared to healthy controls [93]. Elevated KYN/tryptophan ratios and a positive relationship between MDD severity and 3-hydroxyanthranilic acid/KYN ratio were observed in adolescent MDD patients with melancholic features compared to healthy controls [94]. These results indicated a shift in the KYN pathway, more to the arm of 3-hydroxyanthranilic acid, where other neurotoxic metabolites, such as 3-hydroxy-kynurenine and quinolinic acid, can be formed [56]. In animal studies, cytokines, such as TNF, were elevated in mice that showed depressive-like behaviors after bacille Calmette-Guérin (BCG) injections, and the enzyme 3-hydroxyanthranilic acid oxidase was also up-regulated [95]. The activation of 3-hydroxyanthranilic acid oxidase enzyme would enhance the degradation of 3-hydroxyanthranilic acid, which would further enhance the formation of quinolinic acid. Increased density of quinolinic acid positive cells of the anterior cingulate cortex, a brain area which has repeatedly been implicated in the pathology of MDD [96], has been reported in acutely depressed patients who have committed suicide [97]. Increased excitotoxic metabolites can have detrimental effects on the brain, such as decreases in neuronal and glial sizes and densities, which have previously been reported in studies on MDD [98].
CONCLUSIONS
Given the evidence stated above, it is likely that stress increases the activity of the SNS and decreases the activity of the PNS, which increases the levels of E and NE, and decreases the level of ACh. This in turn can increase the level of pro-inflammatory cytokines, such as TNF, IL-1, IL-6, and interferons and decrease the level of anti-inflammatory cytokines, such as IL-10, resulting in a state of inflammation. Inflammatory conditions can induce IDO activity, increase the KYN/tryptophan ratio and cause a shift in the KYN pathway, resulting in an imbalance between neuroprotective and neurotoxic KYN metabolites. This can ultimately lead to neurodegenerative changes of the brain, which may render the brain susceptible to depression, as summarized in Fig. (1). Recent reviews have reported the associations among the KYN pathway, inflammatory cytokines and neurotoxicity of KYN pathway metabolites in depression [99, 100]. Furthermore, we have also considered the role of stress and the autonomic nervous system along with inflammatory cytokines and the KYN pathway in our review, when discussing the pathogenesis of depression.
In accordance with our current understanding of how depression represents a state of inflammation, previous studies have attempted to investigate whether anti-inflammatory drugs could have treatment effects on MDD. Several human and animal studies have suggested that certain anti-inflammatory drugs might play an important adjunctive role in the treatment of major depression [101]. As we consider inflammatory conditions to be caused by an over-driven SNS together with an under-driven PNS induced by stress, treatments that increase the parasympathetic tone and hence strengthen the cholinergic anti-inflammatory pathway [102] may be useful in treating MDD. This may explain why methods that increase parasympathetic tone, such as vagus nerve stimulation, have been suggested to be effective in treating depression [103].
Further research on the use of KYN metabolism related markers in the early diagnosis of depression, and novel therapeutic methods involving the normalization of the ANS, immune system and KYN pathway, will help to develop effective ways to detect and treat MDD.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Leonard B.E. Impact of inflammation on neurotransmitter changes in major depression: an insight into the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:261–267. doi: 10.1016/j.pnpbp.2013.10.018. [http://dx.doi.org/10.1016/j.pnpbp.2013.10.018]. [PMID: 24189118]. [DOI] [PubMed] [Google Scholar]
- 2.Lépine J.P., Briley M. The increasing burden of depression. Neuropsychiatr. Dis. Treat. 2011;7(Suppl. 1):3–7. doi: 10.2147/NDT.S19617. [PMID: 21750622]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard B.E. Inflammation as the cause of the metabolic syndrome in depression. Mod. Trends Pharmacopsychiatry. 2013;28:117–126. doi: 10.1159/000343974. [http://dx.doi.org/10.1159/000343974]. [PMID: 25224895]. [DOI] [PubMed] [Google Scholar]
- 4.Lee K.M., Kim Y.K. The role of IL-12 and TGF-beta1 in the pathophysiology of major depressive disorder. Int. Immunopharmacol. 2006;6(8):1298–1304. doi: 10.1016/j.intimp.2006.03.015. [http://dx.doi.org/10.1016/ j.intimp.2006.03.015]. [PMID: 16782542]. [DOI] [PubMed] [Google Scholar]
- 5.Kim J.W., Kim Y.K., Hwang J.A., Yoon H.K., Ko Y.H., Han C., Lee H.J., Ham B.J., Lee H.S. Plasma Levels of IL-23 and IL-17 before and after Antidepressant Treatment in Patients with Major Depressive Disorder. Psychiatry Investig. 2013;10(3):294–299. doi: 10.4306/pi.2013.10.3.294. [http://dx.doi.org/10.4306/pi.2013.10.3.294]. [PMID: 24302954]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y.K., Suh I.B., Kim H., Han C.S., Lim C.S., Choi S.H., Licinio J. The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol. Psychiatry. 2002;7(10):1107–1114. doi: 10.1038/sj.mp.4001084. [http://dx.doi.org/10. 1038/sj.mp.4001084]. [PMID: 12476326]. [DOI] [PubMed] [Google Scholar]
- 7.Yoon H.K., Kim Y.K., Lee H.J., Kwon D.Y., Kim L. Role of cytokines in atypical depression. Nord. J. Psychiatry. 2012;66(3):183–188. doi: 10.3109/08039488.2011.611894. [http://dx.doi.org/10.3109/08039488.2011.611894]. [PMID: 21936732]. [DOI] [PubMed] [Google Scholar]
- 8.Müller N. Immunology of major depression. Neuroimmunomodulation. 2014;21(2-3):123–130. doi: 10.1159/000356540. [PMID: 24557045]. [DOI] [PubMed] [Google Scholar]
- 9.Chrousos G.P., Gold P.W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [http://dx.doi.org/10.1001/jama. 1992.03480090092034]. [PMID: 1538563]. [PubMed] [Google Scholar]
- 10.Olff M. Stress, depression and immunity: the role of defense and coping styles. Psychiatry Res. 1999;85(1):7–15. doi: 10.1016/s0165-1781(98)00139-5. [http://dx.doi.org/ 10.1016/S0165-1781(98)00139-5]. [PMID: 10195312]. [DOI] [PubMed] [Google Scholar]
- 11.Ader R., Cohen N., Felten D. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet. 1995;345(8942):99–103. doi: 10.1016/s0140-6736(95)90066-7. [http://dx.doi.org/10.1016/ S0140-6736(95)90066-7]. [PMID: 7815892]. [DOI] [PubMed] [Google Scholar]
- 12.Gold P.W., Machado-Vieira R., Pavlatou M.G. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural Plast. 2015 doi: 10.1155/2015/581976. 2015 [http://dx.doi.org/ 10.1155/2015/581976] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weik U., Herforth A., Kolb-Bachofen V., Deinzer R. Acute stress induces proinflammatory signaling at chronic inflammation sites. Psychosom. Med. 2008;70(8):906–912. doi: 10.1097/PSY.0b013e3181835bf3. [http://dx.doi.org/ 10.1097/PSY.0b013e3181835bf3]. [PMID: 18799429]. [DOI] [PubMed] [Google Scholar]
- 14.Maes M., Song C., Lin A., De Jongh R., Van Gastel A., Kenis G., Bosmans E., De Meester I., Benoy I., Neels H., Demedts P., Janca A., Scharpé S., Smith R.S. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [http://dx.doi.org/10.1006/ cyto.1997.0290]. [PMID: 9617578]. [DOI] [PubMed] [Google Scholar]
- 15.Brydon L., Edwards S., Mohamed-Ali V., Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav. Immun. 2004;18(3):281–290. doi: 10.1016/j.bbi.2003.09.011. [http://dx.doi.org/ 10.1016/j.bbi.2003.09.011]. [PMID: 15050655]. [DOI] [PubMed] [Google Scholar]
- 16.Capuron L., Ravaud A., Neveu P.J., Miller A.H., Maes M., Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry. 2002;7(5):468–473. doi: 10.1038/sj.mp.4000995. [http://dx.doi.org/10.1038/sj.mp.4000995]. [PMID: 12082564]. [DOI] [PubMed] [Google Scholar]
- 17.Bonaccorso S., Marino V., Puzella A., Pasquini M., Biondi M., Artini M., Almerighi C., Verkerk R., Meltzer H., Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J. Clin. Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [http://dx.doi.org/10.1097/ 00004714-200202000-00014]. [PMID: 11799348]. [DOI] [PubMed] [Google Scholar]
- 18.Hale M.W., Raison C.L., Lowry C.A. Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol. Ther. 2013;137(1):108–118. doi: 10.1016/j.pharmthera.2012.09.005. [http://dx.doi.org/10.1016/j.pharmthera.2012.09.005]. [PMID: 23017938]. [DOI] [PubMed] [Google Scholar]
- 19.Lapin I.P., Oxenkrug G.F. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet. 1969;1(7586):132–136. doi: 10.1016/s0140-6736(69)91140-4. [http://dx.doi. org/10.1016/S0140-6736(69)91140-4]. [PMID: 4178247]. [DOI] [PubMed] [Google Scholar]
- 20.Maes M., Verkerk R., Bonaccorso S., Ombelet W., Bosmans E., Scharpé S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71(16):1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [http://dx.doi.org/10.1016/S0024-3205(02)01853-2]. [PMID: 12175700]. [DOI] [PubMed] [Google Scholar]
- 21.Myint A.M., Kim Y.K. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med. Hypotheses. 2003;61(5-6):519–525. doi: 10.1016/s0306-9877(03)00207-x. [http://dx.doi.org/10.1016/ S0306-9877(03)00207-X]. [PMID: 14592780]. [DOI] [PubMed] [Google Scholar]
- 22.Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52(4):595–638. [PMID: 11121511]. [PubMed] [Google Scholar]
- 23.Breznitz S., Ben-Zur H., Berzon Y., Weiss D.W., Levitan G., Tarcic N., Lischinsky S., Greenberg A., Levi N., Zinder O. Experimental induction and termination of acute psychological stress in human volunteers: effects on immunological, neuro- endocrine, cardiovascular, and psychological parameters. Brain Behav. Immun. 1998;12(1):34–52. doi: 10.1006/brbi.1997.0511. [http://dx.doi.org/10.1006/ brbi.1997.0511]. [PMID: 9570860]. [DOI] [PubMed] [Google Scholar]
- 24.Cacioppo J.T., Malarkey W.B., Kiecolt-Glaser J.K., Uchino B.N., Sgoutas-Emch S.A., Sheridan J.F., Berntson G.G., Glaser R. Heterogeneity in neuroendocrine and immune responses to brief psychological stressors as a function of autonomic cardiac activation. Psychosom. Med. 1995;57(2):154–164. doi: 10.1097/00006842-199503000-00008. [http://dx.doi. org/10.1097/00006842-199503000-00008]. [PMID: 7792374]. [DOI] [PubMed] [Google Scholar]
- 25.Larson M.R., Ader R., Moynihan J.A. Heart rate, neuro- endocrine, and immunological reactivity in response to an acute laboratory stressor. Psychosom. Med. 2001;63(3):493–501. doi: 10.1097/00006842-200105000-00020. [http:// dx.doi.org/10.1097/00006842-200105000-00020]. [PMID: 11382278]. [DOI] [PubMed] [Google Scholar]
- 26.Jones B.E., Yang T.Z. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J. Comp. Neurol. 1985;242(1):56–92. doi: 10.1002/cne.902420105. [http://dx.doi.org/10.1002/cne.902420105]. [PMID: 2416786]. [DOI] [PubMed] [Google Scholar]
- 27.Lewis D.I., Coote J.H. Excitation and inhibition of rat sympathetic preganglionic neurones by catecholamines. Brain Res. 1990;530(2):229–234. doi: 10.1016/0006-8993(90)91287-q. [http://dx.doi.org/10.1016/0006-8993(90) 91287-Q]. [PMID: 2265354]. [DOI] [PubMed] [Google Scholar]
- 28.Unnerstall J.R., Kopajtic T.A., Kuhar M.J. Distribution of alpha 2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the phar- macologic effects of clonidine and related adrenergic agents. Brain Res. 1984;319(1):69–101. doi: 10.1016/0165-0173(84)90030-4. [http://dx.doi.org/10.1016/0165-0173 (84)90030-4]. [PMID: 6324960]. [DOI] [PubMed] [Google Scholar]
- 29.Reiche E.M., Nunes S.O., Morimoto H.K. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [http://dx.doi.org/10.1016/S1470-2045(04)01597-9]. [PMID: 15465465]. [DOI] [PubMed] [Google Scholar]
- 30.Rees C.A. Lost among the trees? The autonomic nervous system and paediatrics. Arch. Dis. Child. 2014;99(6):552–562. doi: 10.1136/archdischild-2012-301863. [http://dx. doi.org/10.1136/archdischild-2012-301863]. [PMID: 24573884]. [DOI] [PubMed] [Google Scholar]
- 31.Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. Int. Rev. Cytol. 1998;181:213–320. doi: 10.1016/s0074-7696(08)60419-2. [http://dx.doi.org/10.1016/ S0074-7696(08)60419-2]. [PMID: 9522458]. [DOI] [PubMed] [Google Scholar]
- 32.Wank S.A. Cholecystokinin receptors. Am. J. Physiol. 1995;269(5 Pt 1):G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [PMID: 7491953]. [DOI] [PubMed] [Google Scholar]
- 33.McCorry L.K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 2007;71(4):78. doi: 10.5688/aj710478. [http://dx.doi.org/10.5688/ aj710478]. [PMID: 17786266]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlov V.A., Tracey K.J. The cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2005;19(6):493–499. doi: 10.1016/j.bbi.2005.03.015. [http://dx. doi.org/10.1016/j.bbi.2005.03.015]. [PMID: 15922555]. [DOI] [PubMed] [Google Scholar]
- 35.Haskó G., Szabó C. Regulation of cytokine and chemokine production by transmitters and co-transmitters of the autonomic nervous system. Biochem. Pharmacol. 1998;56(9):1079–1087. doi: 10.1016/s0006-2952(98)00153-1. [http:// dx.doi.org/10.1016/S0006-2952(98)00153-1]. [PMID: 9802316]. [DOI] [PubMed] [Google Scholar]
- 36.Bertini R., Garattini S., Delgado R., Ghezzi P. Pharmacological activities of chlorpromazine involved in the inhibition of tumour necrosis factor production in vivo in mice. Immunology. 1993;79(2):217–219. [PMID: 8102118]. [PMC free article] [PubMed] [Google Scholar]
- 37.Spengler R.N., Chensue S.W., Giacherio D.A., Blenk N., Kunkel S.L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 1994;152(6):3024–3031. [PMID: 8144901]. [PubMed] [Google Scholar]
- 38.Chrousos G.P. The stress response and immune function: clinical implications. The 1999 Novera H. Spector Lecture. Ann. N. Y. Acad. Sci. 2000;917:38–67. doi: 10.1111/j.1749-6632.2000.tb05371.x. [http://dx.doi.org/10.1111/j.1749-6632.2000.tb05371.x]. [PMID: 11268364]. [DOI] [PubMed] [Google Scholar]
- 39.Koff W.C., Dunegan M.A. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J. Immunol. 1985;135(1):350–354. [PMID: 2582037]. [PubMed] [Google Scholar]
- 40.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [http:// dx.doi.org/10.1038/35013070]. [PMID: 10839541]. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov V.A., Parrish W.R., Rosas-Ballina M., Ochani M., Puerta M., Ochani K., Chavan S., Al-Abed Y., Tracey K.J. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009;23(1):41–45. doi: 10.1016/j.bbi.2008.06.011. [http://dx.doi.org/10.1016/ j.bbi.2008.06.011]. [PMID: 18639629]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent S., Bluthé R.M., Kelley K.W., Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 1992;13(1):24–28. doi: 10.1016/0165-6147(92)90012-u. [http://dx.doi.org/10.1016/0165-6147(92) 90012-U]. [PMID: 1542935]. [DOI] [PubMed] [Google Scholar]
- 43.Smith R.S. The macrophage theory of depression. Med. Hypotheses. 1991;35(4):298–306. doi: 10.1016/0306-9877(91)90272-z. [http://dx.doi.org/10.1016/0306-9877(91)90272-Z]. [PMID: 1943879]. [DOI] [PubMed] [Google Scholar]
- 44.Meyers C.A. Mood and cognitive disorders in cancer patients receiving cytokine therapy. Adv. Exp. Med. Biol. 1999;461:75–81. doi: 10.1007/978-0-585-37970-8_5. [http://dx.doi.org/10.1007/978-0-585-37970-8_5]. [PMID: 10442168]. [DOI] [PubMed] [Google Scholar]
- 45.Capuron L., Ravaud A., Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J. Clin. Oncol. 2000;18(10):2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [PMID: 10811680]. [DOI] [PubMed] [Google Scholar]
- 46.Capuron L., Ravaud A., Miller A.H., Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav. Immun. 2004;18(3):205–213. doi: 10.1016/j.bbi.2003.11.004. [http://dx. doi.org/10.1016/j.bbi.2003.11.004]. [PMID: 15050647]. [DOI] [PubMed] [Google Scholar]
- 47.Tyring S., Gottlieb A., Papp K., Gordon K., Leonardi C., Wang A., Lalla D., Woolley M., Jahreis A., Zitnik R., Cella D., Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [http://dx.doi.org/ 10.1016/S0140-6736(05)67763-X]. [PMID: 16399150]. [DOI] [PubMed] [Google Scholar]
- 48.Reichenberg A., Yirmiya R., Schuld A., Kraus T., Haack M., Morag A., Pollmächer T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [http://dx.doi.org/10.1001/archpsyc.58.5.445]. [PMID: 11343523]. [DOI] [PubMed] [Google Scholar]
- 49.Strike P.C., Wardle J., Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. J. Psychosom. Res. 2004;57(2):189–194. doi: 10.1016/S0022-3999(03)00569-5. [http://dx.doi.org/10.1016/S0022-3999(03) 00569-5]. [PMID: 15465075]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright C.E., Strike P.C., Brydon L., Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav. Immun. 2005;19(4):345–350. doi: 10.1016/j.bbi.2004.10.003. [http://dx.doi.org/ 10.1016/j.bbi.2004.10.003]. [PMID: 15944074]. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.K., Na K.S., Shin K.H., Jung H.Y., Choi S.H., Kim J.B. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;31(5):1044–1053. doi: 10.1016/j.pnpbp.2007.03.004. [http://dx.doi.org/10.1016/j.pnpbp.2007.03.004]. [PMID: 17433516]. [DOI] [PubMed] [Google Scholar]
- 52.Dhabhar F.S., Burke H.M., Epel E.S., Mellon S.H., Rosser R., Reus V.I., Wolkowitz O.M. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 2009;43(11):962–969. doi: 10.1016/j.jpsychires.2009.05.010. [http://dx.doi.org/10.1016/j.jpsychires.2009.05.010]. [PMID: 19552919]. [DOI] [PubMed] [Google Scholar]
- 53.Russo S., Kema I.P., Fokkema M.R., Boon J.C., Willemse P.H., de Vries E.G., den Boer J.A., Korf J. Tryptophan as a link between psychopathology and somatic states. Psychosom. Med. 2003;65(4):665–671. doi: 10.1097/01.psy.0000078188.74020.cc. [http://dx.doi.org/10.1097/01.PSY. 0000078188.74020.CC]. [PMID: 12883120]. [DOI] [PubMed] [Google Scholar]
- 54.Murakami Y., Hoshi M., Imamura Y., Arioka Y., Yamamoto Y., Saito K. Remarkable role of indoleamine 2,3-dioxygenase and tryptophan metabolites in infectious diseases: potential role in macrophage-mediated inflammatory diseases. Mediators Inflamm. 2013 doi: 10.1155/2013/391984. 391984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Löb S., Königsrainer A., Rammensee H.G., Opelz G., Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat. Rev. Cancer. 2009;9(6):445–452. doi: 10.1038/nrc2639. [http://dx.doi.org/10.1038/nrc2639]. [PMID: 19461669]. [DOI] [PubMed] [Google Scholar]
- 56.Myint A.M., Kim Y.K. Network beyond IDO in psychiatric disorders: revisiting neurodegeneration hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2014;48:304–313. doi: 10.1016/j.pnpbp.2013.08.008. [http://dx. doi.org/10.1016/j.pnpbp.2013.08.008]. [PMID: 24184687]. [DOI] [PubMed] [Google Scholar]
- 57.Maddison D.C., Giorgini F. The kynurenine pathway and neurodegenerative disease. Semin. Cell Dev. Biol. 2015;40:134–141. doi: 10.1016/j.semcdb.2015.03.002. [http://dx.doi.org/10.1016/j.semcdb.2015.03.002]. [PMID: 25773161]. [DOI] [PubMed] [Google Scholar]
- 58.Dang Y., Dale W.E., Brown O.R. Comparative effects of oxygen on indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase of the kynurenine pathway. Free Radic. Biol. Med. 2000;28(4):615–624. doi: 10.1016/s0891-5849(99)00272-5. [http://dx.doi.org/10.1016/S0891-5849(99)00272-5]. [PMID: 10719243]. [DOI] [PubMed] [Google Scholar]
- 59.Gál E.M., Sherman A.D. L-kynurenine: its synthesis and possible regulatory function in brain. Neurochem. Res. 1980;5(3):223–239. doi: 10.1007/BF00964611. [http://dx.doi.org/10.1007/BF00964611]. [PMID: 6154900]. [DOI] [PubMed] [Google Scholar]
- 60.Guillemin G.J., Cullen K.M., Lim C.K., Smythe G.A., Garner B., Kapoor V., Takikawa O., Brew B.J. Characterization of the kynurenine pathway in human neurons. J. Neurosci. 2007;27(47):12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [http://dx.doi.org/10.1523/JNEUROSCI.4101-07. 2007]. [PMID: 18032661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ting K.K., Brew B., Guillemin G. The involvement of astrocytes and kynurenine pathway in Alzheimer’s disease. Neurotox. Res. 2007;12(4):247–262. doi: 10.1007/BF03033908. [http://dx.doi.org/10.1007/BF03033908]. [PMID: 18201952]. [DOI] [PubMed] [Google Scholar]
- 62.Guillemin G.J., Smith D.G., Smythe G.A., Armati P.J., Brew B.J. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [http://dx.doi.org/10.1007/978-1-4615-0135-0_12]. [PMID: 15206722]. [DOI] [PubMed] [Google Scholar]
- 63.Guillemin G.J., Kerr S.J., Smythe G.A., Smith D.G., Kapoor V., Armati P.J., Croitoru J., Brew B.J. Kynurenine pathway metabolism in human astrocytes: a paradox for neuronal protection. J. Neurochem. 2001;78(4):842–853. doi: 10.1046/j.1471-4159.2001.00498.x. [http://dx.doi.org/10.1046/j. 1471-4159.2001.00498.x]. [PMID: 11520905]. [DOI] [PubMed] [Google Scholar]
- 64.Dantzer R., O’Connor J.C., Lawson M.A., Kelley K.W. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36(3):426–436. doi: 10.1016/j.psyneuen.2010.09.012. [http://dx.doi. org/10.1016/j.psyneuen.2010.09.012]. [PMID: 21041030]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Werner-Felmayer G., Werner E.R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta. 1989;1012(2):140–147. doi: 10.1016/0167-4889(89)90087-6. [http://dx.doi.org/10.1016/0167-4889 (89)90087-6]. [PMID: 2500976]. [DOI] [PubMed] [Google Scholar]
- 66.Werner-Felmayer G., Werner E.R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Neopterin formation and tryptophan degradation by a human myelomonocytic cell line (THP-1) upon cytokine treatment. Cancer Res. 1990;50(10):2863–2867. [PMID: 2110500]. [PubMed] [Google Scholar]
- 67.Robinson C.M., Hale P.T., Carlin J.M. The role of IFN-gamma and TNF-alpha-responsive regulatory elements in the synergistic induction of indoleamine dioxygenase. J. Interferon Cytokine Res. 2005;25(1):20–30. doi: 10.1089/jir.2005.25.20. [http://dx.doi.org/10.1089/jir.2005.25.20]. [PMID: 15684619]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mellor A.L., Munn D.H. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol. Today. 1999;20(10):469–473. doi: 10.1016/s0167-5699(99)01520-0. [http://dx.doi.org/10.1016/S0167-5699(99) 01520-0]. [PMID: 10500295]. [DOI] [PubMed] [Google Scholar]
- 69.Heyes M.P., Achim C.L., Wiley C.A., Major E.O., Saito K., Markey S.P. Human microglia convert l-tryptophan into the neurotoxin quinolinic acid. Biochem. J. 1996;320(Pt 2):595–597. doi: 10.1042/bj3200595. [http://dx.doi.org/10.1042/bj3200595]. [PMID: 8973572]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moffett J.R., Blinder K.L., Venkateshan C.N., Namboodiri M.A. Differential effects of kynurenine and tryptophan treatment on quinolinate immunoreactivity in rat lymphoid and non-lymphoid organs. Cell Tissue Res. 1998;293(3):525–534. doi: 10.1007/s004410051145. [http://dx.doi.org/ 10.1007/s004410051145]. [PMID: 9716743]. [DOI] [PubMed] [Google Scholar]
- 71.Moffett J.R., Namboodiri M.A. Tryptophan and the immune response. Immunol. Cell Biol. 2003;81(4):247–265. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [http://dx.doi. org/10.1046/j.1440-1711.2003.t01-1-01177.x]. [PMID: 12848846]. [DOI] [PubMed] [Google Scholar]
- 72.Zunszain P.A., Anacker C., Cattaneo A., Choudhury S., Musaelyan K., Myint A.M., Thuret S., Price J., Pariante C.M. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277. [http://dx.doi.org/10.1038/npp.2011.277]. [PMID: 22071871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harden J.L., Egilmez N.K. Indoleamine 2,3-dioxygenase and dendritic cell tolerogenicity. Immunol. Invest. 2012;41(6-7):738–764. doi: 10.3109/08820139.2012.676122. [http://dx.doi.org/10.3109/08820139.2012.676122]. [PMID: 23017144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Puccetti P., Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat. Rev. Immunol. 2007;7(10):817–823. doi: 10.1038/nri2163. [http://dx.doi.org/10.1038/ nri2163]. [PMID: 17767193]. [DOI] [PubMed] [Google Scholar]
- 75.Melillo G., Cox G.W., Radzioch D., Varesio L. Picolinic acid, a catabolite of L-tryptophan, is a costimulus for the induction of reactive nitrogen intermediate production in murine macrophages. J. Immunol. 1993;150(9):4031–4040. [PMID: 8473748]. [PubMed] [Google Scholar]
- 76.Widner B., Laich A., Sperner-Unterweger B., Ledochowski M., Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav. Immun. 2002;16(5):590–595. doi: 10.1016/s0889-1591(02)00006-5. [http://dx.doi.org/10.1016/S0889-1591(02)00006-5]. [PMID: 12401473]. [DOI] [PubMed] [Google Scholar]
- 77.Moore P., Landolt H.P., Seifritz E., Clark C., Bhatti T., Kelsoe J., Rapaport M., Gillin J.C. Clinical and physiological consequences of rapid tryptophan depletion. Neuropsychopharmacology. 2000;23(6):601–622. doi: 10.1016/S0893-133X(00)00161-5. [http://dx.doi.org/10.1016/S0893-133X(00)00161-5]. [PMID: 11063917]. [DOI] [PubMed] [Google Scholar]
- 78.Van der Does A.J. The effects of tryptophan depletion on mood and psychiatric symptoms. J. Affect. Disord. 2001;64(2-3):107–119. doi: 10.1016/s0165-0327(00)00209-3. [http://dx.doi.org/10.1016/S0165-0327(00)00209-3]. [PMID: 11313078]. [DOI] [PubMed] [Google Scholar]
- 79.O’Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., Kelley K.W., Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [http://dx.doi.org/10.1038/sj.mp.4002148]. [PMID: 18195714]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dunn A.J., Wang J., Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv. Exp. Med. Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [http://dx.doi.org/10.1007/978-0-585-37970-8_8]. [PMID: 10442171]. [DOI] [PubMed] [Google Scholar]
- 81.Raison C.L., Dantzer R., Kelley K.W., Lawson M.A., Woolwine B.J., Vogt G., Spivey J.R., Saito K., Miller A.H. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [http://dx. doi.org/10.1038/mp.2009.116]. [PMID: 19918244]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukui S., Schwarcz R., Rapoport S.I., Takada Y., Smith Q.R. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J. Neurochem. 1991;56(6):2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [http://dx.doi.org/10.1111/j.1471-4159.1991.tb03460.x]. [PMID: 1827495]. [DOI] [PubMed] [Google Scholar]
- 83.Vazquez S., Garner B., Sheil M.M., Truscott R.J. Characterisation of the major autoxidation products of 3-hydroxykynurenine under physiological conditions. Free Radic. Res. 2000;32(1):11–23. doi: 10.1080/10715760000300021. [http://dx.doi.org/10.1080/10715760000300021]. [PMID: 10625213]. [DOI] [PubMed] [Google Scholar]
- 84.Okuda S., Nishiyama N., Saito H., Katsuki H. 3-Hydro- xykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J. Neurochem. 1998;70(1):299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [http://dx.doi.org/10.1046/ j.1471-4159.1998.70010299.x]. [PMID: 9422375]. [DOI] [PubMed] [Google Scholar]
- 85.Goldstein L.E., Leopold M.C., Huang X., Atwood C.S., Saunders A.J., Hartshorn M., Lim J.T., Faget K.Y., Muffat J.A., Scarpa R.C., Chylack L.T., Jr, Bowden E.F., Tanzi R.E., Bush A.I. 3-Hydroxykynurenine and 3-hydroxyanthranilic acid generate hydrogen peroxide and promote alpha-crystallin cross-linking by metal ion reduction. Biochemistry. 2000;39(24):7266–7275. doi: 10.1021/bi992997s. [http://dx.doi.org/10.1021/bi992997s]. [PMID: 10852726]. [DOI] [PubMed] [Google Scholar]
- 86.Stone T.W., Perkins M.N. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur. J. Pharmacol. 1981;72(4):411–412. doi: 10.1016/0014-2999(81)90587-2. [http://dx.doi.org/10.1016/0014-2999(81)90587-2]. [PMID: 6268428]. [DOI] [PubMed] [Google Scholar]
- 87.Tavares R.G., Tasca C.I., Santos C.E., Alves L.B., Porciúncula L.O., Emanuelli T., Souza D.O. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochem. Int. 2002;40(7):621–627. doi: 10.1016/s0197-0186(01)00133-4. [http://dx.doi. org/10.1016/S0197-0186(01)00133-4]. [PMID: 11900857]. [DOI] [PubMed] [Google Scholar]
- 88.Ting K.K., Brew B.J., Guillemin G.J. Effect of quinolinic acid on human astrocytes morphology and functions: implications in Alzheimer’s disease. J. Neuroinflammation. 2009;6:36. doi: 10.1186/1742-2094-6-36. [http://dx.doi.org/10.1186/1742-2094-6-36]. [PMID: 20003262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pérez-De La Cruz V., Carrillo-Mora P., Santamaría A. Quinolinic Acid, an endogenous molecule combining excitotoxicity, oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012;5:1–8. doi: 10.4137/IJTR.S8158. [PMID: 22408367]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guillemin G.J., Smythe G., Takikawa O., Brew B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [http://dx.doi.org/10.1002/glia.20090]. [PMID: 15390107]. [DOI] [PubMed] [Google Scholar]
- 91.Lugo-Huitrón R., Blanco-Ayala T., Ugalde-Muñiz P., Carrillo-Mora P., Pedraza-Chaverrí J., Silva-Adaya D., Maldonado P.D., Torres I., Pinzón E., Ortiz-Islas E., López T., García E., Pineda B., Torres-Ramos M., Santamaría A., La Cruz V.P. On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011;33(5):538–547. doi: 10.1016/j.ntt.2011.07.002. [http://dx.doi.org/10.1016/j.ntt.2011.07.002]. [PMID: 21763768]. [DOI] [PubMed] [Google Scholar]
- 92.Perkins M.N., Stone T.W. An iontophoretic investigation of the actions of convulsant kynurenines and their interaction with the endogenous excitant quinolinic acid. Brain Res. 1982;247(1):184–187. doi: 10.1016/0006-8993(82)91048-4. [http://dx.doi.org/10.1016/0006-8993(82)91048-4]. [PMID: 6215086]. [DOI] [PubMed] [Google Scholar]
- 93.Myint A.M., Kim Y.K., Verkerk R., Scharpé S., Steinbusch H., Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J. Affect. Disord. 2007;98(1-2):143–151. doi: 10.1016/j.jad.2006.07.013. [http://dx.doi.org/10.1016/j.jad.2006.07.013]. [PMID: 16952400]. [DOI] [PubMed] [Google Scholar]
- 94.Gabbay V., Klein R.G., Katz Y., Mendoza S., Guttman L.E., Alonso C.M., Babb J.S., Hirsch G.S., Liebes L. The possible role of the kynurenine pathway in adolescent depression with melancholic features. J. Child Psychol. Psychiatry. 2010;51(8):935–943. doi: 10.1111/j.1469-7610.2010.02245.x. [http://dx.doi.org/10.1111/j.1469-7610.2010.02245.x]. [PMID: 20406333]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Connor J.C., Lawson M.A., André C., Briley E.M., Szegedi S.S., Lestage J., Castanon N., Herkenham M., Dantzer R., Kelley K.W. Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J. Immunol. 2009;182(5):3202–3212. doi: 10.4049/jimmunol.0802722. [http://dx.doi.org/10.4049/ jimmunol.0802722]. [PMID: 19234218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mayberg H.S., Brannan S.K., Mahurin R.K., Jerabek P.A., Brickman J.S., Tekell J.L., Silva J.A., McGinnis S., Glass T.G., Martin C.C., Fox P.T. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8(4):1057–1061. doi: 10.1097/00001756-199703030-00048. [http://dx.doi.org/10.1097/00001756-199703030-00048]. [PMID: 9141092]. [DOI] [PubMed] [Google Scholar]
- 97.Steiner J., Walter M., Gos T., Guillemin G.J., Bernstein H.G., Sarnyai Z., Mawrin C., Brisch R., Bielau H., Meyer zu Schwabedissen L., Bogerts B., Myint A.M. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflammation. 2011;8:94. doi: 10.1186/1742-2094-8-94. [http://dx.doi.org/10.1186/1742-2094-8-94]. [PMID: 21831269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rajkowska G., Miguel-Hidalgo J.J., Wei J., Dilley G., Pittman S.D., Meltzer H.Y., Overholser J.C., Roth B.L., Stockmeier C.A. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry. 1999;45(9):1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [http://dx.doi.org/10.1016/S0006-3223(99)00041-4]. [PMID: 10331101]. [DOI] [PubMed] [Google Scholar]
- 99.Hazari N., Bhad R. Kynurenine pathway (KP) inhibitors: Novel agents for the management of depression. J. Psychopharmacol. (Oxford) 2015;29(10):1133–1134. doi: 10.1177/0269881115599386. [http://dx.doi.org/10.1177/ 0269881115599386]. [PMID: 26253624]. [DOI] [PubMed] [Google Scholar]
- 100.Jo W.K., Zhang Y., Emrich H.M., Dietrich D.E. Glia in the cytokine-mediated onset of depression: fine tuning the immune response. Front. Cell. Neurosci. 2015;9:268. doi: 10.3389/fncel.2015.00268. [http://dx.doi.org/10. 3389/fncel.2015.00268]. [PMID: 26217190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Najjar S., Pearlman D.M., Alper K., Najjar A., Devinsky O. Neuroinflammation and psychiatric illness. J. Neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43. [http://dx.doi.org/10.1186/1742-2094-10-43]. [PMID: 23547920]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavlov V.A. Cholinergic modulation of inflammation. Int. J. Clin. Exp. Med. 2008;1(3):203–212. [PMID: 19079659]. [PMC free article] [PubMed] [Google Scholar]
- 103.Grimonprez A., Raedt R., Baeken C., Boon P., Vonck K. The antidepressant mechanism of action of vagus nerve stimulation: Evidence from preclinical studies. Neurosci. Biobehav. Rev. 2015;56:26–34. doi: 10.1016/j.neubiorev.2015.06.019. [http://dx.doi.org/10.1016/j.neubiorev.2015.06.019]. [PMID: 26116875]. [DOI] [PubMed] [Google Scholar]