Abstract
Interleukin (IL) 16 plays a key role in inflammatory diseases as well as in tumorigenesis of osteosarcoma (OS). The aim of this study was to investigate the association of IL16 polymorphisms and plasma IL16 level with OS risk in a Chinese population. We genotyped IL16 rs4778889, rs11556218, and rs4072111 in 358 patients with OS and 402 controls using a polymerase chain reaction-restriction fragment length polymorphism assay. Plasma IL16 level was measured by enzyme-linked immunosorbent assay. Rs11556218 was associated with an increased risk of OS in heterozygote comparison (adjusted OR = 1.65, 95% CI, 1.23–2.21, P < 0.001), dominant model (adjusted OR = 1.66, 95% CI, 1.24–2.21, P < 0.001), and allele comparison (adjusted OR = 1.44, 95% CI, 1.14–1.81, P = 0.002). Moreover, rs11556218 TG/GG genotypes were associated with higher levels of IL16 as compared to TT genotype (P = 0.03). However, no significant association of rs4778889 and rs4072111 and OS was found. These findings suggest that rs11556218 TG/GG genotypes may be associated with increased susceptibility to OS, probably by increasing the production of IL16 level.
Osteosarcoma (OS), derived from primitive transformed cells of mesenchymal origin, is an aggressive malignant neoplasm in bone and often occurs in children and young adults1,2,3,4. Through improvements of treatment strategies, the 5-year survival rate of localized OS patients has increased to 60–80%, while the survival rate is only 25% in patients with metastases at the time of diagnosis3,5,6,7. It is of great importance, therefore, to uncover the molecular mechanism involved in the tumorigenesis of OS. It has been identified that radiation and chemicals exposure are risk factors for the development of OS8,9. However, only a few individuals exposed to the similar risk factors develop OS, suggesting that OS is a complex, multistep, and multifactorial disease10,11. Previously, several candidate genes have been reported to influence individuals’ susceptibility to OS, such as glutamate receptor metabotropic 4 gene, interleukin (IL) 1β gene, IL6, and IL12 12,13,14,15.
IL16 is a pleiotropic cytokine and functions as a modulator not only in inflammatory processes but also in tumorigenesis. By binding to the CD4 molecule, IL16 can activate monocytes and stimulate the secretion of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL1β, IL6, and IL15. These cytokines were related to the development of OS16,17,18,19,20,21,22,23,24.
IL16, located on chromosome 15q26.3 in the human genome, encodes IL16 cytokine25. Recently, association studies have been carried out to investigate the relationship of single nucleotide polymorphisms (SNPs) in IL16 with risk of a series of cancer types, including colorectal cancer26,27, gastric cancer26,28,29,30, nasopharyngeal carcinoma31,32, hepatocellular carcinoma33, prostate cancer34, renal cell carcinoma35,36, and glioma37. No report, nevertheless, was performed to examine the association between three common SNPs (i.e., rs4778889, rs11556218, and rs4072111) in IL16 and OS risk. In this study, we evaluated the association of the three SNPs in IL16 with susceptibility to OS in a Chinese population. Moreover, the effect of IL16 polymorphisms on plasma level of IL16 was also assessed.
Materials and Methods
Ethics Statement
The study protocol was approved by the Review Boards of Affiliated Hospital of Youjiang Medical College for Nationalities. The study was carried out in accordance with the relevant guidelines. Informed consent was signed by each adult participant or guardians on the behalf of the children participants.
Study population
This study population included 358 OS patients and 402 healthy controls from the Affiliated Hospital of Youjiang Medical College for Nationalities between January 2008 and June 2015. Diagnosis of OS was confirmed by histological examination. Clinical information was extracted from medical records, including age of diagnosis, gender, family history of cancer, tumor location, and metastasis. We excluded those patients with a history of familial cancer from this study. The control subjects were recruited from individuals who underwent routine health examination at the same hospital during the same period. The selection criteria for control subjects were as follows: healthy volunteers without OS, hypertension, and diabetes mellitus; no history of any cancer, cardiovascular diseases, and other inflammatory diseases; no family history of any cancer. The controls were frequency matched to cases in terms of age, gender, and residence area. All subjects were unrelated Han Chinese.
SNPs selection
We selected three SNPs in IL16, which have been identified to be functional previously38,39,40: rs4778889 (c. −475 T > C); rs11556218 (c. 3441 T > G; p. Asn1147Lys); and rs4072111 (c. 1300 C > T; p. Pro434Ser).
Genotyping
Genomic DNA was extracted from peripheral blood using a commercial kit according to the manufacturer’s manuals (Tiangen Inc., Beijing, China). The polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) assay was carried out to genotype IL16 rs4778889, rs11556218, and rs4072111. PCR primer sequences, annealing temperature, restriction enzymes used, and length of PCR products were prepared as described previously26. The genotyping results were confirmed by Sanger sequencing.
Plasma IL16 Level
Blood samples were obtained from patients with OS and healthy controls. After centrifugation at 1000 g for 10 min, the plasma was stored at −80 °C until analysis. Plasma IL16 concentration was measured by enzyme-linked immunosorbent assay (ELISA) (Raybiotech, Norcross, GA, USA) according to the manufacturer’s instructions. The minimum detectable dose of the human IL16 ELISA kit was 5 pg/mL. No cross-reactivity with other cytokines was tested. All the samples were analyzed in duplicate with intra-assay coefficients of variation less than 10%.
Statistical analysis
All statistical analyses were done using SPSS statistical software package version 13.0 (SPSS Inc., Chicago, IL, USA). Differences of age between the study groups were assessed using the Student’s t-test, whereas differences of gender were evaluated using Pearson χ2 test. Hardy-Weinberg equilibrium (HWE) of the three polymorphisms in IL16 was tested using a goodness-of-fit χ2 test. Genotype and allele frequencies between cases and controls were compared using the chi-square test. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed using unconditional logistic regression based on age and gender adjustment. Differences of IL16 plasma level among cases and controls were compared using Mann-Whitney U test. Statistical significance was set at P < 0.05.
Results
Characteristics of the study subjects
The characteristics of patients with OS and controls are summarized in Table 1. The median age in cases was 18.0 years, ranging from 6.0 to 58.0 years. The median age in controls was 20.0 years, ranging from 12.0 to 59.0 years. No significant difference was found between cases and controls with regard to age (P = 0.23) and gender (P = 0.91). Most of the tumors (72.1%) located on long tubular bones. Only 29.3% patients had tumor metastasis.
Table 1. General characteristics of the subjects.
| Characteristics | Osteosarcoma patients (n = 358) | Controls (n = 402) |
|---|---|---|
| Age of diagnosis (median, years) | 18.0 (6.0–58.0) | 20.0 (12.0–59.0) |
| Gender, n (%) | ||
| Male | 216 (60.3) | 241 (60.0) |
| Female | 142 (39.7) | 161 (40.0) |
| Tumor location, n (%) | ||
| Long tubular bones | 258 (72.1) | |
| Axial skeleton | 100 (27.9) | |
| Metastasis, n (%) | ||
| Yes | 105 (29.3) | |
| No | 253 (70.7) | |
SD, standard deviation.
Association of IL16 polymorphisms with OS risk
The distributions of IL16 rs4778889, rs11556218, and rs4072111 in OS patients and healthy controls are shown in Tables 2 and 3. All the three SNPs genotyped were in HWE among control subjects (P > 0.05). The frequencies of rs11556218 TG and TG/GG genotypes were significantly increased in OS patients compared to controls (TG vs. TT: adjusted OR = 1.65, 95% CI, 1.23–2.21, P < 0.001; TG/GG vs. TT: adjusted OR = 1.66, 95% CI, 1.24–2.21, P < 0.001). Similarly, the frequency of rs11556218 G allele was significantly higher in OS cases compared to controls (G vs. T: adjusted OR = 1.44, 95% CI, 1.14–1.81, P = 0.002). However, no significant association between rs4778889 and rs4072111 and OS risk was observed (P > 0.05). When stratification analysis was done according to tumor location and metastasis, no significant difference of IL16 genotype and allele frequencies was detected among any subgroup of OS patients (data not shown). No evidence of linkage disequilibrium was observed for the three SNPs (for rs4778889 and rs11556218, D′ = 0.15; for rs11556218 and rs4072111, D′ = 0.26; and for rs4778889 and rs4072111, D′ = 0.21).
Table 2. Genotype distributions of three SNPs in IL16 between osteosarcoma patients and controls.
| Polymorphisms | Osteosarcoma patients, n = 358 (%) | Controls, n = 402 (%) | Crude OR | Adjusted OR (95% CI)† | Adjusted P value† |
|---|---|---|---|---|---|
| rs4778889 | |||||
| TT | 215 (60.1) | 240 (59.7) | 1.00 (Ref) | 1.00 (Ref) | |
| TC | 127 (35.5) | 140 (34.8) | 1.01 (0.75–1.37) | 1.01 (0.75–1.37) | 0.94 |
| CC | 16 (4.5) | 22 (5.5) | 0.81 (0.42–1.59) | 0.81 (0.41–1.60) | 0.55 |
| Dominant | 0.99 (0.74–1.32) | 0.99 (0.74–1.32) | 0.92 | ||
| Recessive | 0.81 (0.42–1.56) | 0.81 (0.42–1.57) | 0.53 | ||
| rs11556218 | |||||
| TT | 165 (46.1) | 235 (58.5) | 1.00 (Ref) | 1.00 (Ref) | |
| TG | 174 (48.6) | 151 (37.6) | 1.64 (1.22–2.20) | 1.65 (1.23–2.21) | <0.001 |
| GG | 19 (5.3) | 16 (4.0) | 1.69 (0.84–3.39) | 1.69 (0.84–3.40) | 0.14 |
| Dominant | 1.65 (1.23–2.19) | 1.66 (1.24–2.21) | <0.001 | ||
| Recessive | 1.35 (0.68–2.67) | 1.39 (0.70–2.77) | 0.34 | ||
| rs4072111 | |||||
| CC | 218 (60.9) | 229 (57.0) | 1.00 (Ref) | 1.00 (Ref) | |
| CT | 124 (34.6) | 158 (39.3) | 0.82 (0.61–1.11) | 0.82 (0.61–1.11) | 0.19 |
| TT | 16 (4.5) | 15 (3.7) | 1.12 (0.54–2.32) | 1.12 (0.54–2.33) | 0.75 |
| Dominant | 0.85 (0.64–1.14) | 0.85 (0.63–1.13) | 0.26 | ||
| Recessive | 1.21 (0.59–2.48) | 1.22 (0.60–2.52) | 0.58 | ||
SNPs, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
†Adjusted by age and gender.
Table 3. Allele distributions of three SNPs in IL16 between osteosarcoma patients and controls.
| Polymorphisms | Osteosarcoma patients (%) | Controls (%) | Crude OR | Adjusted OR (95% CI)† | Adjusted P value† |
|---|---|---|---|---|---|
| rs4778889 | |||||
| T | 557 (77.8) | 620 (77.1) | 1.00 (Ref) | 1.00 (Ref) | |
| C | 159 (22.2) | 184 (22.9) | 0.96 (0.76–1.22) | 0.96 (0.76–1.23) | 0.76 |
| rs11556218 | |||||
| T | 504 (70.4) | 621 (77.7) | 1.00 (Ref) | 1.00 (Ref) | |
| G | 212 (29.6) | 183 (22.8) | 1.43 (1.13–1.80) | 1.44 (1.14–1.81) | 0.002 |
| rs4072111 | |||||
| C | 560 (78.2) | 616 (76.6) | 1.00 (Ref) | 1.00 (Ref) | |
| T | 156 (21.8) | 188 (23.4) | 0.91 (0.72–1.16) | 0.91 (0.72–1.16) | 0.45 |
SNPs, single nucleotide polymorphisms; OR, odds ratio; CI, confidence interval.
†Adjusted by age and gender.
Plasma IL16 level and polymorphisms
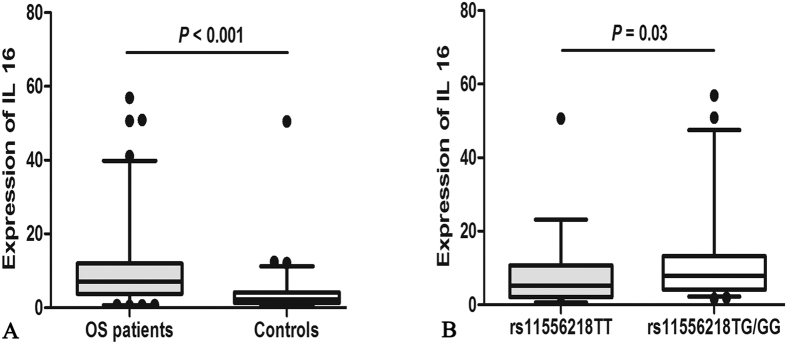
As shown in Fig. 1A, the median plasma IL16 level was 7.02 ng/mL (range 0.48–56.87 ng/mL) in OS patients (n = 82) and 2.21 ng/mL (range 0.09–50.48 ng/mL) in healthy controls (n = 68). The concentration of IL16 in cases was significantly higher than that in controls (P < 0.001). However, there was no significant difference of IL16 level among metastatic patients and non-metastatic patients (P = 0.65). We further compared the correlation between plasma IL16 level and IL16 polymorphisms. We found that patients carrying rs11556218 TG/GG genotypes had higher levels of IL16 than those carrying TT genotype (P = 0.03, Fig. 1B). Nevertheless, we failed to find any relationship between plasma IL16 level and IL16 rs4778889 and rs4072111 (P > 0.05).
Figure 1. ELISA detection of IL16 expression.
(A) plasma level of IL16 in osteosarcoma patients (n = 82) and controls (n = 68). (B) plasma level of IL16 in patients carrying rs11556218 TG/GG genotypes (n = 46) and patients carrying TT genotype (n = 36). The lines inside the boxes denote the medians. The boxes denote the interval between the 25th and 75th percentiles. The whiskers denote the interval between the 5th and 95th percentiles.
Discussion
In this hospital-based case-control study, we investigated the association between three common SNPs in IL16 and risk of OS in a Chinese population. The results revealed that rs11556218 was associated with an increased risk of OS in heterozygote comparison, dominant model, and allele comparison. Moreover, rs11556218 TG/GG genotypes corresponded to higher levels of IL16. These findings indicate that rs11556218 may be responsible for the susceptibility to OS.
The rs11556218, located on exon 6 of IL16, can result in an asparagine to lysine substitution. In 2009, Gao et al. firstly reported that rs11556218 TG genotype was a risk factor for the development of colorectal cancer and gastric cancer26. The positive association was also observed in nasopharyngeal carcinoma31,32, prostate cancer34, and glioma37. Meta-analysis conducted by two independent groups confirmed these findings of IL16 rs11556218 increasing cancer risk41,42. In agreement with these results, we found that the rs11556218 TG/GG genotypes had a 1.66-fold increased risk of developing OS. We further evaluated whether the genetic polymorphism can influence plasma levels of IL16. Compared with rs11556218 TT genotype, rs11556218 TG/GG genotypes were associated with higher levels of IL16. Taken together, these findings indicate that rs11556218 TG/GG genotypes were risk factors for tumorigenesis of OS, probably by increasing the expression of IL16.
The rs4778889, located at position −295 bp in the promoter region of IL16, is related to gene expression and transcriptional activity38,39,40. Previously, the SNP has been studied extensively. Conflicting results, however, were obtained, even in the same ethnic group and cancer type. Some authors reported that IL16 rs4778889 CC genotype was associated with an elevated risk of non-cardia gastric cancer28 and renal cell carcinoma36. On the contrary, some authors reported that IL16 rs4778889 CC genotype was associated with a decreased risk of renal cell carcinoma35 and colorectal cancer27. Some authors reported that IL16 rs4778889 was not associated with the risk of colorectal cancer26, gastric cancer26, nasopharyngeal carcinoma31,32, and glioma37. Our results were consistent with the negative effect. We failed to find any association between IL16 rs4778889 polymorphism and OS risk, which was verified by three independent meta-analyses41,42,43.
With regard to rs4072111 and cancer risk, contradictory results were also observed26,27. Some possibilities should be taken into consideration to explain the discrepancy. SNPs have different roles in different cancer types, especially in diverse ethnicities. As for the conflicting results in the same ethnic group and same cancer type, limited sample size and selection bias may be potential reasons. Therefore, population-based association studies with larger sample sizes are necessary to obtain precise results.
IL16, produced by CD8+T cells, can stimulate the secretion of tumor-related cytokines, such as TNF-α, IL1β, IL6, and IL1516. Serum level of TNF-α was significantly increased in OS patients, with a function of promoting OS progression22,23. Anti-TNF-α therapy can inhibit tumor metastasis24. IL6 can promote cell motility, proliferation, metastasis, and angiogenesis by inducing the expression of some important genes, such as signal transducer and activator of transcription 3 gene, intercellular adhesion molecule-1 gene, and vascular endothelial growth factor (VEGF) gene44,45,46. Knockdown IL6 can reduce VEGF expression and abolish conditional medium-mediated angiogenesis in human OS cells46. Given the key roles of IL16 in OS development, the positive results in this study were biologically reasonable.
Some limitations in this study should be discussed. Both cases and controls were enrolled from a single hospital, which we cannot rule out the possibility of selection bias. However, no deviation from HWE was tested in each SNP, suggesting the possibility is minimal. As is known, OS is composed of complex interactions between genetic and environmental factors. In this study, gene-environment interaction cannot be assessed due to lack of available data. Further gene-environment interaction analysis may provide strong evidence of IL16 polymorphisms in the etiology of OS. Only OS patients were investigated in this study. Therefore, the results cannot be expanded directly to other pediatric cancers until confirmation was made in different cancer types.
In conclusion, we reported for the first time that IL16 rs11556218 TG genotype had an elevated risk of OS in the Chinese population. Further well-designed investigations involving in various populations are warranted to verify these results. In the future, further functional analysis of rs11556218 will help us to clarify the potential biological mechanism of OS.
Additional Information
How to cite this article: Tang, Y.-J. et al. Association of interleukin 16 gene polymorphisms and plasma IL16 level with osteosarcoma risk. Sci. Rep. 6, 34607; doi: 10.1038/srep34607 (2016).
Acknowledgments
We would like to thank Dr. Yesheng Wei for his technical assistance.
Footnotes
Author Contributions Y.-J.T. designed and wrote the manuscript. J.-L.W. collected samples and performed experiments. K.-G.X. performed statistical analysis. C.-G.L. prepared figure. All authors reviewed the manuscript.
References
- Ritter J. & Bielack S. S. Osteosarcoma. Ann Oncol 21 Suppl 7, vii320–vii325, doi: 10.1093/annonc/mdq276 (2010). [DOI] [PubMed] [Google Scholar]
- Dorfman H. D. & Czerniak B. Bone cancers. Cancer 75, 203–210 (1995). [DOI] [PubMed] [Google Scholar]
- Mirabello L., Troisi R. J. & Savage S. A. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer 115, 1531–1543, doi: 10.1002/cncr.24121 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani G. & Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res 152, 3–13, doi: 10.1007/978-1-4419-0284-9_1 (2009). [DOI] [PubMed] [Google Scholar]
- Kager L. et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 21, 2011–2018, doi: 10.1200/JCO.2003.08.132 (2003). [DOI] [PubMed] [Google Scholar]
- Gorlick R. et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res 9, 5442–5453 (2003). [PubMed] [Google Scholar]
- Wittig J. C. et al. Osteosarcoma: a multidisciplinary approach to diagnosis and treatment. American family physician 65, 1123–1132 (2002). [PubMed] [Google Scholar]
- Burningham Z., Hashibe M., Spector L. & Schiffman J. D. The epidemiology of sarcoma. Clinical sarcoma research 2, 14, doi: 10.1186/2045-3329-2-14 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan A. & Iyer N. G. Radiation-induced sarcomas of the head and neck. World journal of clinical oncology 5, 973–981, doi: 10.5306/wjco.v5.i5.973 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee J. V. & Hogendoorn P. C. Molecular pathology of sarcomas: concepts and clinical implications. Virchows Archiv: an international journal of pathology 456, 193–199, doi: 10.1007/s00428-009-0828-5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers M., Zhang W., Lopez-Terrada D., Czerniak B. A. & Lazar A. J. The molecular pathology of sarcomas. Cancer biomarkers: section A of Disease markers 9, 475–491, doi: 10.3233/CBM-2011-0170 (2010). [DOI] [PubMed] [Google Scholar]
- Savage S. A. et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat Genet 45, 799–803, doi: 10.1038/ng.2645 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y. et al. Genetic variations in interleukin-6 polymorphism and the association with susceptibility and overall survival of osteosarcoma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine, doi: 10.1007/s13277-016-4876-6 (2016). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Association of interleukin-12 polymorphisms and serum IL-12p40 levels with osteosarcoma risk. DNA Cell Biol 32, 605–610, doi: 10.1089/dna.2013.2098 (2013). [DOI] [PubMed] [Google Scholar]
- He Y. et al. Genetic polymorphisms of interleukin-1 beta and osteosarcoma risk. International orthopaedics 38, 1671–1676, doi: 10.1007/s00264-014-2374-2 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy N. L. et al. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 100, 63–69 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. et al. Ampelopsin suppresses TNF-alpha-induced migration and invasion of U2OS osteosarcoma cells. Molecular medicine reports, doi: 10.3892/mmr.2016.5124 (2016). [DOI] [PubMed] [Google Scholar]
- Matsuda T., Kondo A., Tsunashima Y. & Togari A. Inhibitory effect of vitamin K(2) on interleukin-1beta-stimulated proliferation of human osteoblasts. Biol Pharm Bull 33, 804–808 (2010). [DOI] [PubMed] [Google Scholar]
- Holzer G., Trieb K., Koschat M., Blahovec H. & Kotz R. Serum concentrations of APO-1/Fas and interleukin-1beta-converting enzyme in osteosarcoma correlate with response to chemotherapy. Anticancer Res 22, 1869–1872 (2002). [PubMed] [Google Scholar]
- Jia S. F. & Kleinerman E. S. Antitumor activity of TNF-alpha, IL-1, and IFN-gamma against three human osteosarcoma cell lines. Lymphokine and cytokine research 10, 281–284 (1991). [PubMed] [Google Scholar]
- Buddingh E. P. et al. Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogeneic and autologous NK cells. Cancer Immunol Immunother 60, 575–586, doi: 10.1007/s00262-010-0965-3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H. et al. Effect of the cytokine levels in serum on osteosarcoma. Tumour Biol 35, 1023–1028, doi: 10.1007/s13277-013-1136-x (2014). [DOI] [PubMed] [Google Scholar]
- Mori T. et al. TNFalpha promotes osteosarcoma progression by maintaining tumor cells in an undifferentiated state. Oncogene 33, 4236–4241, doi: 10.1038/onc.2013.545 (2014). [DOI] [PubMed] [Google Scholar]
- Kato H. et al. Anti-tumor necrosis factor therapy inhibits lung metastasis in an osteosarcoma cell line. Oncology 88, 139–146, doi: 10.1159/000368414 (2015). [DOI] [PubMed] [Google Scholar]
- Kim H. S. Assignment of human interleukin 16 (IL16) to chromosome 15q26.3 by radiation hybrid mapping. Cytogenetics and cell genetics 84, 93, doi: 15224 (1999). [DOI] [PubMed] [Google Scholar]
- Gao L. B. et al. The association of interleukin-16 polymorphisms with IL-16 serum levels and risk of colorectal and gastric cancer. Carcinogenesis 30, 295–299, doi: bgn281 [pii]10.1093/carcin/bgn281 (2009). [DOI] [PubMed] [Google Scholar]
- Azimzadeh P. et al. Interleukin-16 (IL-16) gene polymorphisms in Iranian patients with colorectal cancer. J Gastrointestin Liver Dis 20, 371–376, doi: 9 [pii] (2011). [PubMed] [Google Scholar]
- Zhang T. & Wang H. Variants of interleukin-16 associated with gastric cancer risk. Asian Pac J Cancer Prev 14, 5269–5273 (2013). [DOI] [PubMed] [Google Scholar]
- Kashfi S. M. et al. Interleukin-16 polymorphisms as new promising biomarkers for risk of gastric cancer. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 37, 2119–2126, doi: 10.1007/s13277-015-4013-y (2016). [DOI] [PubMed] [Google Scholar]
- Wang Y. M. et al. Association of genetic polymorphisms of interleukins with gastric cancer and precancerous gastric lesions in a high-risk Chinese population. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 37, 2233–2242, doi: 10.1007/s13277-015-4022-x (2016). [DOI] [PubMed] [Google Scholar]
- Gao L. B. et al. Genetic polymorphism of interleukin-16 and risk of nasopharyngeal carcinoma. Clin Chim Acta 409, 132–135, doi: S0009-8981(09)00484-7 [pii]10.1016/j.cca.2009.09.017 (2009). [DOI] [PubMed] [Google Scholar]
- Qin X. et al. The association of interleukin-16 gene polymorphisms with IL-16 serum levels and risk of nasopharyngeal carcinoma in a Chinese population. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 35, 1917–1924, doi: 10.1007/s13277-013-1257-2 (2014). [DOI] [PubMed] [Google Scholar]
- Li S. et al. Genetic polymorphism of interleukin-16 influences susceptibility to HBV-related hepatocellular carcinoma in a Chinese population. Infect Genet Evol 11, 2083–2088, doi: 10.1016/j.meegid.2011.09.025S1567-1348(11)00355-8[pii] (2011). [DOI] [PubMed] [Google Scholar]
- Batai K. et al. Fine-mapping of IL16 gene and prostate cancer risk in African Americans. Cancer Epidemiol Biomarkers Prev 21, 2059–2068, doi: 10.1158/1055-9965.EPI-12-0707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. et al. IL-16 polymorphism and risk of renal cell carcinoma: association in a Chinese population. Int J Urol 17, 700–707, doi: IJU2559 [pii]10.1111/j.1442-2042.2010.02559.x (2010). [DOI] [PubMed] [Google Scholar]
- Wang Z., Xu Y. & Zhu S. Interleukin-16 rs4778889 polymorphism contributes to the development of renal cell cancer in a Chinese population. International journal of clinical and experimental pathology 8, 15228–15233 (2015). [PMC free article] [PubMed] [Google Scholar]
- Luo Q. S. et al. Interleukin-16 polymorphism is associated with an increased risk of glioma. Genetic testing and molecular biomarkers 18, 711–714, doi: 10.1089/gtmb.2014.0170 (2014). [DOI] [PubMed] [Google Scholar]
- Nakayama E. E., Wasi C., Ajisawa A., Iwamoto A. & Shioda T. A new polymorphism in the promoter region of the human interleukin-16 (IL-16) gene. Genes Immun 1, 293–294, doi: 10.1038/sj.gene.6363672 (2000). [DOI] [PubMed] [Google Scholar]
- Glas J., Torok H. P., Unterhuber H., Radlmayr M. & Folwaczny C. The -295T-to-C promoter polymorphism of the IL-16 gene is associated with Crohn’s disease. Clin Immunol 106, 197–200 (2003). [DOI] [PubMed] [Google Scholar]
- Burkart K. M. et al. Association of asthma with a functional promoter polymorphism in the IL16 gene. J Allergy Clin Immunol 117, 86–91, doi: S0091-6749(05)02266-9 [pii]10.1016/j.jaci.2005.10.011 (2006). [DOI] [PubMed] [Google Scholar]
- Mo C. J. et al. Positive association between IL-16 rs11556218 T/G polymorphism and cancer risk: a meta-analysis. Asian Pacific journal of cancer prevention: APJCP 15, 4697–4703 (2014). [DOI] [PubMed] [Google Scholar]
- Zhao Y. et al. Interleukin-16 gene polymorphisms rs4778889, rs4072111, rs11556218, and cancer risk in Asian populations: a meta-analysis. Genetic testing and molecular biomarkers 18, 174–182, doi: 10.1089/gtmb.2013.0386 (2014). [DOI] [PubMed] [Google Scholar]
- Xu L. L., Song Z. C., Shang K., Zhao L. Q. & Zhu Z. S. Non-association of IL-16 rs4778889 T/C polymorphism with cancer risk in Asians: a meta-analysis. Asian Pacific journal of cancer prevention: APJCP 15, 803–805 (2014). [DOI] [PubMed] [Google Scholar]
- Tu B., Du L., Fan Q. M., Tang Z. & Tang T. T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett 325, 80–88, doi: 10.1016/j.canlet.2012.06.006 (2012). [DOI] [PubMed] [Google Scholar]
- Lin Y. M., Chang Z. L., Liao Y. Y., Chou M. C. & Tang C. H. IL-6 promotes ICAM-1 expression and cell motility in human osteosarcoma. Cancer Lett 328, 135–143, doi: 10.1016/j.canlet.2012.08.029 (2013). [DOI] [PubMed] [Google Scholar]
- Tzeng H. E. et al. Interleukin-6 induces vascular endothelial growth factor expression and promotes angiogenesis through apoptosis signal-regulating kinase 1 in human osteosarcoma. Biochem Pharmacol 85, 531–540, doi: 10.1016/j.bcp.2012.11.021 (2013). [DOI] [PubMed] [Google Scholar]