Abstract
Abstract: Background
Propofol is a sedative agent that at clinical concentrations acts by allosterically activating or potentiating the γ-aminobutyric acid type A (GABAA) receptor. Mutational, modeling, and photolabeling studies with propofol and its analogues have identified potential interaction sites in the transmembrane domain of the receptor. At the “+” of the β subunit, in the β-α interface, meta-azipropofol labels the M286 residue in the third transmembrane domain. Substitution of this residue with tryptophan results in loss of potentiation by propofol. At the “-” side of the β subunit, in the α-β interface (or β-β interface, in the case of homomeric β receptors), ortho-propofol diazirine labels the H267 residue in the second transmembrane domain. Structural modeling indicates that the β(H267) residue lines a cavity that docks propofol with favorable interaction energy.
Method
We used two-electrode voltage clamp to determine the functional effects of mutations to the “+” and “-” sides of the β subunit on activation of the α1β3 GABAA receptor by propofol.
Results
We found that while the individual mutations had a small effect, the combination of the M286W mutation with tryptophan mutations of selected residues at the α-β interface leads to strong reduction in gating efficacy for propofol.
Conclusion
We conclude that α1β3 GABAA receptors can be activated by propofol interactions with the β-β, α-β, and β-α interfaces, where distinct, non-equivalent regions control channel gating. Any interface can mediate activation, hence substitutions at all interfaces are required for loss of activation by propofol.
Keywords: Activation, binding site, GABAA receptor, mutation, propofol, structure
INTRODUCTION
The γ-aminobutyric acid type A (GABAA) receptor is the major inhibitory transmitter-gated ion channel in the brain. In mature neurons, activation of the GABAA receptor results in increased membrane conductance for Cl- leading to hyperpolarization of the cell or reduction of the effects of excitatory channels, thereby having an inhibitory effect on overall brain activity. Drugs capable of augmenting GABAA receptor activity can be clinically useful as sedatives or anticonvulsants.
The GABAA receptor is a pentameric membrane protein. Each of the five homologous subunits contains a large aminoterminal extracellular domain followed by four transmembrane domains and a short carboxyterminal end at the extracellular side of the membrane. The two transmitter binding sites are located in the extracellular domain, at the interfaces between the β and α subunits. Heteromeric GABAA receptors, consisting of two α subunits, two β subunits, and a fifth subunit, e.g., a γ or δ subunit can be gated by the transmitter GABA, and directly activated and modulated by several allosteric ligands (e.g., neuroactive steroids, propofol, etomidate, barbiturates) [1, 2]. Homomeric GABAA receptors containing five β subunits do not respond to GABA but are functional in the presence of some allosteric activators, e.g., propofol and barbiturates [3, 4]. The allosteric activators exert their effects via interactions with allosteric binding sites, distinct from binding sites for the transmitter, GABA [5].
Propofol (2,6-diisopropylphenol) is widely used clinically to induce and maintain general anesthesia. The major advantage of propofol is its favorable pharmacokinetics, i.e., rapid onset and offset of the effect. While its hepatic elimination half-life is in hours, its sedative effects after a single dose terminate in minutes, due to redistribution of the drug to peripheral tissue.
The sedative effects of propofol are mediated by actions on the GABAA receptor. Mice harboring a single point mutation (N265M) in the GABAA receptor β3 subunit are resistant to suppression of noxious-evoked movements by propofol and display a drastic reduction in the duration of loss-of-righting reflex following administration of propofol [6].
Current responses from native and heterologously-expressed GABAA receptors are potentiated when micromolar concentrations of propofol are coapplied with a subsaturating concentration of transmitter [7, 8]. Miniature inhibitory postsynaptic currents are prolonged in the presence of micromolar concentrations of propofol [9]. Propofol, especially at higher concentrations, is also an efficacious agonist of the GABAA receptor. It is considered that the same sites in the receptor mediate direct gating and potentiation [10].
Here, we discuss the structural aspects of propofol interaction with the GABAA receptor. We focus on published mutational, functional, and modeling studies, and introduce novel data from our laboratories. The major conclusion is that non-equivalent interaction sites at the various intersubunit interfaces control GABAA receptor activation by propofol.
COMPARATIVE MOLECULAR PHARMACOLOGICAL STUDIES
Mutational studies based on comparing the effects of propofol and other general anesthetics on mammalian GABAA vs. glycine and Drosophila GABA receptors that are not modulated by these drugs have revealed the involvement of the transmembrane domains in the actions of propofol (reviewed in [11]). Of interest, it was shown that α2β1 and α2β1γ2 receptors containing the β1(M286W) mutation in the third transmembrane domain are not potentiated by propofol [12]. A later work finding that small amino acid substitutions at β(M286) are permissive for potentiation by propofol while the tryptophan substitution does not eliminate the ability of smaller analogues, e.g., 2,6,-dimethylphenol, to potentiate the receptor, proposed that the β(M286W) mutation decreases the volume of the putative binding cavity below propofol cutoff [13, 14].
Direct involvement of the β(M286) residue in the binding of propofol was later demonstrated through the substituted-cysteine accessibility method (SCAM). This approach is based on examining the functional effect of modification of a cysteine residue introduced to the region of interest with a sulphydryl-specific reagent [15]. Bali and Akabas [16] demonstrated that propofol sterically protects the cysteine residue substituted for β(M286) from modification by the sulfhydryl-modifying agent p-chloromercuribenzenesulfonate.
An observation that β3, but not β1, homomeric GABAA receptors are directly activated by propofol led to the discovery that activation by propofol critically depends on the nature of the amino acid residue in the second transmembrane domain, at position 265 [17]. The β3 subunit contains an asparagine while the β1 subunit contains a serine residue at this position. A substitution of asparagine with serine in β3 generates a receptor that is insensitive to propofol whereas the opposite mutation, S265N, in β1 produces a receptor that can be activated by propofol [17]. In α1β2γ2 and α2β3γ2 heteromeric GABAA receptors, an asparagine-to-methionine switch at position 265 leads to drastic reduction in potentiation and a loss of direct activation by propofol [18, 19]. It is, however, unlikely that the N265 residue directly interacts with propofol, because SCAM studies have shown that propofol does not protect modification of the N265C residue by p-chloromer-curibenzenesulfonate [16]. Furthermore, in β3 homomeric receptors, the N265S mutation has a dominant-negative effect, i.e., the presence of a single mutation in the homopentameric receptor is sufficient to render the receptor nonresponsive to propofol [17].
PHOTOLABELING STUDIES CONFIRM THE INVOLVEMENT OF TRANSMEMBRANE DOMAINS
Recent studies employing photoactivatable analogues of propofol support the idea that propofol binds in the transmembrane region of the GABAA receptor. In α1β3 receptors, a photoreactive propofol analogue 2-isopropyl-5-[3-(trifluoromethyl)-3H-diazirin-3-yl]phenol (meta-azipropofol) labels the β3(M286) and α1(M236) residues at the β-α interface [20]. Labeling of these residues is inhibited by propofol, as well as etomidate and a photoactivatable analogue of barbiturate. And conversely, photolabeling of homologous residues at the β-α and γ-β interfaces by etomidate and barbiturate analogues is inhibited by propofol, albeit at relatively high concentrations (IC50 in tens of micromolar). A binding model emerges from these findings where various anesthetic compounds interact with equivalent sites at the α-β, β-β, and β-α interfaces, although the binding affinities and selectivity of a compound for each of the interfaces can be different [20]. As expected, meta-azipropofol retains the anesthetic potency of the parent compound propofol in the Xenopus tadpole loss-of-righting reflex assay, although it exhibits a somewhat smaller degree of potentiation of heterologously-expressed α1β2γ2L GABAA receptors [21].
In another study, Yip and coworkers [22] employed a propofol photoactivatable analogue ortho-propofol diazirine, labeling the β3(H267) residue in β3 homomeric and α1β3 heteromeric GABAA receptors. The β3(H267) residue resides in a hydrophobic cleft near the interface between the extracellular and transmembrane domains. The cavity lies in a triangular structure formed by the first and second transmembrane domains at the “-” side of a β subunit and the second transmembrane domain at the “+” side of the neighboring subunit (an α subunit in α1β3 receptors or another β subunit in β3 homomeric receptors).
Substitution of the 2-isopropyl group in the propofol molecule with trifluoromethyl diazirine in ortho-propofol diazirine had a relatively benign effect on the compound's biological activity, including inhibition of [35S]t-butylbi- cyclophosphorothionate binding to β3 GABAA receptors, potentiation of GABA-elicited currents from heterologously-expressed α1β3 receptors, and the loss-of-righting reflex in rats [22].
By reasoning that anesthetics, and in fact all activators, bind more tightly in the open state than in the closed state, and are therefore likely to bind in regions that show conformational changes upon channel opening, Franks [23] compared open and closed state structures from the structurally-related proton-gated Gloeobacter ligand-gated ion channel (GLIC) and the glutamate-gated chloride channel (GluCl) from Caenorhabditis elegans. The greatest conformational difference between the open and closed states was evident in the region near the top of the second and third transmembrane domains. In the β3 GABAA receptor, this region contains two cavities that are adjacent to the photolabeled β3(H267) residue. From docking calculations propofol is predicted to bind in the two cavities with affinities near concentrations at which it acts on the GABAA receptor [23].
ELUCIDATION OF THE FUNCTIONAL ROLE OF LABELED RESIDUES IN Β3 HOMOMERIC RECEPTORS
To determine the functional role of residues in the putative binding cavity at the interface between the transmembrane and extracellular domains, Eaton and coworkers [24] examined the effects of tryptophan-substitutions at β3(H267) and nearby residues. The major finding was that while the mutation of the photolabeled β3(H267) residue was without effect on activation by propofol, substitutions at several nearby locations had a profound effect on activation of β3 receptors by propofol but not by another allosteric activator, pentobarbital.
The standard electrophysiological concentration-response data were analyzed in the Monod-Wyman-Changeux (MWC) allosteric protein framework. This approach enables determination of equilibrium affinity of the closed receptor to the activator (KC) and a measure of gating efficacy (d) (more details below). It was shown that the β3(H267W) mutation had minimal effect on KC or d. However, substitutions at other locations lining the cavity had a drastic effect on propofol activation. The β3(Y143W) mutation strongly reduced gating efficacy. The β3(F221W), β3(Q224W), and β3(T266W) mutations resulted in receptors that showed no current responses in the presence of propofol. The mutations, however, had a relatively small effect on activation by pentobarbital indicative of a selective effect on activation by propofol [24]. The MWC analysis supported a model where the mutated residues interact with the propofol molecule in the active state where the tryptophan side chain results in an unfavorable interaction with the propofol molecule.
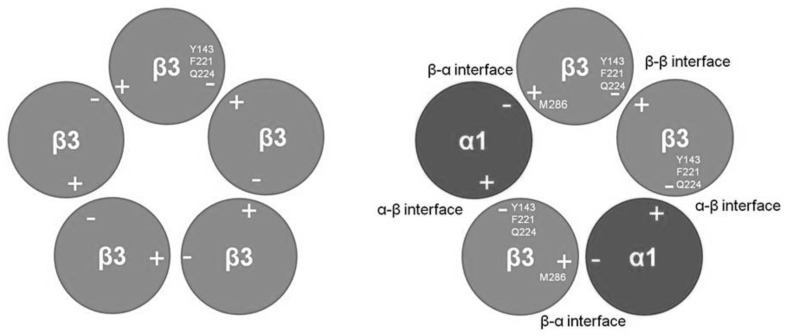
The Y143, F221, and Q224 residues are located at the “-” side of the β3 subunit (Fig. 1). In the homomeric β3 receptor there are five such interfaces and, accordingly, five identical interaction sites.
Fig. (1).
Top view of the β3 homomeric (left) and α1β3 heteromeric (right) GABAA receptor. The putative propofol binding sites in the β3 receptor are located at each of the five β-β interfaces, predominantly in the subunit contributing the “-” side of the interface. Tryptophan-substitutions of Y143, F221, and Q224 drastically reduce or abolish activation by propofol. We propose that in the α1β3 receptor, the propofol binding sites are located at the β-β and α-β where Y143, F221, and Q224 control drug interactions with the receptors, and at the β-α interfaces where the M286 defines the putative binding site.
The lack of effect of tryptophan-mutation of the photolabeled β3(H267) residue is surprising, but may be an artifact of the photolabeling mechanism. The relatively long half-life of the quinone methide [25], a major photo product of photolysis of ortho-propofol diazirine, may mean that the activated compound had diffused a short distance. However, the fact that of the numerous water accessible nucleophilic amino acids only β3(H267) was labeled indicates that ortho-propofol diazirine is concentrated near this residue.
The experiments did not directly address how the tryptophan substitutions affect receptor activation by propofol. Previous works probing structural features of neurosteroid and etomidate binding sites have proposed that introduction of a large hydrophobic sidechain in a strategic location may mimic the presence of a ligand in the site, and manifest as reduction in GABA EC50 and enhanced level of spontaneous activity [26, 27]. Indeed, spontaneous activity was strongly increased in β3(Y143W), β3(F221W), and β3(T266W). The tryptophan substitution may have decreased the volume of the binding cavity as was proposed for the β(M286W) mutation that retained sensitivity to 2,6,-dimethylphenol but not propofol (2,6-diisopropylphenol) in α1β2γ2 receptors [14]. In agreement with this notion, it was shown that activation of the β3(Y143W) receptor by smaller propofol analogues 2-isopropylphenol and 2,6-dimethylphenol was unchanged [24].
ELUCIDATION OF THE FUNCTIONAL ROLE OF LABELED RESIDUES IN Α1Β3 HETEROMERIC RECEPTORS
The initial study [24] examining the functional role of putative binding site residues near the top of the second transmembrane domain reported a controversy. The β3(T266W) mutation, that in β3 homomeric receptors abolished activation by propofol, was without effect in α1β3 heteromeric receptors. Furthermore, when β3(T266W) was combined with a tryptophan-substitution at the homologous location (I271) in the α1 subunit, the resulting α1(I271W) β3(T266W) receptors exhibited near-normal sensitivity to propofol [24].
A possible explanation is that α1β3 receptors contain additional interaction site(s) for propofol, that were not affected by mutations to the β3(T266) or α1(I271) residues. One potential candidate site for propofol interactions with heteromeric receptors is the β3(M286) residue that is photolabeled by the propofol analogue meta-azipropofol [20]. In this hypothetical model, propofol interacts with the “-” side of the β3 subunit in the cavity centered around the β3(H267) residue, and with the “+” side of the β3 subunit at the site defined by the M286 residue (Fig. 1).
To test this hypothesis, we generated doubly-mutated β3 subunits, where the M286W mutation was combined with one of Y143W, F221W, or Q224W. The mutated β3 subunits were co-expressed with the wild-type α1 subunit. The data reveal that mutagenesis of the defining residues in either putative site (M286 vs. Y143, F221, or Q224) alone had a relatively small effect on channel activation by propofol. The combination of mutations to both the “+” and “-” sides of the β3 subunit (i.e., M286W+Y143W or M286W+F221W) essentially abolished activation by propofol.
To reach these conclusions, we analyzed the data in the MWC allosteric model framework [28, 29]. This analysis has the strength that it can account independently for any effects on the inherent energy barrier for activation of the receptor, and the efficacy of an agonist for promoting activation. It provides estimates for the affinity of the resting receptor for an agonist (KC) and for the ratio of the affinity of the active receptor to that of the resting receptor (d = KO/KC), while taking into consideration the ability of receptor to undergo activation in the absence of activator. The latter is expressed as L0, the gating equilibrium constant of unliganded receptor, and calculated as (1-Po,spont)/Po,spont from experimental data. The parameter d measures the efficacy of the agonist for channel gating; a small value denotes a very large increase of affinity when the channel opens (high efficacy) while a value of 1 indicates that the affinities are identical for closed and open channels and no ability of the agonist to stabilize open channels.
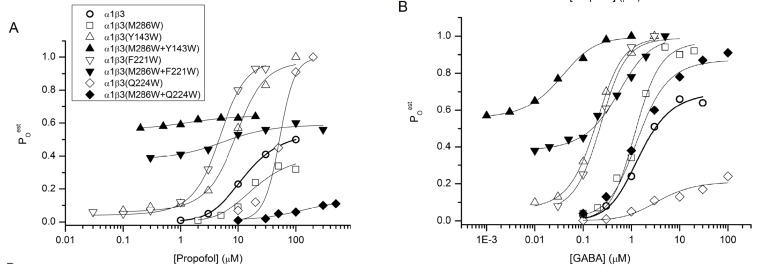
For analysis in the MWC framework, the standard normalized concentration-response data were converted to units of open probability. It should be pointed out that the standard activation curves showed a relatively small functional effect of any mutation or a combination of mutations. The EC50 for activation of wild-type α1β3 by propofol was 13 µM. The midpoints of the activation concentration-response curves for mutant receptors ranged from 2 to 69 µM with no clear systematic effect by any combination of mutations (Table 1).
Table 1.
Concentration-response properties for wild-type and mutant α1β3 GABAA receptors.
| Receptor | Po,spontest | Propofol | GABA | ||||
|---|---|---|---|---|---|---|---|
| EC50 (µM) | nH | Po,maxest | EC50 (µM) | nH | Po,maxest | ||
| α1β3 | 0 | 12.5±1.9 | 1.7±0.3 | 0.50±0.02 | 1.4±0.1 | 1.5±0.1 | 0.65±0.04 |
| α1β3(Y143W) | 0.06±0.01 | 12.8±2.9 | 1.6±0.1 | 1 | 0.18±0.02 | 1.7±0.1 | 1 |
| α1β3(F221W) | 0.04±0.01 | 5.0±0.5 | 1.9±0.3 | 1 | 0.24±0.01 | 1.5±0.1 | 1 |
| α1β3(Q224W) | 0 | 50±2 | 2.9±0.6 | 1 | 3.5±0.6 | 1.0±0.1 | 0.24±0.03 |
| α1β3(M286W) | 0 | 14.0±0.7 | 2.8±0.3 | 0.33±0.04 | 1.0±0.2 | 1.8±0.2 | 0.94±0.01 |
| α1β3(M286W+Y143W) | 0.56±0.03 | 1.6±0.2 | 1.5±0.2 | 0.64±0.02 | 0.06±0.01 | 0.8±0.1 | 0.96±0.09 |
| α1β3(M286W+F221W) | 0.38±0.04 | 6.6±0.8 | 1.1±0.1 | 0.60±0.04 | 0.65±0.11 | 1.1±0.1 | 1 |
| α1β3(M286W+Q224W) | 0.01±0.003 | 69±9 | 1.9±0.1 | 0.11±0.01 | 1.3±0.3 | 1.1±0.1 | 0.87±0.02 |
The table shows Po,spontest, and EC50, nH values and Po,max (mean ± S.E.M.) for propofol and GABA, from at least 4 cells under each condition. The concentration-response data were fitted, individually for each cell, with the following equation: Y=Ymax*([drug]nH/([drug]nH+EC50nH))
where EC50 is the concentration of drug producing a half-maximal effect, nH describes the slope of relationship, and Ymax is the high concentration asymptote.
The estimated open probability of spontaneously-active receptors (Po,spontest) was calculated by comparing holding current to the current levels in the presence of 100 µM picrotoxin (assumed Po = 0) and saturating GABA in the presence of 100 µM pentobarbital or 1 µM alphaxalone (assumed Po = 1). Po,spontest of 0 indicates that no consistent change in holding current was observed in the presence of 100 µM picrotoxin. Maximal open probability (Po,maxest) was determined by comparing peak responses to saturating propofol, saturating GABA, and saturating GABA in the presence of 100 µM pentobarbital or 1 µM alphaxalone. Po,maxest of 1 indicates that no increase in peak current was observed when either potentiator was co-applied with saturating GABA. In cases where Po,maxest < 1, the observed fold potentiation can be calculated as (Po,maxest)-1. The errors in estimating channel open probability may rise from incomplete blockade of spontaneously open channels during application of picrotoxin and the inability to reach a Po of 1 during application of saturating GABA and potentiator.
All shown mutations were made in the β3 subunit using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). Electrophysiological experiments were conducted using the two-electrode voltage clamp technique as described previously [24].
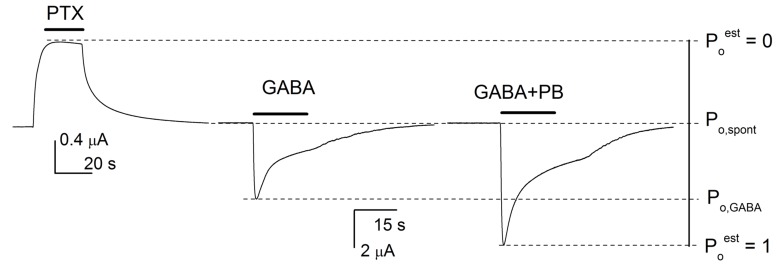
The conversion to units of open probability re-estimates current responses to an activator taking into consideration spontaneous activity, and maximal open probability that may be unattainable even in the presence of saturating concentrations of the activator. To estimate open probability of spontaneously active receptors (Po,spontest), receptors in the absence of activator were exposed to 100 µM picrotoxin, a blocker of the GABAA receptor. Under these conditions, any change in the holding current is an indication of block of spontaneous activity. Maximal open probability was obtained by exposing receptors to a saturating concentration of GABA in the presence of a potentiator (100 µM pentobarbital or 1 µM alphaxalone). The drug combination that produced the largest response was considered to have Po,maxest of 1. A graphic demonstration of the conversion is given in Fig. 2.
Fig. (2).
Graphic presentation of estimation of channel open probability. To estimate channel open probability for a given agonist, we first determined Po of spontaneously active receptors (Po,spontest) and conditions required to attain the maximal open probability (Poest of 1). The current level corresponding to estimated open probability of 0 was attained by exposing receptors to 100 µM picrotoxin (PTX). The current level corresponding to Poest of 1 was obtained by activating receptors with a saturating concentration of GABA in the presence of 100 µM pentobarbital (GABA + PB). All other current levels, including the holding current and the peak current in the presence of GABA or propofol, were compared to this range to obtain estimates of Po,spont, and Po in the presence of various concentrations of GABA or propofol. For illustrative purposes, the data traces were obtained from different receptors (block by picrotoxin from α1β3(M286W+F221W), potentiation by pentobarbital from α1β3 wild-type). The human α1β3 GABAA receptors were expressed in Xenopus oocytes. Harvesting of oocytes was conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The protocol was approved by the Animal Studies Committee of Washington University in St. Louis. Current traces were recorded using standard two-electrode voltage clamp as described previously [24].
Fitting the pooled Poest data showed that the individual mutations β3(Y143W), β3(F221W) and β3(M286W) have a relatively modest effect on KC and d for propofol. In the wild-type α1β3 receptor, the KC was 4.7 µM and d was 0.24 (KO can be calculated as 4.7 μM x 0.24 = 1.1 μM). In mutant receptors, affinity estimates ranged from 6 µM (M286W) to 23 µM (Y143W), and d between 0.23 (F221W) and 0.27 (both Y143W and M286W). The β3(Q224W) mutation was unusual in that it resulted in lower affinity to propofol (increased KC) but higher efficacy (reduced d).
The true effect of the double mutations becomes apparent in inspection of Fig. 3. Wild-type α1β3 and single mutant receptors exhibited relatively low levels of unliganded gating and little change in the maximal activation by propofol, so there is a large difference between Po,spontest and maximal Poest. In contrast, most of the double mutant receptors showed an increase in Po,spont with a reduction in maximal Poest. For example, receptors containing β3(M286W+Y143W) or β3(M286W+F221W) had Po,spontest of 0.56 or 0.38, respectively. Yet, enhanced unliganded gating was not accompanied by an increase in Po,maxest (0.64 and 0.60, respectively). In other words, for the double mutants there was a much smaller ability of propofol to elicit opening. The effects of the β3(M286W+Q224W) mutation appear somewhat different from the other two mutations. The mutated receptor had a low Po,spontest (0.01) but also a greatly reduced Po,maxest (0.11) in the presence of propofol.
The effect of mutation pairs manifested as an increase in d (KO/KC) rather than KC (Table 2). Propofol KC values in double mutants were generally similar to those in single mutants. In contrast, the ratios of open to closed receptor affinities (d) were strongly increased in all combinations of double mutants. In α1β3(M286W+Y143W), d was 0.93, indicating that the open receptor binds propofol less than 10% more tightly than the closed receptor. In α1β3 (M286W+F221W), d was 0.84. The double mutant receptor containing β3(M286W+Q224W) also had impaired gating with d (0.58) that was greater than that in wild-type (0.24), or in the β3(M286W) (0.27) or β3(Q224W) (0.04) single mutants. Hence, the data are most consistent with a model where propofol binding to the closed receptor is essentially unaffected by the mutations, while the tryptophan substitutions interfere with the ability of propofol to interact with these sites when the channel is open. It seems less likely that the substitutions strongly affect the conformational changes during channel gating per se, as the effects on activation by GABA were less marked. The data also suggest that the β-α and α-β/β-β interfaces, or more precisely the effects of tryptophan substitutions at selected sites in these interfaces, are energetically equivalent.
Table 2.
Summary of analysis of electrophysiological data from α1β3 receptors in the MWC allosteric protein framework.
| Receptor | L0 | Propofol | GABA | ||
|---|---|---|---|---|---|
| KC (µM) | d | KC (µM) | d | ||
| α1β3 | 1000 | 4.7±0.1 | 0.24±0.01 | 1.6±0.2 | 0.02±0.001 |
| α1β3(Y143W) | 15.7 | 23±6 | 0.27±0.03 | 2.8±1.6 | 0.02±0.001 |
| α1β3(F221W) | 24 | 13.1±1.5 | 0.23±0.01 | 2.0±1.8 | 0.03±0.02 |
| α1β3(Q224W) | 1000 | 336±435 | 0.04±0.05 | 2.0±0.7 | 0.06±0.01 |
| α1β3(M286W) | 1000 | 6.0±1.4 | 0.27±0.01 | 6.6±3.4 | 0.005±0.002 |
| α1β3(M286W+Y143W) | 0.79 | 1.4±0.3 | 0.93±0.01 | 0.6±0.4 | 0.08±0.05 |
| α1β3(M286W+F221W) | 1.63 | 5.7±1.6 | 0.84±0.01 | 8±7 | 0.07±0.05 |
| α1β3(M286W+Q224W) | 99 | 61±14 | 0.58±0.01 | 2.9±0.6 | 0.04±0.004 |
The effects of mutations on receptor affinity to the activator and gating efficacy were determined within the Monod-Wyman-Changeux allosteric model framework [28, 29]. This approach involves estimating the relationship between channel open probability and concentration of the activator. A graphic presentation of how open probability was estimated is shown in Fig. 2.
Parameters for binding and gating in the presence of propofol or GABA were derived from fitting the Poest from pooled data to the following equation [30, 31]:
Poest=(1+L0((1+[agonist]/KC)/(1+[agonist]/dKC))n)-1
where L0 is the ratio of the equilibrium occupancy of closed receptors to the equilibrium occupancy of open receptors in the absence of agonist, KC stands for the closed receptor equilibrium dissociation constant for a given agonist (propofol or GABA), d is a measure of efficacy expressed as the ratio of open receptor dissociation constant to closed receptor dissociation constant, and n is an integer that corresponds to the number of binding sites that need to be occupied to produce activation. The fitting results shown were obtained with n of 2 for GABA and 5 for propofol. The value for L0 was experimentally determined as (1-Po,spontest)/Po,spontest. Measurable spontaneous currents were not observed consistently with α1β3, α1β3(Q224W), and α1β3(M286W); accordingly L0 was held at an arbitrarily chosen value of 1000. KC and d were free parameters. Pooled Poest data from at least four cells were used for fitting.
Poest=(1+L0((1+[agonist]/KC)/(1+[agonist]/dKC))n)-1
where L0 is the ratio of the equilibrium occupancy of closed receptors to the equilibrium occupancy of open receptors in the absence of agonist, KC stands for the closed receptor equilibrium dissociation constant for activator, d is a measure of efficacy expressed as the ratio of open receptor dissociation constant to closed receptor dissociation constant, and n is the number of binding sites for the activator. The number of sites for propofol was constrained to 5. Additional fitting (not shown), conducted with n = 4 binding sites, did not show consistent and significant improvement in the goodness of the fit. The number of sites for GABA was constrained to 2. Additional details of analysis and the fitting results are given in Table 2.
As is evident from the data in Table 2 and Fig. 3, double mutant receptors retained some of their sensitivity to propofol. This indicates that tryptophan substitutions at sites studied are not fully effective at blocking activation or that additional, hitherto unidentified, sites mediate residual activation. We do not favor the latter possibility because of lack of drastic changes in slopes of activation curves in mutated receptors. In any case, residual activation in double mutants is negligible and changes in slopes poorly defined.
Fig. (3).
Functional results from studies of α1β3 GABAA receptors. The data points show averaged values for estimated open probability (Poest) of wild-type and mutant α1β3 receptors activated by propofol (A) or GABA (B). The Poest values were obtained as described in Fig. 2. The curves were generated by fitting the following equation:
The Discovery studio 2.5.5 molecular modeling package (Accelrys, Inc.) was used as described previously [33] to dock propofol in potential anesthetic binding pockets in a homology model of a human α1β3 GABAA receptor with a β3-α1-β3-α1-β3 subunit order. The previously described model [19] is based on the published structure of a human homopentameric β3 GABAA receptor [32]. The CHARMm-based molecular dynamics simulated-annealing program CDOCKER was used to dock propofol in the models. To determine whether propofol could be docked stably in the potential binding pockets that exist above and below position M2-17’ (β3(H267) or α1(S272) at the “-” side of β or α), a binding site sphere was centered at M2-17’. The binding sphere had a radius of 14 Å, to accommodate a potential binding pocket without allowing the ligand access to less constrained regions, i.e., outside the receptor structure. To produce a more favorable binding pocket near M2-17' at the α-β triplet site, the side chain of α1(I271) (α1M2-16') was rotated to match the orientation of β3(T266) (β3M2-16') in the crystal structure. Five propofol molecules of different orientations were separately seeded into each pocket and docking was performed for each seeded ligand using 50 random starting orientations for 50 high-temperature molecular-dynamics induced conformations (thus 2,500 individual simulated annealings with full potential minimization for each seeded ligand). The 100 lowest energy solutions were collected for each starting propofol molecule. Docking near β3(M286) was described previously [20] using a 12 Å radius sphere centered at the level of β3(F289) in a β3-α1 interface.
In contrast to the effects on activation by propofol, there were relatively small effects on activation by GABA. The affinities for the closed receptor were distributed around the value for the wild-type receptor. The values for d tended to be increased, but only from the wild-type value of 0.02 to a maximum of 0.08, as opposed to the essential loss of efficacy for propofol. Overall, the data indicate that the combination of β3(M286W) with one of β3(Y143W), β3(F221W) or β3(Q224W) strongly reduces gating efficacy for propofol but not GABA.
We note that these residues are conserved among the subtypes of the β subunit. Accordingly, the effects of mutations are expected to be similar in receptors containing the β1 or β2 subunits.
A STRUCTURAL MODEL EMERGING FROM FUNCTIONAL DATA
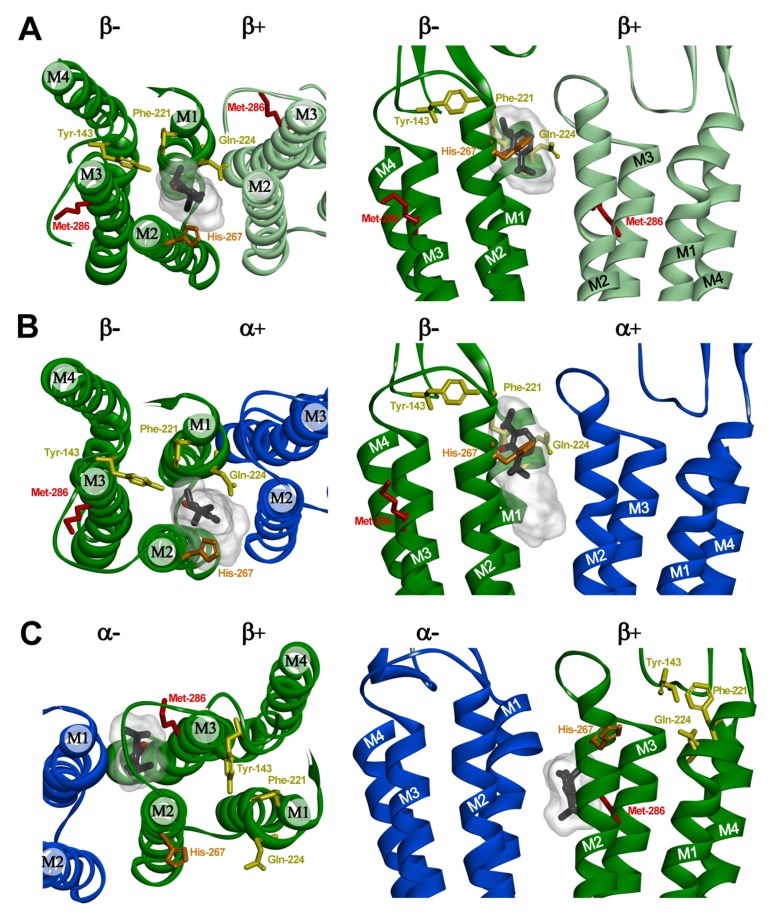
Molecular modeling was used to predict whether propofol could bind within the pocket containing the triplet mutated residues at the α1-β3 interface or at homologous pockets at the β3-β3 and β3-α1 interfaces. We used a homology model of a β3-α1-β3-α1-β3 receptor [20] based on the published structure of a human pentameric β3 receptor [32]. In this model, the three β3 subunits retained the crystallographic structures with only the two α1 subunits requiring homology replacement. Propofol was predicted to bind stably and with similar energies in the pockets between the extracellular and transmembrane domains in proximity to the triplet mutated residues at the β-β (Fig. 4A) and α-β (Fig. 4B) interfaces, as well as at the β-α interface (not shown). Within the β-β pocket, the lowest energy solution for propofol was 4.7 Å from β3(Y143), 3.5 Å from β3(F221), and 3.3 Å from β3(Q224) (Fig. 4A). For docking at the α-β triplet pocket, one of the top 20 solutions was 4.8 Å from β3(Y143), while the other docking solutions extended down into the transmembrane domain (Fig. 4B). The lowest energy solutions for docking at the homologous β-α interface triplet pocket (not shown) were positioned below the 17' residue in the second transmembrane domain (M2-17’), 9.6 Å from α1(F146) (homologous to β3(Y143)) and between the adjacent M2 helices, with less favorable interaction energies than either the β-β or α-β triplet pocket solutions. Computational docking predicted previously [20] that in the α1β3 GABAA receptor β3(M286) contributes to pockets at the β-α and β-β interfaces with appropriate size to bind propofol stably. This pocket is located in the transmembrane domain below the level of M2-17 between the second and third transmembrane helices of the “+” side of β and first and second transmembrane helices of the “-” side of α subunit (Fig. 4C). Based upon CDOCKER interaction energies, propofol is predicted to bind more favorably (by ~2-3 kcal/mol) to the β-β and α-β pockets containing the triplet mutated residues than to the pocket containing β3(M286).
Fig. (4).
Predicted propofol binding sites at intersubunit interfaces in an α1β3 GABAA receptor homology model. The figure shows views from the top with pore at the bottom (left panels) and sideviews from the pore for β-β (A), α-β (B), and β-α (C) interfaces (right panels). Note that the interfaces are referred to with the subunit providing the “+” side of the interface named first. The α1 subunit is shown in blue and the β3 subunits are shown in green and light green. The putative propofol binding site residues that were mutated to tryptophan at the β “-” surface (Y143, F221, Q224) are colored in yellow, and the residue photolabeled with ortho-propofol diazirine (H267) in orange. The defining propofol site residue mutated at the β “+” surface (M286) is shown in red. Propofol is shown in stick format color-coded by atom type (carbon, black; oxygen red) enclosed in a transparent Connolly surface covering the 20 lowest energy docking solutions for each pocket. Propofol is shown docked in its lowest energy solution at the β-β (A) and β-α (C) interfaces. At the α-β interface (B), the propofol molecule is shown in the lowest energy solution which best agrees with the functional data. The images in the bottom panels were derived from previous docking studies [20].
CONCLUSION
The data support the involvement of a region near the β3(M286) residue, originally identified by photolabeling with meta-azipropofol [20], at the β-α interface. The functional data for the “-” side of the β subunit (α-β and β-β interfaces) indicate that propofol gating is mediated by the region near β3(H267), previously identified with photolabeling with ortho-propofol diazirine [22], and involving residues β3(Y143), β3(F221), and β3(Q224).
From the analysis of electrophysiological data, conducted in the Monod-Wyman-Changeux allosteric protein framework, we propose a model where receptor activation is produced by propofol interactions with the β-α interface near the β3(M286) residue, and the β-β and α-β interfaces near the β3(Y143), β3(F221) or β3(Q224) residues. Any interface can mediate activation; hence substitutions at all interfaces are required to produce loss of activity in the presence of propofol.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health National Institute of General Medical Sciences to J.B. Cohen (GM058448), A.S. Evers (GM108799), and G. Akk (GM108580), and by funds from the Taylor Family Institute for Innovative Psychiatric Research to A.S. Evers and G. Akk.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Belelli D., Callachan H., Hill-Venning C., Peters J.A., Lambert J.J. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br. J. Pharmacol. 1996;118(3):563–576. doi: 10.1111/j.1476-5381.1996.tb15439.x. [http:// dx.doi.org/10.1111/j.1476-5381.1996.tb15439.x]. [PMID: 8762079]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison N.L., Simmonds M.A. Modulation of the GABA receptor complex by a steroid anaesthetic. Brain Res. 1984;323(2):287–292. doi: 10.1016/0006-8993(84)90299-3. [http://dx.doi.org/10.1016/0006-8993(84)90299-3]. [PMID: 6098342]. [DOI] [PubMed] [Google Scholar]
- 3.Cestari I.N., Uchida I., Li L., Burt D., Yang J. The agonistic action of pentobarbital on GABAA β-subunit homomeric receptors. Neuroreport. 1996;7(4):943–947. doi: 10.1097/00001756-199603220-00023. [http://dx.doi.org/10.1097/ 00001756-199603220-00023]. [PMID: 8724679]. [DOI] [PubMed] [Google Scholar]
- 4.Krishek B.J., Moss S.J., Smart T.G. Homomeric β 1 γ-aminobutyric acid A receptor-ion channels: evaluation of pharmacological and physiological properties. Mol. Pharmacol. 1996;49(3):494–504. [PMID: 8643089]. [PubMed] [Google Scholar]
- 5.Amin J., Weiss D.S. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366(6455):565–569. doi: 10.1038/366565a0. [http://dx.doi.org/ 10.1038/366565a0]. [PMID: 7504783]. [DOI] [PubMed] [Google Scholar]
- 6.Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K.E., Ledermann B., Antkowiak B., Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor β3 subunit. FASEB J. 2003;17(2):250–252. doi: 10.1096/fj.02-0611fje. [PMID: 12475885]. [DOI] [PubMed] [Google Scholar]
- 7.Hales T.G., Lambert J.J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br. J. Pharmacol. 1991;104(3):619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [http://dx.doi.org/10.1111/j.1476-5381.1991.tb12479.x]. [PMID: 1665745]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam D.W., Reynolds J.N. Modulatory and direct effects of propofol on recombinant GABAA receptors expressed in xenopus oocytes: influence of α- and γ2-subunits. Brain Res. 1998;784(1-2):179–187. doi: 10.1016/s0006-8993(97)01334-6. [http://dx.doi.org/10.1016/S0006-8993(97)01334-6]. [PMID: 9518600]. [DOI] [PubMed] [Google Scholar]
- 9.Orser B.A., Wang L.Y., Pennefather P.S., MacDonald J.F. Propofol modulates activation and desensitization of GABAA receptors in cultured murine hippocampal neurons. J. Neurosci. 1994;14(12):7747–7760. doi: 10.1523/JNEUROSCI.14-12-07747.1994. [PMID: 7996209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruesch D., Neumann E., Wulf H., Forman S.A. An allosteric coagonist model for propofol effects on α1β2γ2L γ-aminobutyric acid type A receptors. Anesthesiology. 2012;116(1):47–55. doi: 10.1097/ALN.0b013e31823d0c36. [http:// dx.doi.org/10.1097/ALN.0b013e31823d0c36]. [PMID: 22104494]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belelli D., Pistis M., Peters J.A., Lambert J.J. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol. Sci. 1999;20(12):496–502. doi: 10.1016/s0165-6147(99)01405-4. [http:// dx.doi.org/10.1016/S0165-6147(99)01405-4]. [PMID: 10603492]. [DOI] [PubMed] [Google Scholar]
- 12.Krasowski M.D., Koltchine V.V., Rick C.E., Ye Q., Finn S.E., Harrison N.L. Propofol and other intravenous anesthetics have sites of action on the γ-aminobutyric acid type A receptor distinct from that for isoflurane. Mol. Pharmacol. 1998;53(3):530–538. doi: 10.1124/mol.53.3.530. [PMID: 9495821]. [DOI] [PubMed] [Google Scholar]
- 13.Krasowski M.D., Jenkins A., Flood P., Kung A.Y., Hopfinger A.J., Harrison N.L. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of γ-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J. Pharmacol. Exp. Ther. 2001;297(1):338–351. [PMID: 11259561]. [PubMed] [Google Scholar]
- 14.Krasowski M.D., Nishikawa K., Nikolaeva N., Lin A., Harrison N.L. Methionine 286 in transmembrane domain 3 of the GABAA receptor β subunit controls a binding cavity for propofol and other alkylphenol general anesthetics. Neuropharmacology. 2001;41(8):952–964. doi: 10.1016/s0028-3908(01)00141-1. [http://dx.doi.org/10.1016/S0028-3908(01)00141-1]. [PMID: 11747900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3(2):102–114. doi: 10.1038/nrn731. [http://dx.doi. org/10.1038/nrn731]. [PMID: 11836518]. [DOI] [PubMed] [Google Scholar]
- 16.Bali M., Akabas M.H. Defining the propofol binding site location on the GABAA receptor. Mol. Pharmacol. 2004;65(1):68–76. doi: 10.1124/mol.65.1.68. [http://dx.doi.org/10.1124/mol.65.1.68]. [PMID: 14722238]. [DOI] [PubMed] [Google Scholar]
- 17.Cestari I.N., Min K.T., Kulli J.C., Yang J. Identification of an amino acid defining the distinct properties of murine β1 and β3 subunit-containing GABA(A) receptors. J. Neurochem. 2000;74(2):827–838. doi: 10.1046/j.1471-4159.2000.740827.x. [http://dx.doi.org/10.1046/j.1471-4159.2000.740827.x]. [PMID: 10646536]. [DOI] [PubMed] [Google Scholar]
- 18.Siegwart R., Jurd R., Rudolph U. Molecular determinants for the action of general anesthetics at recombinant α(2)β(3)γ(2)γ-aminobutyric acid(A) receptors. J. Neurochem. 2002;80(1):140–148. doi: 10.1046/j.0022-3042.2001.00682.x. [http://dx.doi.org/10.1046/j.0022-3042.2001.00682.x]. [PMID: 11796752]. [DOI] [PubMed] [Google Scholar]
- 19.Siegwart R., Krähenbühl K., Lambert S., Rudolph U. Mutational analysis of molecular requirements for the actions of general anaesthetics at the gamma-aminobutyric acidA receptor subtype, alpha1beta2gamma2. BMC Pharmacol. 2003;3:13. doi: 10.1186/1471-2210-3-13. [http://dx.doi. org/10.1186/1471-2210-3-13]. [PMID: 14613517]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayakar S.S., Zhou X., Chiara D.C., Dostalova Z., Savechenkov P.Y., Bruzik K.S., Dailey W.P., Miller K.W., Eckenhoff R.G., Cohen J.B. Multiple propofol-binding sites in a γ-aminobutyric acid type A receptor (GABAAR) identified using a photoreactive propofol analog. J. Biol. Chem. 2014;289(40):27456–27468. doi: 10.1074/jbc.M114.581728. [http://dx.doi.org/10.1074/jbc.M114.581728]. [PMID: 25086038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall M.A., Xi J., Lor C., Dai S., Pearce R., Dailey W.P., Eckenhoff R.G. m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J. Med. Chem. 2010;53(15):5667–5675. doi: 10.1021/jm1004072. [http://dx.doi.org/10.1021/jm1004072]. [PMID: 20597506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip G.M., Chen Z.W., Edge C.J., Smith E.H., Dickinson R., Hohenester E., Townsend R.R., Fuchs K., Sieghart W., Evers A.S., Franks N.P. A propofol binding site on mammalian GABAA receptors identified by photolabeling. Nat. Chem. Biol. 2013;9(11):715–720. doi: 10.1038/nchembio.1340. [http://dx.doi.org/10.1038/nchembio.1340]. [PMID: 24056400]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franks N.P. Structural comparisons of ligand-gated ion channels in open, closed, and desensitized states identify a novel propofol-binding site on mammalian γ-aminobutyric acid type A receptors. Anesthesiology. 2015;122(4):787–794. doi: 10.1097/ALN.0000000000000588. [http://dx.doi.org/10.1097/ ALN.0000000000000588]. [PMID: 25575161]. [DOI] [PubMed] [Google Scholar]
- 24.Eaton M.M., Cao L.Q., Chen Z., Franks N.P., Evers A.S., Akk G. Mutational analysis of the putative high-affinity propofol binding site in human β3 homomeric GABAA receptors. Mol. Pharmacol. 2015;88(4):736–745. doi: 10.1124/mol.115.100347. [http://dx.doi.org/10.1124/ mol.115.100347]. [PMID: 26206487]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva Gd., Bozzelli J.W. Quantum chemical study of the thermal decomposition of o-quinone methide (6-methylene-2,4-cyclohexadien-1-one). J. Phys. Chem. A. 2007;111(32):7987–7994. doi: 10.1021/jp073335c. [http://dx.doi.org/10.1021/jp073335c]. [PMID: 17645323]. [DOI] [PubMed] [Google Scholar]
- 26.Akk G., Li P., Bracamontes J., Reichert D.E., Covey D.F., Steinbach J.H. Mutations of the GABA-A receptor α1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol. Pharmacol. 2008;74(3):614–627. doi: 10.1124/mol.108.048520. [http://dx.doi.org/10.1124/mol.108.048520]. [PMID: 18544665]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart D., Desai R., Cheng Q., Liu A., Forman S.A. Tryptophan mutations at azi-etomidate photo-incorporation sites on α1 or β2 subunits enhance GABAA receptor gating and reduce etomidate modulation. Mol. Pharmacol. 2008;74(6):1687–1695. doi: 10.1124/mol.108.050500. [http://dx.doi.org/10.1124/mol.108.050500]. [PMID: 18805938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monod J., Wyman J., Changeux J.P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [http://dx.doi.org/10.1016/S0022-2836(65)80285-6]. [PMID: 14343300]. [DOI] [PubMed] [Google Scholar]
- 29.Forman S.A. Monod-Wyman-Changeux allosteric mechanisms of action and the pharmacology of etomidate. Curr. Opin. Anaesthesiol. 2012;25(4):411–418. doi: 10.1097/ACO.0b013e328354feea. [http://dx.doi.org/10.1097/ ACO.0b013e328354feea]. [PMID: 22614249]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y., Weiss D.S. Allosteric activation mechanism of the α 1 β 2 γ 2 γ-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophys. J. 1999;77(5):2542–2551. doi: 10.1016/s0006-3495(99)77089-x. [http://dx.doi.org/10.1016/S0006-3495(99)77089-X]. [PMID: 10545355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rüsch D., Zhong H., Forman S.A. Gating allosterism at a single class of etomidate sites on α1β2γ2L GABA A receptors accounts for both direct activation and agonist modulation. J. Biol. Chem. 2004;279(20):20982–20992. doi: 10.1074/jbc.M400472200. [http://dx.doi.org/10.1074/jbc.M400472200]. [PMID: 15016806]. [DOI] [PubMed] [Google Scholar]
- 32.Miller P.S., Aricescu A.R. Crystal structure of a human GABAA receptor. Nature. 2014;512(7514):270–275. doi: 10.1038/nature13293. [http://dx.doi.org/ 10.1038/nature13293]. [PMID: 24909990]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiara D.C., Jayakar S.S., Zhou X., Zhang X., Savechenkov P.Y., Bruzik K.S., Miller K.W., Cohen J.B. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human α1β3γ2 γ-aminobutyric acid type A (GABAA) receptor. J. Biol. Chem. 2013;288(27):19343–19357. doi: 10.1074/jbc.M113.479725. [http://dx. doi.org/10.1074/jbc.M113.479725]. [PMID: 23677991]. [DOI] [PMC free article] [PubMed] [Google Scholar]