Abstract
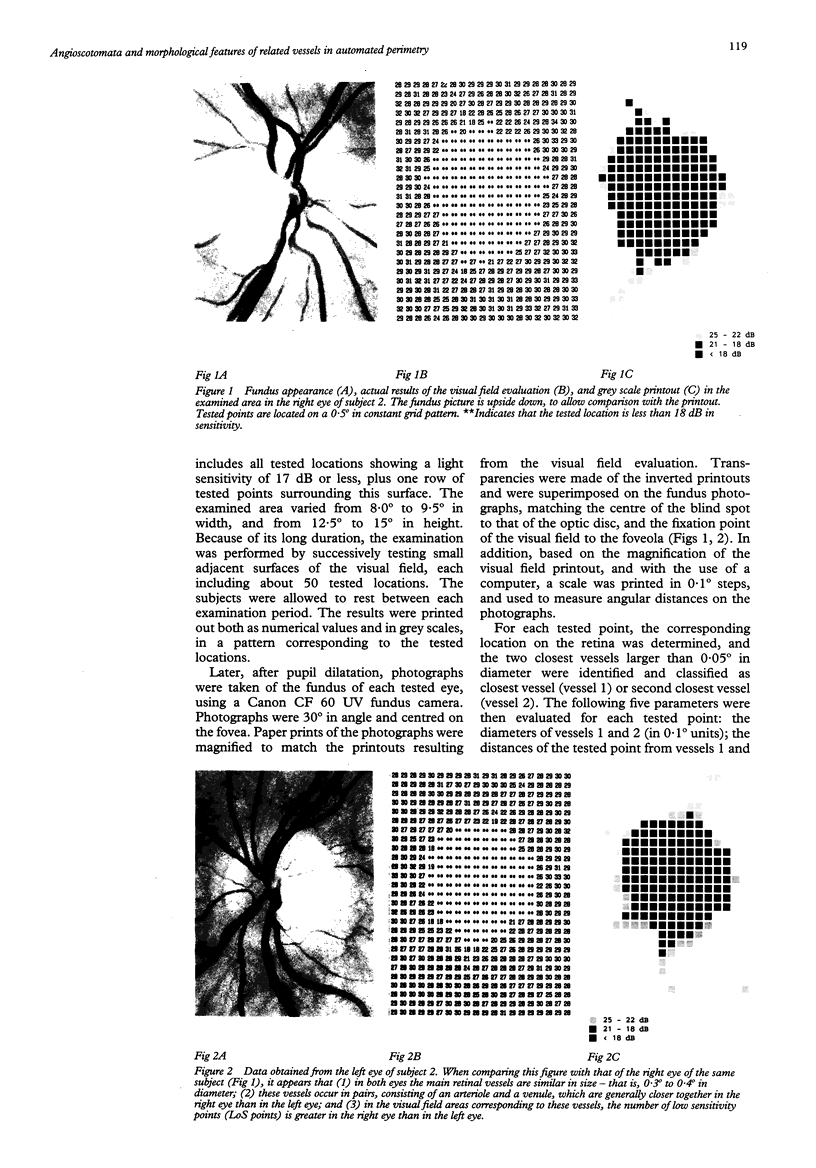
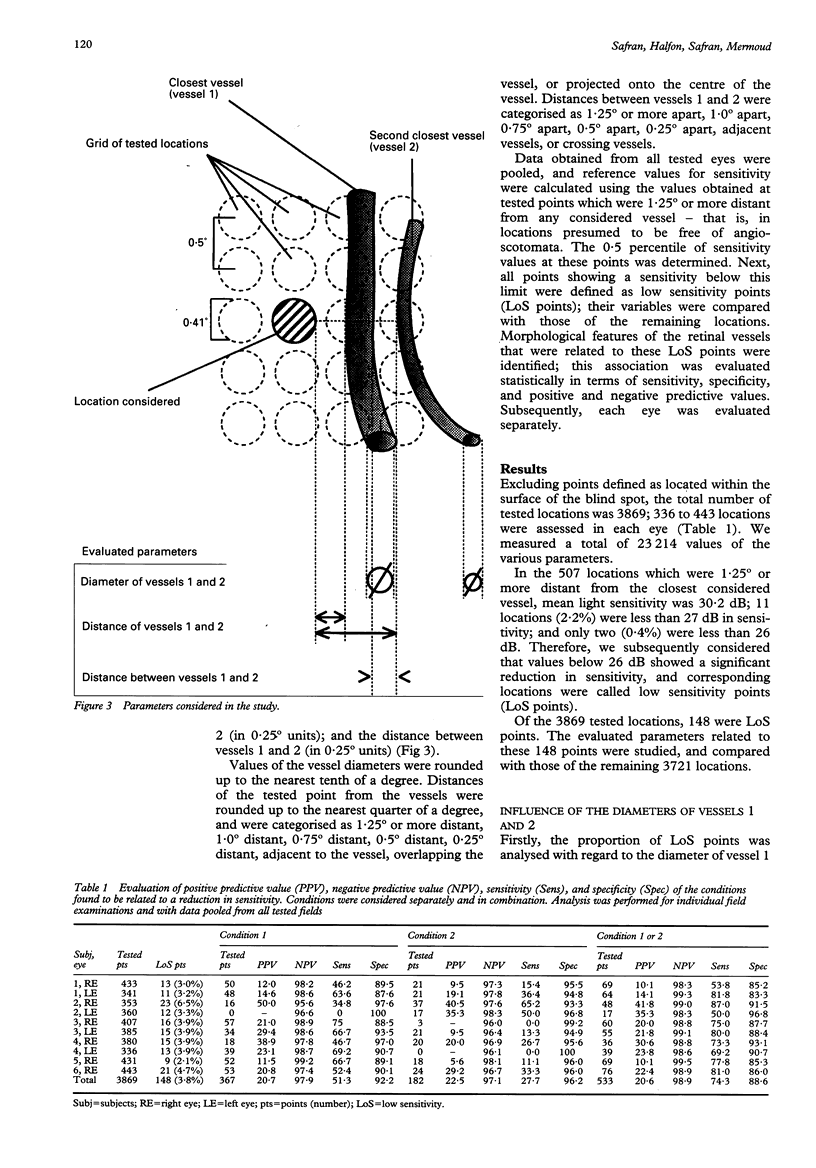
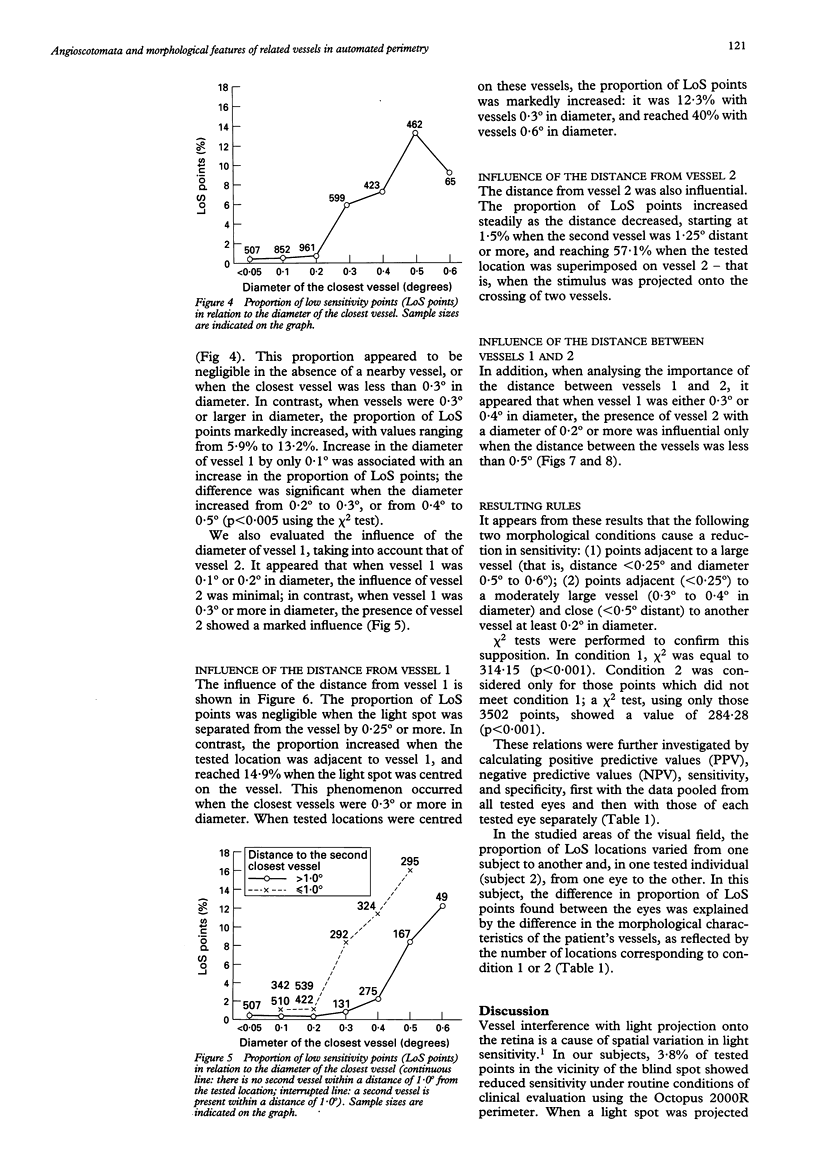
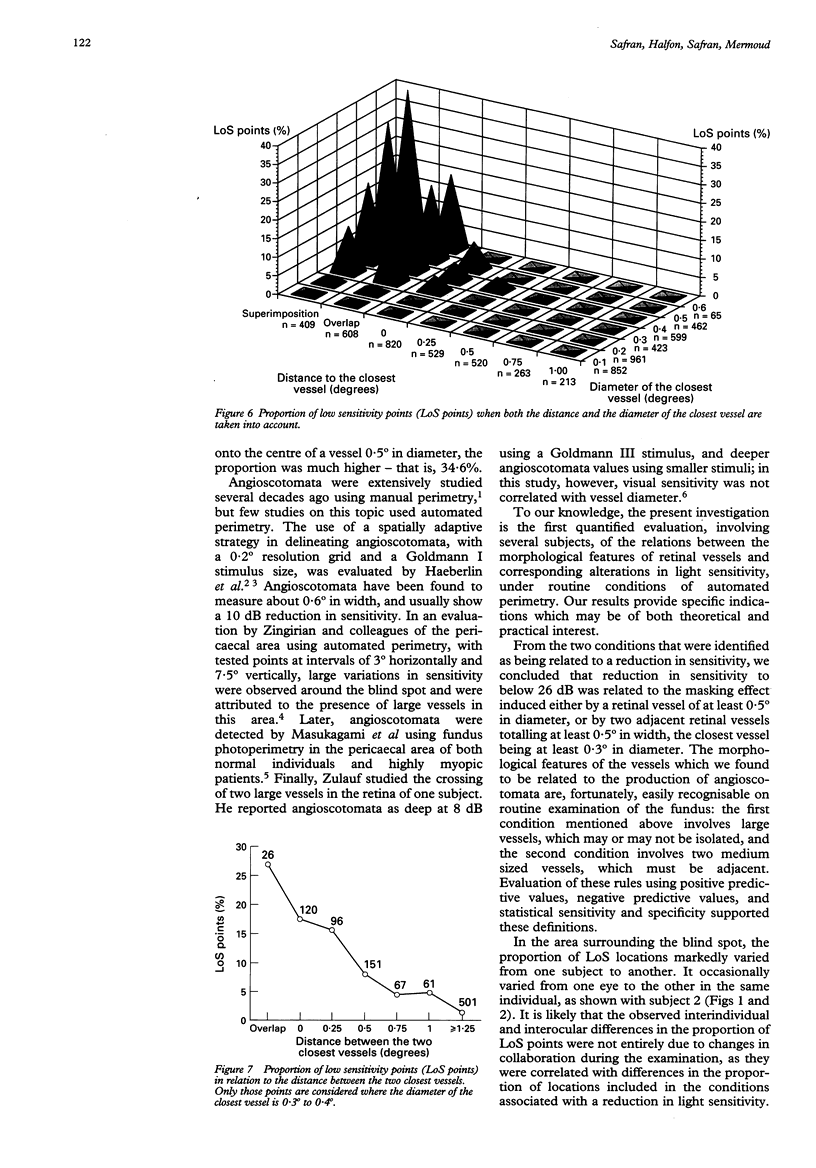
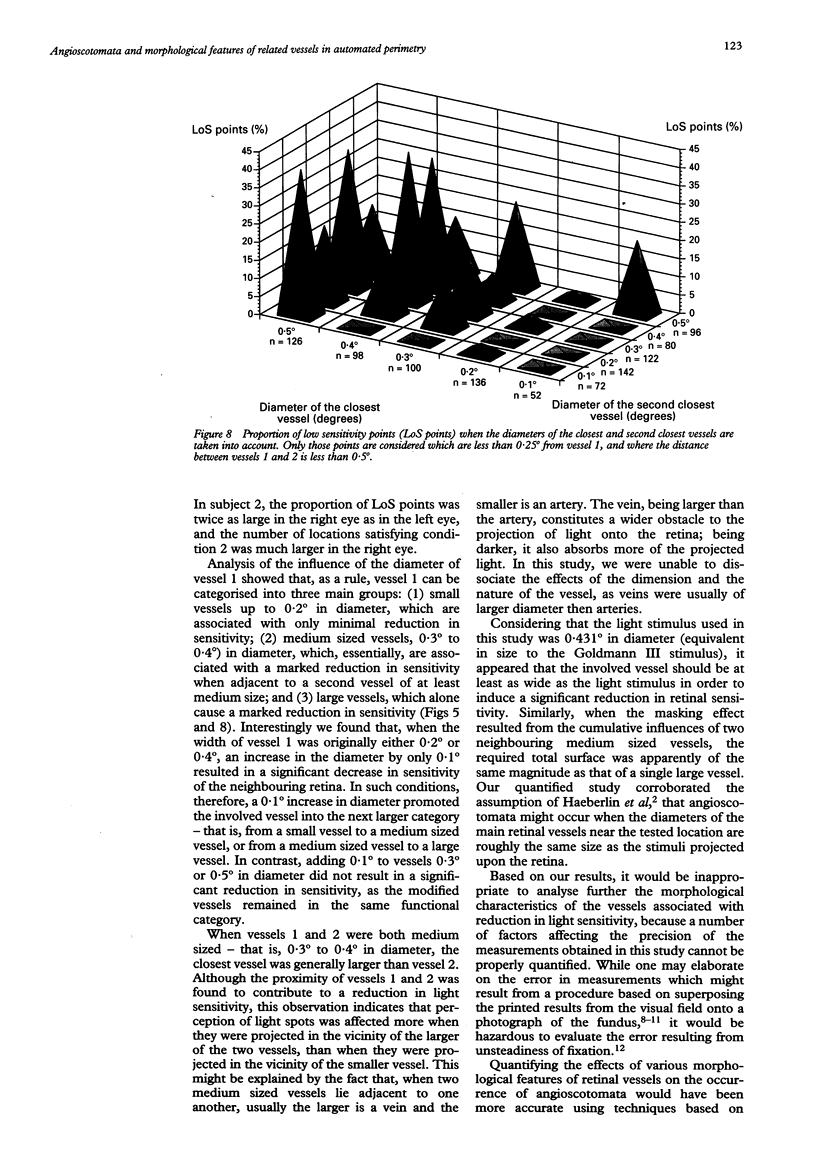
AIMS--To determine principles which regulate the occurrence of angioscotomata in automated static perimetry, variations in light sensitivity were correlated with the location and diameter of neighbouring retinal vessels. METHODS--Ten normal eyes were tested with the Octopus 2000R, using a 0.431 degree light stimulus. Sensitivity was quantified in points located around the blind spot, according to a regular, 0.5 degree constant, grid pattern. From 336 to 443 locations were tested in each eye. The resulting printouts were superimposed on corresponding fundus photographs. At each tested point, the following five additional variables were evaluated: the diameters of the closest and the second closest vessel (in 0.1 degree units); the distances of the apparent location of the tested point to the closest and the second closest vessel (in 0.25 degree units); and the distance between the two closest vessels (in 0.25 degree units). Altogether, 3869 locations were tested and 23,214 values were quantified. RESULTS--The following two conditions were found to be related to a reduction in sensitivity: (1) proximity (< 0.25 degree) to a large vessel (> or = 0.5 degree in diameter); (2) proximity (< 0.25 degree) to one of two adjacent (< 0.5 degree distant), moderately large vessels (0.3 degree to 0.4 degree in diameter). In condition 1, sensitivity was 51.3% and specificity was 92.2%; in condition 2, sensitivity was 16.2% and specificity was 98.3%; and with a combination of conditions 1 and 2, sensitivity was 67.6% and specificity was 90.5%. Increase by 0.1 degree of an adjacent vessel which was 0.4 degree in diameter markedly affected light sensitivity. CONCLUSION--Modifications in vessel diameter are observed in a number of circumstances, including adaptive vascular response to changes in ambient conditions and obstructive disorders of retinal vessels. These findings indicate that changes in vessel diameter over time can result in fluctuation of sensitivity. It is concluded that, in contrast with what is commonly stated, when ocular media are unaltered and the subject's collaboration is adequate, temporal variations in measured thresholds do not necessarily reflect functional changes in nervous tissues in the visual pathways.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bek T. Localised scotomata and types of vascular occlusion in diabetic retinopathy. Acta Ophthalmol (Copenh) 1991 Feb;69(1):11–18. doi: 10.1111/j.1755-3768.1991.tb01984.x. [DOI] [PubMed] [Google Scholar]
- Bek T., Lund-Andersen H. Accurate superimposition of perimetry data onto fundus photographs. Acta Ophthalmol (Copenh) 1990 Feb;68(1):11–18. doi: 10.1111/j.1755-3768.1990.tb01642.x. [DOI] [PubMed] [Google Scholar]
- Eizenman M., Trope G. E., Fortinsky M., Murphy P. H. Stability of fixation in healthy subjects during automated perimetry. Can J Ophthalmol. 1992 Dec;27(7):336–340. [PubMed] [Google Scholar]
- Frisén L. The cartographic deformations of the visual field. Ophthalmologica. 1970;161(1):38–54. doi: 10.1159/000306090. [DOI] [PubMed] [Google Scholar]
- Kirkham T. H., Meyer E. Visual field area on the Goldmann hemispheric perimeter surface. Correction of cartographic errors inherent in perimetry. Curr Eye Res. 1981;1(2):93–99. doi: 10.3109/02713688109001732. [DOI] [PubMed] [Google Scholar]
- Timberlake G. T., Mainster M. A., Webb R. H., Hughes G. W., Trempe C. L. Retinal localization of scotomata by scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 1982 Jan;22(1):91–97. [PubMed] [Google Scholar]
- Tsacopoulos M., David N. J. The effect of arterial PCO 2 on relative retinal blood flow in monkeys. Invest Ophthalmol. 1973 May;12(5):335–347. [PubMed] [Google Scholar]