Abstract
The development of point-of-care clinical chemistry analyzers has enabled the implementation of these ancillary tests in field laboratories in resource-limited outbreak areas. The Eternal Love Winning Africa (ELWA) outbreak diagnostic laboratory, established in Monrovia, Liberia, to provide Ebola virus and Plasmodium spp. diagnostics during the Ebola epidemic, implemented clinical chemistry analyzers in December 2014. Clinical chemistry testing was performed for 68 patients in triage, including 12 patients infected with Ebola virus and 18 infected with Plasmodium spp. The main distinguishing feature in clinical chemistry of Ebola virus–infected patients was the elevation in alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and γ-glutamyltransferase levels and the decrease in calcium. The implementation of clinical chemistry is probably most helpful when the medical supportive care implemented at the Ebola treatment unit allows for correction of biochemistry derangements and on-site clinical chemistry analyzers can be used to monitor electrolyte balance.
Keywords: Ebola virus, clinical chemistry, West Africa
The availability of small, easy-to-operate point-of-care clinical chemistry analyzers has enabled the implementation of this equipment in field laboratories and patient care settings in resource-limited outbreak areas [1]. The Eternal Love Winning Africa (ELWA) outbreak diagnostic laboratory provided Ebola virus and Plasmodium spp. diagnostics in Monrovia, Liberia, from the height of the outbreak in Liberia in August 2014 until 2 weeks after the World Health Organization declared Liberia Ebola virus free for the first time in May 2015 [2, 3]. In an attempt to aid the improvement of clinical care at the ELWA3 Ebola treatment unit, clinical chemistry was implemented in the ELWA laboratory in December 2014. In addition to blood sample collection in ethylenediaminetetraacetic acid for Ebola virus and Plasmodium spp. diagnostics, a lithium-heparinized whole-blood sample was collected from patients who were entered into triage at the ELWA3 Ebola treatment unit.
Blood samples were analyzed using 2 Piccolo Xpress chemistry analyzers (Abaxis) within 1 hour of collection. To ensure safe operation, the 2 analyzers were placed in a negatively pressurized, high-efficiency particulate arrestance–filtered flexible film isolator (Coy Lab Products). Owing to problems with overheating of the systems at ambient temperatures (27°C–32°C) in Monrovia, the flexible film isolator containing the analyzers was placed in an air-conditioned space with constant temperatures of approximately 25°C during operation. For most patients, 2 test panels were performed: the BioChemistry Panel Plus (Abaxis), to measure alanine aminotransferase, albumin, alkaline phosphatase (ALP), amylase, aspartate aminotransferase, C-reactive protein (CRP), calcium (Ca2+), creatinine, γ-glutamyltransferase, glucose, total protein, blood urea nitrogen (BUN), and uric acid, and the renal function panel, to detect albumin, calcium, chloride, creatinine, glucose, phosphorus, potassium, sodium, total carbon dioxide, and BUN.
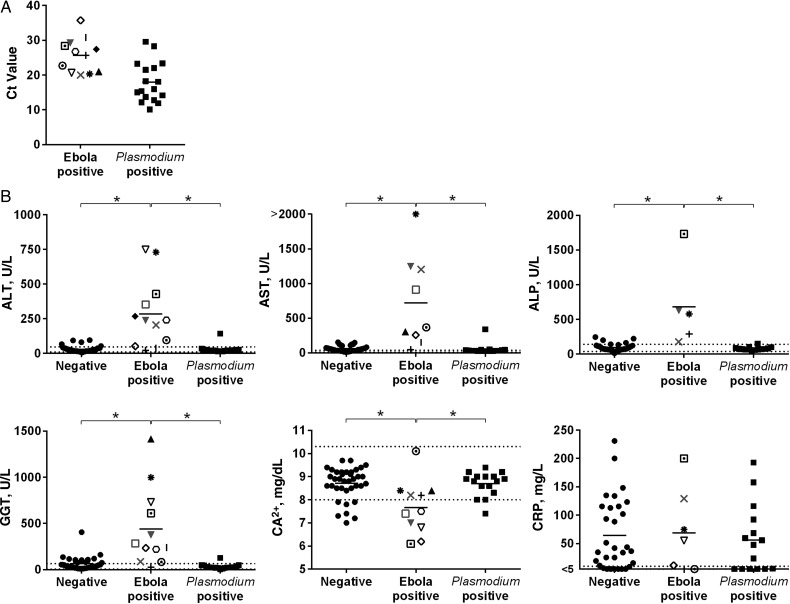
In total, clinical chemistry testing was performed for 68 patients in triage. Follow-up samples from Ebola virus–positive patients were rarely submitted. Of 68 patients in whom clinical chemistry analysis was performed, 9 were positive for Ebola virus and negative for Plasmodium spp., 3 patients were positive for Ebola virus but their Plasmodium spp. status was not determined, 18 were negative for Ebola virus but positive for Plasmodium spp., and 38 were negative for both Ebola virus and Plasmodium spp. The Ebola viral load and Plasmodium spp. parasite load, as determined by cycle threshold (Ct) value, are shown in Figure 1A.
Figure 1.
Ebola viral load, Plasmodium spp. parasite load, and clinical chemistry data in patients admitted into triage at the Eternal Love Winning Africa (ELWA) 3 Ebola treatment unit. A, Ebola virus and Plasmodium spp. Cycle threshold (Ct) values detected with quantitative reverse-transcription polymerase chain reaction in individual patients are plotted as a proxy for viral and parasite load. B, Analytes that differed significantly between Ebola virus–infected and Ebola virus–negative patients. Dotted lines indicate the normal range. Individual symbols for the Ebola virus–positive patients correlate to the same patient in each panel in Figures 1 and 2. Negative indicates patients negative for both Ebola virus and Plasmodium spp.; Ebola positive, patients positive for Ebola virus and negative or unknown for Plasmodium spp.; Plasmodium positive, patients negative for Ebola virus and positive for Plasmodium spp. Gray symbols in the Ebola-positive column indicate that Plasmodium spp. status was not determined for these patients; black symbols, patients who were Plasmodium spp. negative. *P < .01 (1-way analysis of variance). Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; Ca2+, calcium; CRP, C-reactive protein; GGT, γ-glutamyltransferase.
The main distinguishing feature in the clinical chemistry of Ebola virus–infected patients was an elevation in the liver enzymes alanine aminotransferase, aspartate aminotransferase, ALP, and γ-glutamyltransferase, which was not observed in the Plasmodium spp.–positive patients or in those in whom both diagnostic tests were negative (Figure 1B). The only other analyte with statistically significant values outside the normal range was Ca2+, with values below the normal range (Figure 1B). When the calcium concentration was corrected for changes in albumin concentration, the Ca2+ concentration in Ebola virus–infected patients remained significantly lower than that in patients with Plasmodium spp. parasitemia or those not infected with either Ebola virus or Plasmodium spp. (data not shown; 1-way analysis of variance, P < .05). However, the lowest corrected Ca2+ concentration measured in an Ebola virus–positive patients was 7.7 mg/dL (data not shown; normal range, 8.5–10.5 mg/dL), and thus the clinical relevance of this low Ca2+ concentration is unclear.
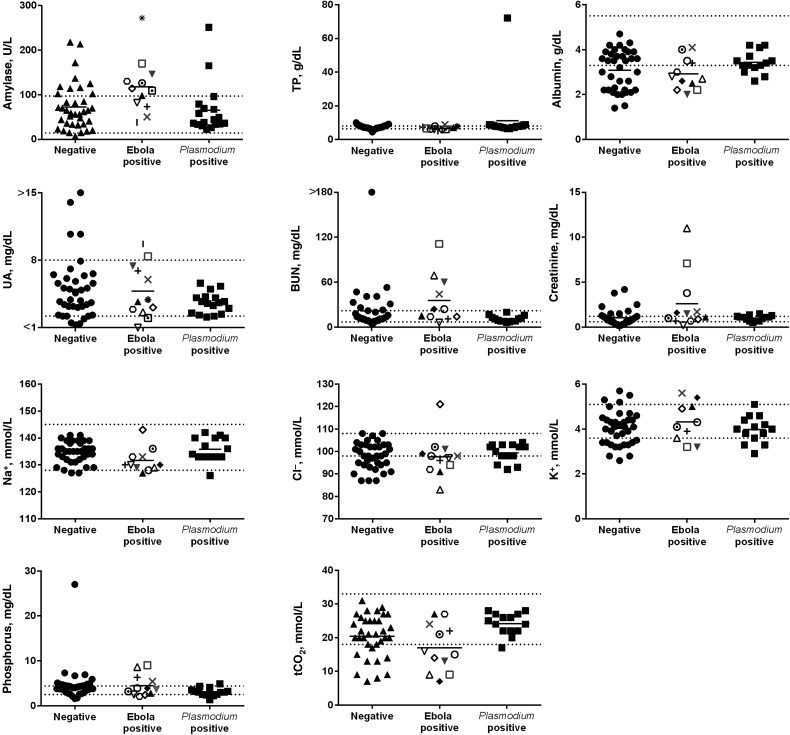
The Plasmodium spp.–positive patients could not be distinguished from those negative for both Ebola virus and Plasmodium spp., because analytes were within the normal range for most of these patients (Figure 1B and data not shown). However, CRP levels were elevated in the majority of patients (Figure 1B), independent of the outcome of the diagnostic Ebola virus and Plasmodium spp. tests, indicating that most patients negative for Ebola virus and Plasmodium spp. were experiencing an inflammatory response that was probably indicative of an ongoing or recent infection. Although other analytes were outside the normal range in some Ebola virus–infected patients, similar abnormalities were detected in those negative for Ebola virus and those positive for Plasmodium spp. (Figure 2).
Figure 2.
Clinical chemistry data in patients admitted into triage at the Eternal Love Winning Africa (ELWA) 3 Ebola treatment unit. Analytes that did not differ significantly between Ebola virus–infected and Ebola virus–negative patients are shown. Dotted lines indicate the normal range. Individual symbols for the Ebola virus–positive patients correlate to the same patient in each panel in Figures 1 and 2. Negative indicates patients negative for Ebola virus and Plasmodium spp.; Ebola positive, patients positive for Ebola virus and negative or unknown for Plasmodium spp.; and Plasmodium positive, patients negative for Ebola virus and positive for Plasmodium spp. Gray symbols in the Ebola-positive column indicate that Plasmodium spp. status was not determined for these patients; black symbols, patients who were Plasmodium spp. negative. Abbreviations: BUN, blood urea nitrogen; Cl−, chlorine; K+, potassium; Na+, sodium; tCO2, total carbon dioxide; TP, total protein; UA, uric acid.
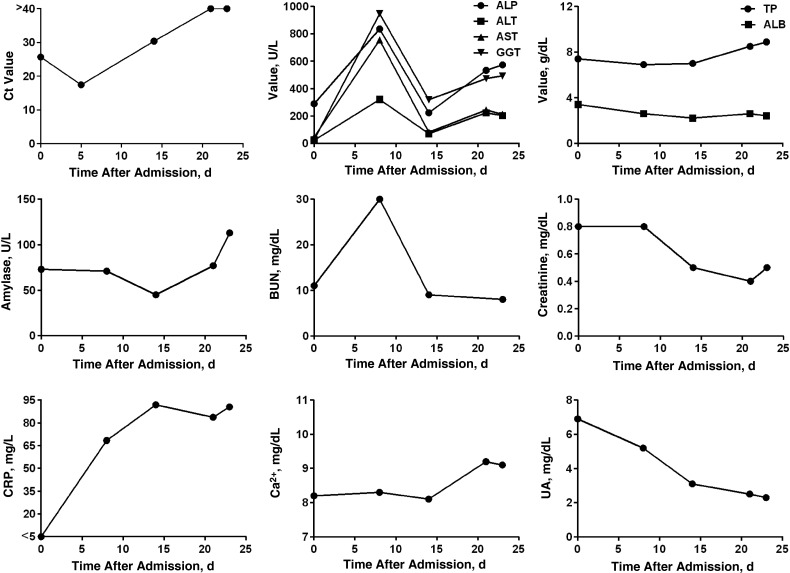
Although very few follow-up samples were submitted from Ebola virus–infected patients, 1 patient was followed up throughout the disease course. This patient was a 13-year old boy, who was admitted into triage 4 days after the onset of symptoms. At this time, the viral quantitative reverse-transcription polymerase chain reaction Ct value in his whole-blood sample was 25.68, and his ALP level was elevated, but all other analytes had values within the normal range (Figure 3). Five days later, his viral load had increased to a Ct value of 17.46; this increased viral load corresponded to increases in liver enzymes, BUN, and CRP on day 8 after admission. The patient was discharged after 2 negative Ebola diagnostic tests 27 days after symptom onset; by this time, his liver enzyme levels had decreased but were not yet within the normal range (Figure 3).
Figure 3.
Clinical chemistry in a single Ebola virus–infected patient over time. A 13-year old male patient was admitted to the Eternal Love Winning Africa (ELWA) 3 Ebola treatment unit 4 days after onset of symptoms and discharged 23 days later after 2 negative Ebola diagnostic test results. Normal ranges as indicated in the user manual: alkaline phosphatase (ALP), 53–128 U/L; alanine aminotransferase (ALT), 10–47 U/L; aspartate aminotransferase (AST), 11–38 U/L; γ-glutamyltransferase (GGT), 5–65 U/L; total protein (TP), 6.4–8.1 g/dL; albumin (ALB), 3.3–5.5 g/dL; amylase, 14–97 U/L; blood urea nitrogen (BUN), 7–22 mg/dL; creatinine, 0.6–1.2 mg/dL; C-reactive protein (CRP), <7.5 mg/L; calcium (Ca2+), 8–10.3 mg/dL; and uric acid (UA) 3.6–8 mg/dL. Abbreviation: Ct, cycle threshold.
It has been suggested that parenteral supportive therapy is key to surviving Ebola virus infection by countering dehydration and electrolyte imbalance due to excessive vomiting and diarrhea [4–6]. Although electrolyte levels were not consistently outside the normal range in the patients in our cohort, the potassium level was above or below the normal range in several Ebola virus–infected patients. The tested samples were obtained on admission, and thus it may have been too early in the disease course to observe additional abnormalities. The implementation of clinical chemistry on site can certainly allow monitoring of such biochemistry derangements, in particular electrolyte imbalances, and therefore has its greatest benefit in allowing a much-needed tailored treatment approach to individual patients who otherwise would have to be receive standard treatment provided to all patients, which could even result in negative effects.
Although the clinical chemistry analyzer was easy to use, the implementation of clinical chemistry in a field diagnostic laboratory during an Ebola outbreak was not without challenges. First, an additional flexible film isolator needed to be installed, to safely test potentially Ebola virus positive samples and to prevent contamination of diagnostic samples. Second, although analysis of samples does not require extensive handling or processing, it took about 15 minutes to analyze a single sample. Combined with the fact that blood samples have to be analyzed within 1 hour of collection, a limited number of samples can be tested. Thus, during the height of the outbreak, implementation of clinical chemistry would only have been possible if additional staff and equipment were added to the laboratory. Alternatively, it may be possible to implement these tests at the point of care, rather than in the diagnostic laboratory. Third, the analyzers were very sensitive to temperatures >25°C and high humidity; one of them malfunctioned within 3 months of first use.
The clinical chemistry data from Ebola virus–infected patients in our cohort were consistent with previously published data from the current outbreak as well as from another Ebolavirus outbreak (species Sudan ebolavirus) in Uganda in 2000–2001 [7–9]. The main findings in those patients also included the elevation in liver enzyme levels [7–9] and Ca2+ levels below the normal range in fatal cases [7]. Despite the constraints of temperature, humidity, and limited space in the field diagnostic laboratory, biochemistry testing could be provided rapidly to help the clinical staff understand the clinical condition of their patients.
Notes
Acknowledgments. We thank Mulbah Jallah, Monrovia, Liberia, for his excellent support and assistance in operating the Eternal Love Winning Africa (ELWA) laboratory; Livia Tampellini of Médecins Sans Frontières; Clayton Onyango, Bonventure Juma, and Barry Fields of the Centers for Disease Control and Prevention in Nairobi, Kenya; Melvin Ochieng, Victor Omballa, and Collins Owuor of the Kenya Medical Research Institute in Nairobi; Galina Zemtsova, Joshua Self, Shannon Emery, Thomas Rowe, Ute Stroeher, Mark Rayfield, and Stuart Nichol of the US Centers for Disease Control and Prevention for technical and logistical support of the ELWA laboratory; Kay Menk, Dawn Clifton, and Les Shupert (all National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) for logistical support; and Daniel Sturdevant (NIAID, NIH) for help with statistics. We also acknowledge the World Health Organization Headquarters Geneva, the World Health Organization Regional Office for Africa, the Ministry of Health and Social Welfare, Liberia, the US Centers for Disease Control and Prevention, the NIH, and ELWA. Finally, we would like to thank the people of Liberia for their hospitality and cooperation.
Ethics committee approval. The human samples and accompanying metadata were collected as diagnostic samples for public health surveillance and not human subjects research, and institutional review board review and approval were not required. The data were transferred to an honest broker for removal of all personal identifiable information at the request of the NIH Office of Human Subjects Research. For data analysis, the authors had access only to the database that was redacted by the honest broker; analysis of the redacted database was deemed “not human subjects research” by the NIH Office of Human Subjects Research.
Financial support. The work was supported by the Intramural Research Program of the NIAID, NIH.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cotte J, Janvier F, Cordier PY, Bordes J, Kaiser E; Conakry Healthcare Worker Ebola Treatment Center. Organ support in Ebola virus disease: utility of point-of-care blood tests. Anaesth Crit Care Pain Med 2015; 34:363–4. [DOI] [PubMed] [Google Scholar]
- 2.de Wit E, Falzarano D, Onyango C et al. . The merits of malaria diagnostics during an Ebola virus disease outbreak. Emerg Infect Dis 2016; 22:323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Wit E, Munster VJ, Rosenke K et al. . Ebola laboratory response at the Eternal Love Winning Africa Campus, Monrovia, Liberia 2014–2015. J Infect Dis 2016; doi:10.1093/infdis/jiw216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chertow DS, Kleine C, Edwards JK, Scaini R, Giuliani R, Sprecher A. Ebola virus disease in West Africa—clinical manifestations and management. N Engl J Med 2014; 371:2054–7. [DOI] [PubMed] [Google Scholar]
- 5.Lamontagne F, Clement C, Fletcher T, Jacob ST, Fischer WA II, Fowler RA. Doing today's work superbly well—treating Ebola with current tools. N Engl J Med 2014; 371:1565–6. [DOI] [PubMed] [Google Scholar]
- 6.Perner A, Fowler RA, Bellomo R, Roberts I. Ebola care and research protocols. Intensive Care Med 2015; 41:111–4. [DOI] [PubMed] [Google Scholar]
- 7.Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis 2007; 196(suppl 2):S364–71. [DOI] [PubMed] [Google Scholar]
- 8.Schieffelin JS, Shaffer JG, Goba A et al. . Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sissoko D, Laouenan C, Folkesson E et al. . Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]