Abstract
The interferon-gamma release assay (IGRA) is useful for diagnosing latent tuberculosis infection (LTBI), however the rate of negative conversion is high, especially in dialysis patients. Few studies have focused on predicting persistently positive patients who are at high risk of tuberculosis reactivation. We screened dialysis patients, and used QuantiFERON-TB Gold In-tube (QFT-GIT) to identify LTBI. Of the 157 participants who had initially positive QFT-GIT, 82 had persistently positivity and 75 had negative conversion. The persistently positive group were younger, more were current smokers, and had higher plasma level of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) and QFT-GIT responses than the negative conversion group. Multivariate logistic regression for persistent positivity revealed that high plasma sTREM-1 and QFT-GIT response, young age and TB contact history were independent factors. Currently smoking had borderline significance. The area under the receiver operating characteristic curve using the multi-factor model was 0.878, higher than 0.821 by QFT-GIT response of 0.95 IU/ml. In conclusion, dialysis patients with persistent LTBI status may be associated with a young age, high plasma sTREM-1, strong QFT-GIT response, currently smoking, and TB contact history. If resources are limited, these five predictors can be used to prioritize QFT-GIT-positive dialysis patients for LTBI treatment.
Tuberculosis (TB) remains one of the most important infectious diseases worldwide. According to World Health Organization (WHO) estimates, there were 9.6 million new TB cases and 1.5 million related deaths in 20141. Control strategies include early treatment to prevent transmission and treatment of latent TB infection (LTBI) to reduce its reactivation2. Patients with renal failure undergoing dialysis are at an increased risk of TB due to attenuated cellular immunity3,4, and it has been reported that the risk of developing active TB is 7.8–25 times higher5,6,7 in dialysis patients compared to the general population. However, the diagnosis of TB is usually delayed because of frequent extra-pulmonary manifestations8,9. Thus, early LTBI detection in this specific group is important2.
Currently, interferon-gamma release assays (IGRAs) are used to diagnose LTBI. However, positive results are not 100% accurate for LTBI, and problems with variations in results have been reported10, IGRAs have several advantages11,12,13, including their application in immuno-compromised patients14, patients who have received the Bacillus Calmette–Guérin (BCG) vaccine15, and in areas where NTM is highly prevalent16. The IGRA has been shown to have a positive rate of around 21–40% in patients undergoing hemodialysis17,18,19,20. However, recent reports have shown a high negative conversion rate with the quantiFERRON Gold In-tube (QFT-GIT) test, a kind of IGRA, in cohorts of health care workers (33% after 18 weeks)21, subjects with close TB contact (35% after 6 months)22, and in patients undergoing dialysis (46% after 6 months)10.
Although inter-experiment variations have been reported to account for 8% of cases of negative conversion23, many other dynamic changes are also involved. Even healthy subjects with close TB contacts have been reported to have a 35% six-month negative conversion rate without preventive treatment22. These findings question the clinical significance of a single positive IGRA result, especially in patients undergoing dialysis. Many reports have suggested increasing the threshold of the QFT-GIT to avoid discordant results in the range of uncertainty10,24. On the other hand, follow-up IGRA testing has also been reported to be useful, and persistently positive IGRA results are more convincing25.
However, performing serial IGRAs has several disadvantages including the time required for follow-up, increased cost, and uncertainty until the results of the second test. Thus, identifying predictors of persistently positive IGRA results at the outset is important to classify those at high risk. Even though increasing the initial QFT-GIT threshold has been reported to predict persistent positivity, the sensitivity is only about 79%10. Thus, the aim of this study was to analyze clinical characteristics and serum cytokines from patients on dialysis with initial positive QFT-GIT test results to establish a model for predicting persistently positive LTBI results.
Methods
We conducted this cohort study at National Taiwan University Hospital, a tertiary referral center, and its branches, regional teaching hospitals, and a local hemodialysis clinic. All of the study sites were located in northern Taiwan except for one in southern Taiwan. The Institutional Review Board of National Taiwan University Hospital approved the study. The study was conducted in accordance with approved guidelines, and all of the participants provided written informed consent.
Between May 2011 and April 2016, adult patients (age ≥20 years) with renal failure and treated with long-term (>3 months) dialysis were prospectively identified. Those with human immunodeficiency virus infection, liver cirrhosis of Child-Pugh class C26, cancer or autoimmune disease receiving chemotherapy within the last 3 months, and active TB within the last 3 years were excluded.
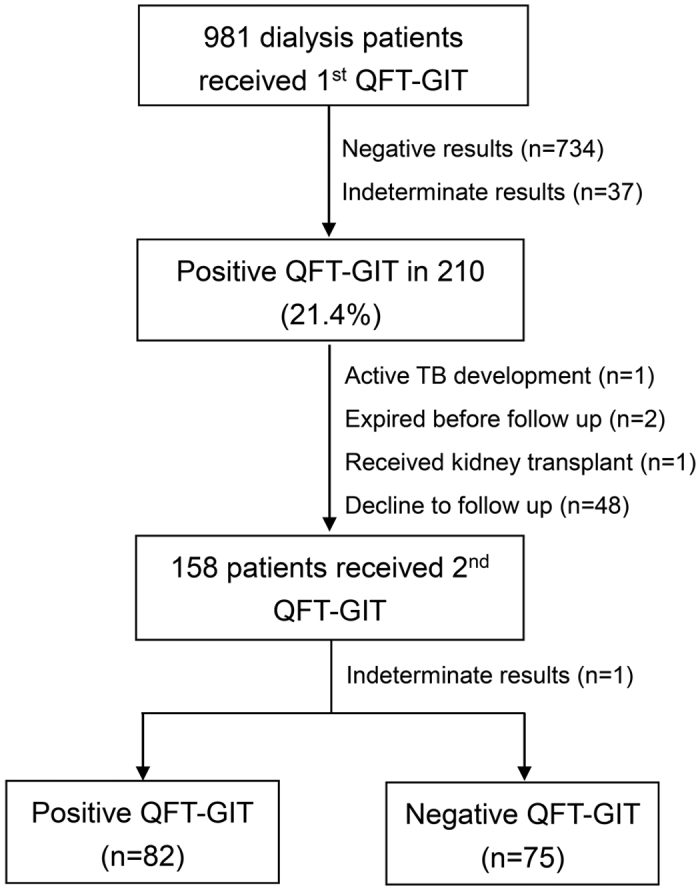
Peripheral blood was collected from the 981 participants at baseline. LTBI status was determined using a QuantiFERON-TB Gold In-tube assay (QFT-GIT) (Celestis, Australia) according to the manufacturer’s instructions27. We used a three-tube QFT-GIT kit, and QFT-GIT responses were calculated by subtracting the level of interferon-γ in the reaction supernatants of the negative control tube from that in TB-antigen tube. The results were defined as positive, negative, or indeterminate28,29.
According to the initial QFT-GIT test, 210 (21.4%) of the 981 enrolled cases had positive results. Among them, 158 cases had follow up of QFT-GIT, including 82 persistent positive results, 75 negative conversions and one becoming indeterminate (Fig. 1). We measured levels of cytokines in all plasma samples from all of the participants except for 24 in whom the markers were measured in the supernatant of the QFT-GIT NIL tube due to a small amount of collected plasma. Inflammatory markers including interferon-gamma (IFN-γ) (R&D Systems, Inc., MN, USA) and soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) (Aviscera Bioscience, Inc., CA, USA), and anti-inflammatory markers (interleukin-10 [IL-10] [R&D Systems, Inc., MN, USA], and decoy receptor 3 [DcR3] [R&D Systems Europe, Abingdon, UK]) were measured using enzyme-linked immunosorbent assays.
Figure 1. The flow chart of participant enrollment.
Data Collection
The demographic and clinical data, including age, sex, underlying co-morbidities, prior TB history, respiratory and constitutional symptoms, smoking status, blood hemoglobin and serum albumin levels were recorded in a standardized case report form. Cough ≥3 weeks was defined as chronic cough. Current smokers were defined as those who had smoked >100 cigarettes, with the last time of smoking within 1 month prior to the study30. A history of TB household contact was defined as previously sharing the same living space with an active pulmonary TB patient.
The radiographic findings were classified into “no lung parenchymal lesions”; “lung lesions not compatible with TB”; “lung lesions compatible with prior TB”, or “lung lesions, cannot be excluded for TB”. “Lung lesions compatible with TB” was defined as new patches of consolidation, collapse, lymphadenopathy, mass or nodule, or cavitary lesion without any other proven etiology7. Prior TB was defined radiographically as fibrotic infiltrates with pleural thickening or calcified nodules over the upper lung fields, or other fibrotic lesions documented from previous TB31.
Statistical Analysis
Inter-group differences were analyzed using the Student’s t test for numerical variables and the chi-square test for categorical variables. Multivariate logistic regression analysis was used to identify factors associated with persistently positive IGRA results. All potential predictors were included in backward conditional stepwise selection. A two-sided p < 0.05 was considered to be statistically significant. The discriminative power of each biomarker for persistently positive QFT-GIT was determined using receiver operating characteristic (ROC) curve and area under the curve (AUC) analyses. The optimal cut-off value, defined as the one with the least (1 − sensitivity)2 + (1 − specificity)2, was used to calculate sensitivity and specificity. All analyses were performed using SPSS software version 19.0 (SPSS, Chicago, IL).
Results
Among the 157 dialysis patients with initial positive QFT-GIT, 82 had persistently positive QFT-GIT results and 75 had negative conversions. The average age of the persistently positive group was 60.2 years, which was lower than that in the negative conversion group (64.0 years, p = 0.032) (Table 1). The persistently positive group had a higher proportion of current smokers (27% vs. 13%, p = 0.036). Other clinical characteristics including sex, dialysis mode and duration, history of prior TB or TB contact, underlying diabetes mellitus, radiographic lesions, and respiratory symptoms were comparable between the two groups. With regards to the laboratory data (Table 2), blood hemoglobin was similar between the two groups, however albumin level was higher in the persistently positive group (4.1 vs. 4.0 g/dL, p = 0.042). Plasma level of the sTREM-1 was higher in the persistently positive group (594.8 vs. 183.1 pg/ml, p = 0.047), however there were no significant differences in anti-inflammatory markers (IL-10 and DcR3) and plasma IFN-γ between the two groups. In addition, the QFT-GIT response, calculated by subtracting the IFN-γ level in the negative control tube from the TB-antigen tube, was higher in the persistently positive group (3.8 vs. 1.1 IU/ml, p < 0.001).
Table 1. Baseline clinical characteristics.
| Persistently positive QFT-GIT (n = 82) | Negative conversion of QFT-GIT (n = 75) | p value | |
|---|---|---|---|
| Age, year | 60.2 (10.9) | 64.0 (10.9) | 0.032 |
| Male sex | 50 (61%) | 39 (52%) | 0.257 |
| Current smoking | 22 (27%) | 10 (13%) | 0.036 |
| Dialysis mode, HD | 73 (89%) | 63 (84%) | 0.356 |
| Dialysis age, year | 6.4 (5.4) | 5.7 (5.5) | 0.417 |
| Diabetes mellitus | 26 (32%) | 23 (31%) | 0.888 |
| Kidney transplant | 2 (2%) | 0 | 0.173 |
| Prior TB history | 9 (11%) | 5 (8%) | 0.344 |
| History of TB household contact | 9 (11%) | 3 (4%) | 0.100 |
| Radiological lesions* | 10 (11%) | 7 (9%) | 0.670 |
| Presence of symptomsǂ | 20 (24%) | 13 (17%) | 0.278 |
Abbreviations: HD, hemodialysis; QFT-GIT, quantiFERON-TB Gold In-tube; TB, tuberculosis.
Data are presented as number (%) or mean (standard deviation).
*Represents radiological lesions, compatible with prior TB or TB cannot be excluded.
ǂIndicates chronic cough, dyspnea, fever, and other constitutional symptoms.
Table 2. Laboratory results based on the status of following quantiFERON-TB Gold In-tube (QFT-GIT).
| Persistently positive QFT-GIT (n = 82) | Negative conversion of QFT-GIT (n = 75) | p value | |
|---|---|---|---|
| Hemoglobin, g/dL | 10.7 (1.4) | 10.8 (1.5) | 0.654 |
| Serum albumin, g/dL | 4.1 (0.3) | 4.0 (0.3) | 0.042 |
| DcR3, pg/ml | 1622.7 (1182.8) | 1406.6 (1399.9) | 0.298 |
| Interferon-gamma, pg/ml | 117.2 (417.4) | 68.7 (327.5) | 0.424 |
| sTREM-1, pg/ml | 594.8 (1824.5) | 183.1 (300.8) | 0.047 |
| Interleukin-10, pg/ml | 18.5 (29.3) | 32.0 (178.6) | 0.504 |
| QFT-GIT response, IU/ml | 3.8 (3.3) | 1.1 (1.6) | <0.001 |
Abbreviations: DcR3, decoy receptor 3; sTREM-1, soluble triggering receptor expressed on myeloid cells-1.
Data are presented as mean (standard deviation).
In multivariate analysis for the predictors of persistently positive QFT-GIT results (Table 3), a higher level of serum sTREM-1 (OR: 1.001, 95% CI: 1.000–1.002, per 1 pg/ml increment), history of TB contact (OR: 5.040, 95% CI: 1.049–24.201, versus absence), age (OR: 0.939, 95% CI: 0.901–0.979, per year increment), and QFT-GIT response (OR: 2.450, 95% CI: 1.672–3.590, per 1 IU/ml increment) were significantly associated with persistent positivity. Although currently smoking (OR: 2.460, 95% CI: 0.896–6.759, versus ex- or non-smoking) was only borderline statistically significant, it was entered into the final multivariate model. The probability of persistently positive QFT-GIT results was calculated according to the model equation (1):
Table 3. Multivariate logistic regression analysis for predicting persistently positive quantiFERON-TB Gold In-tube (QFT-GIT) among patients with initially positive results.
| Characteristics | Multivariate |
|
|---|---|---|
| p value | OR (95% C.I.) | |
| Age, year | 0.003 | 0.939 (0.901–0.979) |
| Sex, male vs. female | 0.643 | |
| Smoking, current vs. non-smoking | 0.081 | 2.460 (0.896–6.759) |
| Diabetes mellitus, presence vs. absence | 0.950 | |
| Dialysis mode, PD vs. HD | 0.251 | |
| Prior TB history, presence vs. none | 0.402 | |
| History of TB contact, presence vs. none | 0.043 | 5.040 (1.049–24.201) |
| Radiologic lesions*, presence vs. none | 0.597 | |
| Symptomsǂ, presence vs. none | 0.559 | |
| Hemoglobin, g/dL | 0.350 | |
| Serum albumin, g/dL | 0.505 | |
| DcR3, pg/ml | 0.153 | |
| Interferon-gamma, pg/ml | 0.875 | |
| sTREM-1 | 0.050 | 1.001 (1.000†–1.002) |
| Interleukin-10, pg/ml | 0.729 | |
| QFT-GIT response, IU/ml | <0.001 | 2.450 (1.672–3.590) |
Abbreviations: DcR3, decoy receptor 3; HD, hemodialysis; PD, peritoneal dialysis; sTREM-1, soluble triggering receptor expressed on myeloid cells-1; TB, tuberculosis.
*Represents radiological lesions, compatible with prior TB or TB cannot be excluded.
ǂIndicates chronic cough, dyspnea, fever, and other constitutional symptoms.
†1.00000018.
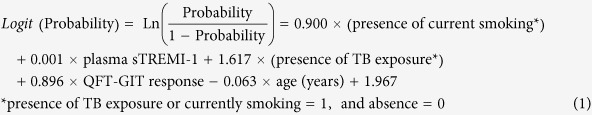 |
Using QFT-GIT response and the 5-factor model to predict persistently positive IGRA results, we plotted receiver operating characteristic (ROC) curves (Fig. 2). The probability of the 5-factor model had an area under the curve (AUC) of 0.878, which was higher than that 1 by QFT-GIT response (0.821). The optimal values were 0.95 IU/ml and 0.42 for the initial QFT-GIT response and the probability of the 5-factor model, respectively. Cost and performance analyses are shown in Table 4. Using a higher QFT-GIT cut-off value of 0.95 IU/m could detect 79% of the persistently positive case in follow-up QFT-GIT. The 5-factor model could also improve the sensitivity and specificity with a smaller increase in cost (5.7 USD) than the QFT-GIT assay alone.
Figure 2. The receiver operating characteristic (ROC) curves according to different factors for predicting persistently positive quantiFERON-TB Gold In-tube results initially and 6 months later.
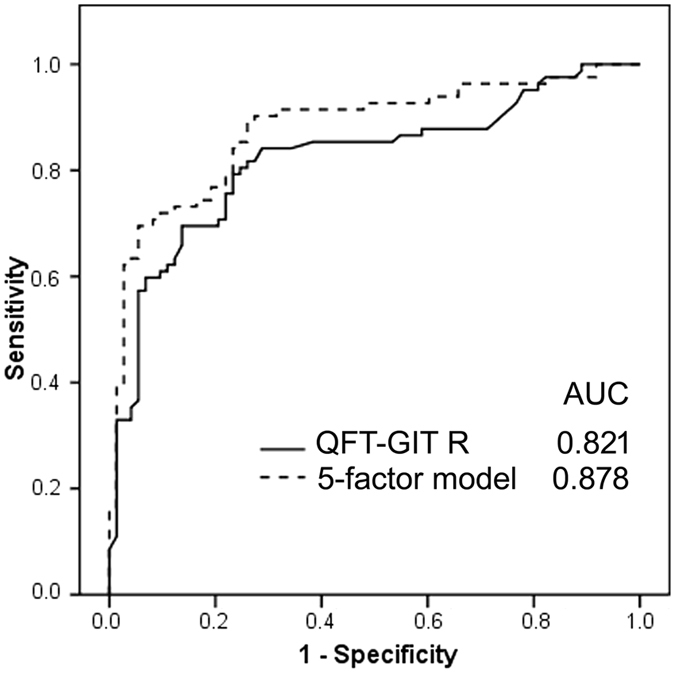
AUC, area under the curve; QFT-GIT R, response of quantiFERON-TB Gold In-tube; 5-factor Model including serum sTREM-1, QFT-GIT R, history of TB contact, age and currently smoking.
Table 4. Cost and effectiveness of different predictive markers.
| Markers | Cost* (USD) | Cut-off value | Sen. | Spe. | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|---|---|
| 1st QFT-GIT R | 47.5 | 0.95 IU/ml | 79% | 76% | 78% | 77% | 3.30 (2.18–5.02) | 0.275 (0.18–0.42) |
| 5-factor modelǂ | 53.2 | 0.42 | 84% | 77% | 80% | 80% | 3.56 (2.32–5.46) | 0.22 (0.14–0.36) |
Abbreviations: QFT-GIT R, the response of quantiFERON-TB Gold In-tube; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value.
*Including assay cost only.
ǂIncludes QFT-GIT response, plasma sTREM-1, history of TB contact, age and currently smoking.
USD is calculated at an exchange rate of 31.6 to NTD on March 16, 2015.
Discussion
In the present study, we followed dialysis patients with an initially positive QFT-GIT result, and found a high negative conversion rate of 48% after 6 months. Compared to those with negative conversion of the QFT-GIT result, those with persistently positive results were younger and had a higher rate of current smokers as well as higher levels of albumin, plasma sTREM-1 and QFT-GIT responses than those with negative conversion. In multivariate analysis, QFT-GIT response, sTREM-1, age and history of TB exposure were independent risk factors for a persistently positive result. The multi-factor model could predict persistently positive IGRA results with a sensitivity of 84% and specificity of 77%.
The high negative conversion rate in this study is comparable to previous reports10,21,22, and deeply influences the application of the IGRA because the temporal positivity might be considered to exclude patients from preventive therapy. Due to the high cost and time of repeat IGRAs, some markers at the first assay have been suggested to predict persistent positivity. Among them, a high initial QFT-GIT response has been reported to be an indicator of a reduction in “gray-zone” variations22. However, the sensitivity was only 79% in the present study, similar to a previous report10. We also evaluated clinical factors and laboratory markers, and found that a younger age, history of TB contact, and plasma sTREM-1 level were significant predictors as well as QFT-GIT response.
The AUC of the QFT-GIT response was 0.821 in the present study, which is comparable to a previous study (AUC 0.815)10. In comparison, the 5-factor model had an AUC of 0.878. This means that measuring the level of sTREM-1, at a cost of 5.7 USD, and recording data on age, smoking status and history of TB contact in addition to the QFT-GIT assay could increase the predictive accuracy of persistent positivity by 5% sensitivity more than using QFT-GIT alone while maintaining a similar specificity (77%). The positive and negative predicted values reached 80%, and it could save 47.5 USD per case for a repeat IGRA 6 months later. With regards to selecting targeted LTBI population for preventive therapy, good negative likelihood ratio (0.22) by the 5-factor model could screen out transiently positive LTBI (negative conversion) and save cost of LTBI treatment.
The inflammatory marker sTREMI-1 was significantly associated with persistent positivity in QFT-GIT. This may be because a higher inflammatory status with LTBI may persist for longer. TREM-1 is expressed on neutrophils and macrophages and is up-regulated during bacterial infections. In addition, it can trigger and amplify inflammatory responses. In contrast, although the level of IFN-γ was relatively higher in the persistently positive group, it was not significant in uni- or multivariate analysis. This is consistent with a previous report which showed that sTREM-1 was more specific than IFN-γ for mycobacteria infections32. Anti-inflammatory cytokines such as IL-10 and immuno-modulator of DcR3 did not seem to be statistically significant, which may be due to a less significant role in inflammation in LTBI.
TB household contact is a factor for both LTBI and active TB. Although few studies have reported a correlation with LTBI persistence, we suggest that definite TB household contact may result in a longer duration of exposure than those without TB contact, and this may explain the correlation with LTBI persistence. Currently smoking has been proven to be an important factor for LTBI and active TB20,30. However, it was only borderline significant for LTBI persistence in this study, and further studies are necessary to clarify its role. Old age is also a known risk factor for LTBI, however it had an opposite effect on persistent LTBI in the present study. A possible explanation for the lower persistence of positive QFT-GIT responses in the older dialysis patients may be due to immune dysfunction, which leads to a shorter period of positive IGRA results. This is an important issue for the application of IGRAs in dialysis patients, because the average age of dialysis patients is usually older.
The present study has some limitations. First, the case number is small and firm conclusions require future large-scale validation. Second, external generalization is limited in dialysis patients, and the use of the prediction model for other immune-compromised population needs further investigation.
In conclusion, the negative conversion rate of positive IGRA results in dialysis patients was as high as 48% in this study. To save cost and avoid a long duration of follow-up, the focus should be on those with a young age, high QFT-GIT response, high level of plasma sTREM-1, currently smoking and those with a history of TB household contact, as they have a high probability of being persistently positive in QFT-GIT.
Additional Information
How to cite this article: Shu, C.-C. et al. Inflammatory markers and clinical characteristics for predicting persistent positivity of interferon gamma release assay in dialysis population. Sci. Rep. 6, 34577; doi: 10.1038/srep34577 (2016).
Acknowledgments
The authors thank the staff of the Eighth Core Lab of the Department of Medical Research of National Taiwan University Hospital for their technical support as well as the Department of Medical Research and Information Technology Office. This study was funded by grants from the Research Center for Biotechnology and Medicine Policy in Taiwan and the Center of Disease Control, Ministry of Health and Welfare, Taiwan (MOHW105-CDC-C-114-000103, 4-3). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Parts of the study results were presented as a poster discussion in the 2015 ATS meeting.
Footnotes
Author Contributions Dr. C.-C.S. conceptualized the study. Drs C.-C.S., C.-L.H., C.-Y.L., V.-C.W., J.-Y.W. and F.-J.Y. participated in the sample and clinical data collection. Drs C.-C.S., Prof. L.-N.L. and Prof. C.-J.Y. were involved in the data analysis and manuscript writing.
References
- Laura Anderson et al. Global Tuberculosis Report 2015 20th edition (World Health Organization, 2015). [Google Scholar]
- Rose D. N. Benefits of screening for latent Mycobacterium tuberculosis infection. Arch Intern Med 160, 1513–1521 (2000). [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R. Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 350, 2060–2067 (2004). [DOI] [PubMed] [Google Scholar]
- Li S. Y. et al. Mycobacterium tuberculosis infection of end-stage renal disease patients in Taiwan: a nationwide longitudinal study. Clin Microbiol Infect 17, 1646–1652 (2011). [DOI] [PubMed] [Google Scholar]
- Chen D. Y., Shen G. H., Hsieh T. Y., Hsieh C. W. & Lan J. L. Effectiveness of the combination of a whole-blood interferon-gamma assay and the tuberculin skin test in detecting latent tuberculosis infection in rheumatoid arthritis patients receiving adalimumab therapy. Arthritis Rheum 59, 800–806 (2008). [DOI] [PubMed] [Google Scholar]
- Lundin A. P., Adler A. J., Berlyne G. M. & Friedman E. A. Tuberculosis in patients undergoing maintenance hemodialysis. Am J Med 67, 597–602 (1979). [DOI] [PubMed] [Google Scholar]
- Smirnoff M., Patt C., Seckler B. & Adler J. J. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis. Chest 113, 25–27 (1998). [DOI] [PubMed] [Google Scholar]
- Venkata R. K., Kumar S., Krishna R. P., Kumar S. B. & Padmanabhan S. Tuberculosis in chronic kidney disease. Clin Nephrol 67, 217–220 (2007). [DOI] [PubMed] [Google Scholar]
- Fang H. C. et al. Tuberculosis in patients with end-stage renal disease. Int J Tuberc Lung Dis 8, 92–97 (2004). [PubMed] [Google Scholar]
- Shu C. C. et al. Dynamic changes in positive interferon-gamma release assay in a dialysis population: An observational cohort study. J Infect 67, 529–535 (2013). [DOI] [PubMed] [Google Scholar]
- Simsek H. et al. Comparison of tuberculin skin testing and T-SPOT.TB for diagnosis of latent and active tuberculosis. Jpn J Infect Dis 63, 99–102 (2010). [PubMed] [Google Scholar]
- Brock I., Weldingh K., Lillebaek T., Follmann F. & Andersen P. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am J Respir Crit Care Med 170, 65–69 (2004). [DOI] [PubMed] [Google Scholar]
- Diel R., Loddenkemper R., Meywald-Walter K., Niemann S. & Nienhaus A. Predictive value of a whole blood IFN-gamma assay for the development of active tuberculosis disease after recent infection with Mycobacterium tuberculosis. Am J Respir Crit Care Med 177, 1164–1170 (2008). [DOI] [PubMed] [Google Scholar]
- Mazurek G. H. et al. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 59, 1–25 (2010). [PubMed] [Google Scholar]
- Yu M. C. et al. Annual risk of tuberculous infection in Taiwan, 1996–1998. J Formos Med Assoc 98, 496–499 (1999). [PubMed] [Google Scholar]
- Bai K. J. et al. Tuberculous empyema. Respirology 3, 261–266 (1998). [DOI] [PubMed] [Google Scholar]
- Triverio P. A. et al. Interferon-gamma release assays versus tuberculin skin testing for detection of latent tuberculosis in chronic haemodialysis patients. Nephrol Dial Transplant 24, 1952–1956 (2009). [DOI] [PubMed] [Google Scholar]
- Lee S. S. et al. High prevalence of latent tuberculosis infection in patients in end-stage renal disease on hemodialysis: Comparison of QuantiFERON-TB GOLD, ELISPOT, and tuberculin skin test. Infection 37, 96–102 (2009). [DOI] [PubMed] [Google Scholar]
- Lee S. S. et al. High prevalence of latent tuberculosis infection in dialysis patients using the interferon-gamma release assay and tuberculin skin test. Clin J Am Soc Nephrol 5, 1451–1457 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y. et al. Interferon-gamma release assay and Rifampicin therapy for household contacts of tuberculosis. J Infect 64, 291–298 (2012). [DOI] [PubMed] [Google Scholar]
- Ringshausen F. C. et al. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect Dis 10, 220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetifa I. M. et al. Interferon-gamma ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am J Respir Crit Care Med 187, 439–445 (2013). [DOI] [PubMed] [Google Scholar]
- Metcalfe J. Z. et al. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 187, 206–211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalesi F. et al. Serial QuantiFERON TB-gold in-tube testing during LTBI therapy in candidates for TNFi treatment. J Infect 66, 346–356 (2013). [DOI] [PubMed] [Google Scholar]
- Shu C. C. et al. Risk of Tuberculosis Among Patients on Dialysis: The Predictive Value of Serial Interferon-Gamma Release Assay. Medicine (Baltimore) 95, e3813 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh R. N., Murray-Lyon I. M., Dawson J. L., Pietroni M. C. & Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60, 646–649 (1973). [DOI] [PubMed] [Google Scholar]
- Lalvani A. et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med 163, 824–828 (2001). [DOI] [PubMed] [Google Scholar]
- Dyrhol-Riise A. M. et al. Diagnosis and follow-up of treatment of latent tuberculosis; the utility of the QuantiFERON-TB Gold In-tube assay in outpatients from a tuberculosis low-endemic country. BMC Infect Dis 10, 57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banach D. B. & Harris T. G. Indeterminate QuantiFERON(R)-TB Gold results in a public health clinic setting. Int J Tuberc Lung Dis 15, 1623–1630 (2011). [DOI] [PubMed] [Google Scholar]
- Lin H. H., Ezzati M., Chang H. Y. & Murray M. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 180, 475–480 (2009). [DOI] [PubMed] [Google Scholar]
- Jasmer R. M. et al. Twelve months of isoniazid compared with four months of isoniazid and rifampin for persons with radiographic evidence of previous tuberculosis: an outcome and cost-effectiveness analysis. Am J Respir Crit Care Med 162, 1648–1652 (2000). [DOI] [PubMed] [Google Scholar]
- Shu C. C. et al. Use of soluble triggering receptor expressed on myeloid cells-1 in non-tuberculous mycobacterial lung disease. Int J Tuberc Lung Dis 15, 1415–1420 (2011). [DOI] [PubMed] [Google Scholar]


