Abstract
A licensed vaccine against Ebola virus (EBOV) remains unavailable, despite >11 000 deaths from the 2014–2016 outbreak of EBOV disease in West Africa. Past studies have shown that recombinant vaccine viruses expressing EBOV glycoprotein (GP) are able to protect nonhuman primates (NHPs) from a lethal EBOV challenge. However, these vaccines express the viral GP–based EBOV variants found in Central Africa, which has 97.3% amino acid homology to the Makona variant found in West Africa. Our previous study showed that a recombinant adenovirus serotype 5 (Ad5)–vectored vaccine expressing the Makona EBOV GP (MakGP) was safe and immunogenic during clinical trials in China, but it is unknown whether the vaccine protects against EBOV infection. Here, we demonstrate that guinea pigs immunized with Ad5-MakGP developed robust humoral responses and were protected against exposure to guinea pig–adapted EBOV. Ad5-MakGP also elicited specific B- and T-cell immunity in NHPs and conferred 100% protection when animals were challenged 4 weeks after immunization. These results support further clinical development of this candidate and highlight the utility of Ad5-MakGP as a prophylactic measure in future outbreaks of EBOV disease.
Keywords: Ebola virus, adenovirus virus, vaccine, guinea pigs, nonhuman primates
The 2014–2016 outbreak of Ebola virus (EBOV) disease, centered in Guinea, Sierra Leone, and Liberia, was the largest in history. As of February 2016, >11 000 of >28 000 infected people had died from EBOV disease stemming from this outbreak [1]. Widespread and intense EBOV transmission was observed in the 3 countries mentioned above, but infected cases imported into other countries through travel or repatriation also resulted in small outbreak clusters, including those observed in Nigeria, Mali, the United States, and Spain [1]. Additionally, the case-fatality rate of EBOV infections may reach up to 90% if they are untreated, and moribund humans can shed high titers of virus during advanced and terminal stages of illness [2]. Vaccinations constitute an important countermeasure to save lives and prevent further infections, but a licensed prophylactic agent against EBOV remains unavailable.
An effective strategy against EBOV has been to use the reverse genetic systems of various viruses to generate recombinant viral vaccines expressing EBOV glycoprotein (GP). Past studies using this approach showed that nonhuman primates (NHPs) were protected from lethal EBOV exposure after immunization [3–10]; these platforms constitute some of the most promising vaccine candidates in development. Currently, experimental vaccines based on vesicular stomatitis Indiana virus (VSV) and chimpanzee adenovirus type 3 (chAd3) platforms are in clinical trials [11, 12]. In addition, vaccines based on the human adenovirus serotype 5 (Ad5) vector were shown in a previous clinical trial to be safe and immunogenic in humans [13]. The VSV-, chAd3-, and Ad5-vectored vaccines express GP derived from EBOV that emerged in central Africa during 1976 (Mayinga variant; GenBank accession number AY142960) or 1995 (Kikwit variant; GenBank accession number AY354458). The EBOV found in Western Africa (Makona variant; GenBank accession number KJ660346) shares 97.3% amino acid homology with the GP of EBOV Mayinga and 98.7% homology with the GP of EBOV Kikwit. While protection against infection with EBOV Makona has been observed in NHPs by using a VSV-vectored vaccine expressing the Kikwit GP [14], the immunity conferred by vaccination is likely to be more robust if the antigen included in the vaccine is matched to that of the circulating outbreak virus, with decreased possibilities for unwanted side effects.
Our previous phase 1 clinical trial in China demonstrated the safety and immunogenicity of an Ad5-vectored vaccine expressing the GP of EBOV Makona (Ad5-MakGP) in humans. Individuals who received a low dose (4 × 1010 viral particles) or a high dose (1.6 × 1011 viral particles) of Ad5-MakGP via the intramuscular route had robust humoral and cell-mediated adaptive immune responses. The vaccine was considered safe since there were no serious adverse events, and the most commonly reported adverse reaction was mild pain at the vaccine injection site [15]. Furthermore, the increased vaccine dose appears to overcome preexisting antibodies against the Ad5 vector, as EBOV GP–specific antibodies were detected in all participants of the high-dose group [15]. However, without phase 2 efficacy trials, it is not possible to know whether Ad5-MakGP is protective against EBOV infection [16].
In this study, we sought to investigate whether Ad5-MakGP can protect guinea pigs against a lethal infection with guinea pig–adapted EBOV (GA-EBOV) and NHPs against wild-type Makona EBOV. Since a GA-EBOV based on Makona EBOV is not yet available, the GA-EBOV based on the Mayinga variant was used instead as a proof of concept for protection. Specific humoral immune responses, which correlate with vaccine-induced protection against EBOV [17], were determined for guinea pigs after vaccination and for NHPs after vaccination and challenge, whereas specific cell-mediated immunity was also assayed in NHPs after vaccination and challenge and will be presented herein.
MATERIALS AND METHODS
Ethics Statement
Guinea pig and NHP experiments were performed at the National Microbiology Laboratory in Winnipeg, Canada. These studies were approved by the Animal Care Committee at the Canadian Science Center for Human and Animal Health, following guidelines provided by the Canadian Council on Animal Care.
Preparation of Adenovirus-Vectored Vaccine
The replication-detective Ad5-MakGP vaccine was developed and prepared by the Beijing Institute of Biotechnology and Tianjin CanSino Biotechnology, as described in a previous study [15].
Guinea Pig Experiments
Female Hartley guinea pigs aged 4–8 weeks (Charles River) were randomized into groups of 6 animals. Animals were vaccinated intramuscularly with Ad5-MakGP. Two doses were used: 4 × 109 viral particles per animal (low dose) or 4 × 1010 viral particles per animal (high dose). A group of 3 control animals were given a high dose of recombinant Ad5 expressing lacZ (Ad5-lacZ) as a mock treatment. Four weeks after vaccination, animals were infected intraperitoneally with 1000 times the 50% lethal dose (LD50; 22 plaque-forming units [PFU]) of EBOV VECTOR/C.porcellus-lab/COD/1976/Mayinga-GPA-p7 (GA-EBOV) [25]. Guinea pigs were weighed daily after exposure for 16 days and were monitored for survival and clinical symptoms for an additional 12 days. Sera were harvested weekly from individual guinea pigs 14, 21, and 28 days after vaccination for serologic studies.
NHP Experiments
Eight cynomolgus macaques (Macaca fascicularis) of Chinese and Mauritian origin (Resonant BioPharma) were used in this study. Animals ranged in weight from 3.1 kg to 7.8 kg and ranged in age from 3 years to 10 years. Animals were randomized into 2 treatment groups (3 animals [2 males and 1 female] per group) and 1 control group (2 animals, both female) and were fed monkey chow, fruits, vegetables, and treats ad libitum. At day −28, NHPs were vaccinated intramuscularly with either a high (2 × 1011 viral particles in 1 mL of sterile water) or low (4 × 1010 viral particles in 1 mL of sterile water) dose of Ad5-MakGP. One animal from the control group received an intramuscular injection of Ad5-lacZ (2 × 1011 viral particles in 1 mL of sterile water), and the other received the same dose but intranasally. Four weeks after vaccination, all NHPs were challenged intramuscularly with 1000 times the LD50 (1000 PFU) of Ebola virus H.sapiens-tc/GIN/2014/Gueckedou-C07 (EBOV-C07) [26]. Animals were scored daily after challenge for observable signs of disease, in addition to changes in food and water consumption. Blood was collected at days 0, 3, 7, 10, 14, 21, and 28 after infection for complete blood counts, blood biochemistry analyses, determination of viral load by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis and live virus titration, and measurement of B- and T-cell responses. At the same time points, oral, rectal, and nasal swab specimens were collected to assess virus shedding by qRT-PCR and live virus titration. The experiment was not blinded, and all animals were included in the analysis.
Serologic Analyses
Immunoglobulin M (IgM) and immunoglobulin G (IgG) enzyme-linked immunosorbent assays (ELISAs) were performed on harvested sera, using a His-tagged recombinant GP as the capture antigen [27]. High-binding, half-well, flat-bottomed polystyrene microtiter plates (Thermo Scientific) were coated with 30 ng of recombinant Ebola GPΔTM (IBT Services) and incubated overnight at 4°C. Plates were then washed with phosphate-buffered saline (PBS) containing 0.1% Tween 20, blocked with PBS containing 5% skim milk, and incubated for 1 hour at 37°C. Sera samples, serially diluted in PBS containing 2% skim milk, were then added in triplicate and incubated for 1 hour at 37°C. After washing with PBS containing 0.1% Tween 20, horseradish peroxidase (HRP)–conjugated goat anti-guinea pig IgG (KPL) or IgM (Innovative Research) was diluted 1:2000 in PBS containing 2% skim milk and incubated with guinea pig samples for 1 hour at 37°C. After washing with PBS containing 0.1% Tween 20, the substrate (ABTS plus H2O2; KPL) was added and incubated for 30 minutes at 37°C. ELISA plates were read on a Versamax Microplate reader (Molecular Devices) at 405 nm, and data were analyzed with SoftMaxPro 6.1 software. Samples were assayed in triplicate, and the average reciprocal dilution of IgG or IgM was reported. Positive dilutions were defined using a method previously described [28], using a significance threshold of 95% (for IgG) and 99.5% (for IgM).
For the NHPs, the method was based on a quantification method described in a previous article [29]. Coating was done with 1.25 µg/mL of GPΔTM (IBT Services) overnight at 4°C. This was followed by a blocking incubation for 2 hours at 37°C in PBS containing 5% skim milk. The samples and in-house standards were diluted in PBS containing 2% skim milk. Thirty microliters of each sample/standard was loaded on the plate (in duplicate), and the plate was then incubated for 2 hours at 37°C. The plate was washed 4 times with PBS containing 0.1% Tween 20 (Sigma). The secondary antibody, HRP-conjugated goat anti-human IgG (KPL) or goat anti-monkey IgM (Rockland), was added to the plate (30 µL per well, with 0.5 µg/mL of PBS containing 2% skim milk) and incubated at 37°C for 1 hour. The plate was washed again 4 times with PBS containing 0.1% Tween 20. TMB single solution (Thermo Scientific) was used as the revealing solution (50 µL per well). The reaction was allowed to proceed for 30 minutes at room temperature before plates were read at 650 nm on a VersaMax plate reader (Molecular Devices). The sample concentrations were calculated from the standard on each plate. The standard was fit to a 5-parameter logistic curve, using Microsoft R Open (3.2.3) and Stan (v 2.8.0) [30]. The upper and lower limits of detection for each curve were defined by the first sample (starting from the center of the curve, moving out) to have a back-calculated concentration that varied by >20% from its expected value. Samples for which all dilutions fell outside the limits of detection were reevaluated at a lower or higher dilution (as appropriate). The error bars correspond to the 95% highest density intervals, which include both uncertainty from the curve and from the dilutions (see the article by Kruschke [31] for an explanation of the highest density intervals).
Concentrations of EBOV-specific neutralizing antibodies (nAbs) were also measured using NHP sera. Samples were serially diluted 2-fold (in a volume of 50 µL) and incubated at 37°C for 1 hour with an equal volume of Dulbecco's modified Eagle's medium (DMEM) containing 100 50% tissue culture infective doses (TCID50) of EBOV-eGFP per well. The virus-serum mixture (100 µL total) was then added to Vero E6 cells in a 96-well flat-bottomed plate and incubated for 1 hour at 37°C. A total of 100 µL of DMEM supplemented with 4% fetal bovine serum was then added to each well and incubated for 2 days at 37°C. Titers of nAbs in each sample were reported as the reciprocal of the highest dilution with a reduction in eGFP fluorescence of at least 50% [32]. All samples were processed in triplicate.
RESULTS
Ad5-MakGP Is Immunogenic and Protective in Guinea Pigs
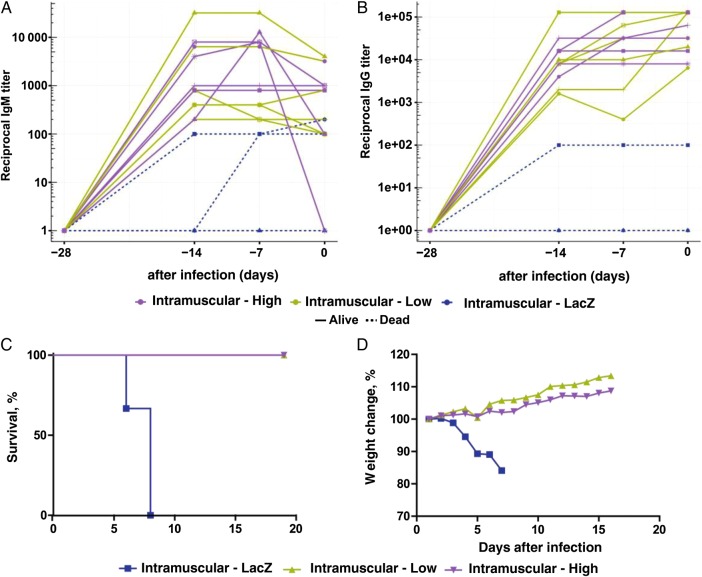
Animals (6 per group) were vaccinated with a low (4 × 109 viral particles) or high (4 × 1010 viral particles) dose of Ad5-MakGP via the intramuscular route. Control animals (n = 3) were vaccinated with Ad5-lacZ via the intramuscular route at a dose equivalent to that received by the high-dose Ad5-MakGP group. In guinea pigs vaccinated intramuscularly, animals given Ad5-MakGP were positive for EBOV GP–specific IgM by day 14 after vaccination. At 28 days after immunization (ie, the day of infection with GA-EBOV), reciprocal dilution titers ranged from 100 to 3200 for both low-dose (geometric mean titer [GMT], 523.1) and high-dose (GMT, 252.0) Ad5-MakGP recipients, with one animal in the high-dose group testing negative for IgM at the time of challenge. Control animals had IgM titers of 100 and 200, with one animal testing negative (Figure 1A). EBOV GP–specific IgG could also be readily detected by day 14 after vaccination. At 28 days after immunization, IgG titers ranged from 6400 to 128 000 for low-dose Ad5-MakGP recipients (GMT, 45 255) and from 8000 to 128 000 for high-dose Ad5-MakGP recipients (GMT, 40 317). One control animal had an IgG titer of 100, with 2 animals testing negative (Figure 1B).
Figure 1.
Humoral responses in individual guinea pigs after adenovirus type 5 (Ad5) Ebola virus (EBOV) variant Makona glycoprotein (MakGP) vaccination and survival and weight change after guinea pig–adapted EBOV challenge. Concentrations of EBOV GP–specific antibodies were measured in guinea pig sera harvested weekly 0–4 weeks after vaccination. Immunoglobulin G (IgG; A) and immunoglobulin M (IgM; B) for vaccinated animals. Survival (C) and weight loss (D) for vaccinated animals. Titers were expressed as reciprocal dilutions of IgG or IgM. LacZ, control guinea pigs that received recombinant Ad5 expressing lacZ.
Control animals in both experiments died of disease 6–8 days after infection with GA-EBOV, with an average weight loss of up to 20% by the time of death. In the intramuscular vaccination groups, all animals survived challenge (Figure 1C), without substantial (ie, >5%) weight loss (Figure 1D).
Ad5-MakGP Elicits Specific Humoral and Cell-Mediated Immune Responses in NHPs
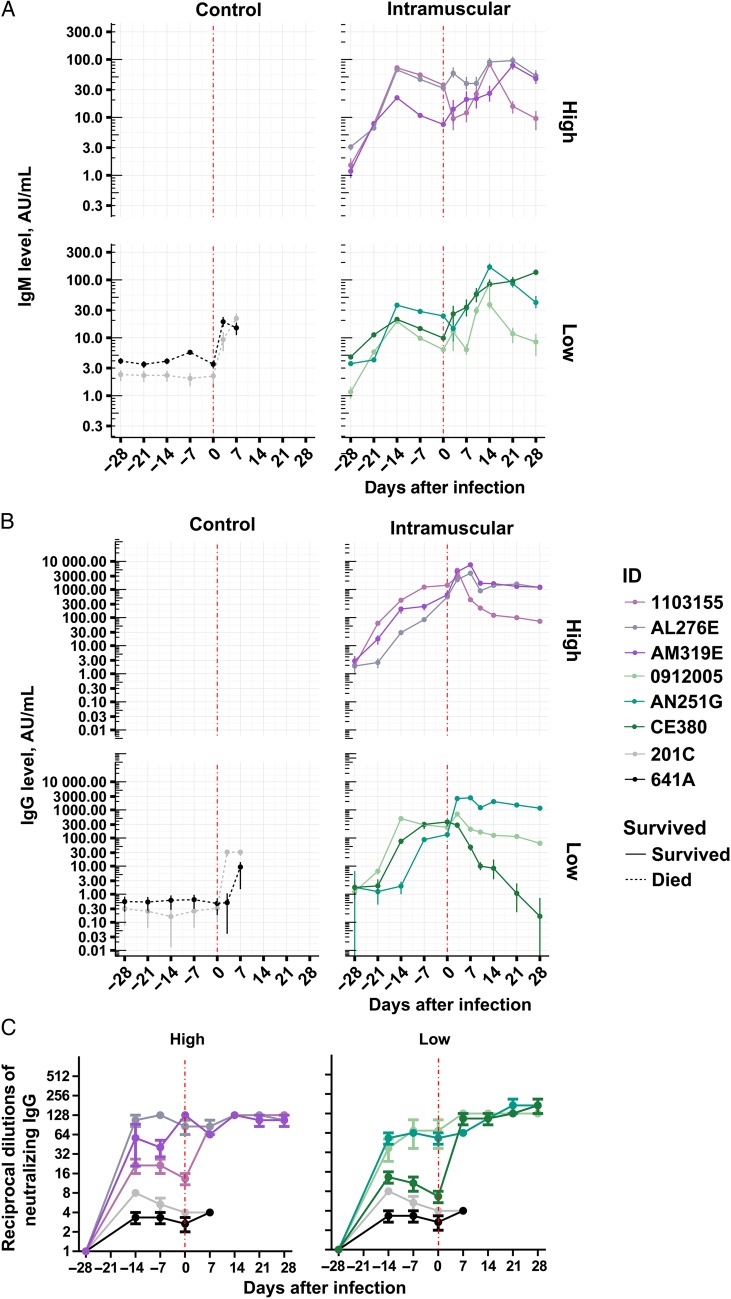
A total of 6 NHPs (3 per group) were vaccinated with a low (4 × 1010 viral particles) or high (2 × 1011 viral particles) dose of Ad5-MakGP via the intramuscular route. EBOV GP–specific IgM and IgG were detected by day 14 after vaccination, and antibody concentrations were maintained until the time of challenge. At 28 days after immunization (ie, on the day of EBOV challenge), IgM concentrations reached 6–36 AU/mL in both the low- and high-dose groups (GMT, 11.4 in the low-dose group and 20.6 in the high-dose group; Figure 2A), while IgG levels were 132–372 AU/mL (GMT, 228) in the low-dose group and 541–1417 AU/mL (GMT, 792) in the high-dose group (Figure 2B). Only background levels of IgM and IgG (<10 AU/mL and <1 AU/mL, respectively) were observed with control animals receiving Ad5-lacZ (Figure 2A and 2B). High titers of nAbs against EBOV were detected in Ad5-MakGP–immunized animals by day 14 after vaccination, increased to 39 reciprocal dilutions (range, 6–128) at the day of challenge, and remained elevated in all surviving animals during the course of infection (Figure 2C). Only background levels of nAbs (<10 reciprocal dilutions) were detected with control animals (Figure 2C). After challenge with a wild-type Makona EBOV from West Africa (EBOV-C07), low titers (9–32 AU/mL) of IgM and IgG were observed in control NHPs at 7 days after infection (Figure 2A and 2B). In vaccinated NHPs, IgM and IgG concentrations were sustained at levels comparable to those detected at the time of EBOV-C07 challenge (Figure 2A and 2B).
Figure 2.
Humoral responses in individual nonhuman primates (NHPs) vaccinated intramuscularly with adenovirus type 5 (Ad5) Ebola virus variant Makona glycoprotein (MakGP). Immunoglobulin M (IgM; A) and immunoglobulin G (IgG; B) response of NHPs vaccinated with Ad5-MakGP intramuscularly. The error bars represent 95% highest density intervals. C, Neutralizing antibody levels of NHPs vaccinated with Ad5-MakGP intramuscularly. The error bars represent the standard error of the mean.
EBOV-specific cell-mediated responses elicited by Ad5-MakGP immunization were assayed by flow cytometry and intracellular cytokine staining. CD4+ and CD8+ T cells were costained with antibodies for the cytotoxic degranulation marker CD107a and the intracellular cytokines CD69, interferon γ (IFN-γ), interleukin 2 (IL-2), interleukin 4 (IL-4), and tumor necrosis factor α (TNF-α). At 14 days after vaccination, secretion of CD69, IFN-γ, and TNF-α from CD4+ T cells was detected in a majority of vaccinated NHPs but had largely decreased to background levels by the day of challenge (Supplementary Figure 1A). Secretion of CD69, IFN-γ, IL-2, and TNF-α from CD8+ T cells were detected in a majority of vaccinated animals by 14 days after Ad5-MakGP vaccination (Supplementary Figure 1B). After EBOV-C07 challenge, the CD4+ T-cell response was particularly robust in Ad5-MakGP animals, with secretion of IFN-γ, IL-2, IL-4, and TNF-α detected between 14 and 28 days after infection (Supplementary Figure 1A), whereas IL-4 secretion from CD8+ T cells was detected in a majority of vaccinated NHPs between 14 and 28 days after infection (Supplementary Figure 1B). Neither CD4+ nor CD8+ T-cell responses were detected in control NHPs after infection (Supplementary Figure 1A and 1B).
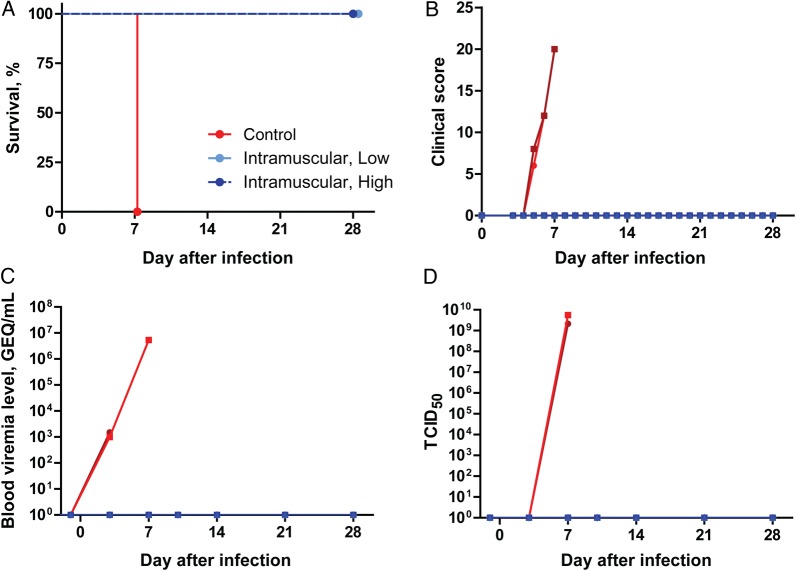
Ad5-MakGP Vaccination Is 100% Protective in NHPs Against EBOV-C07 Challenge
After infection with EBOV-C07, control NHPs died of infection within 7 days (Figure 3A), with clinical signs (Figure 3B), as well as fever and weight loss (Supplementary Figure 1A and 1B). These animals showed decreased white blood cell and platelet counts after challenge (Supplementary Figure 2C and 2D, respectively) and also developed liver and kidney dysfunction, as indicated by considerably elevated activities/concentrations of serum alkaline phosphatase, alanine aminotransferase, total bilirubin, blood urea nitrogen, and creatinine between 3 and 7 days after infection (Figure 2E–2I). In contrast, all animals from both the low- and high-dose Ad5-MakGP groups survived the challenge (Figure 3A). Clinical symptoms, fever, and weight loss were not observed for any animals over the course of the experiment (Figure 3B and Supplementary Figure 2A and 2B), indicating that there were no signs of overt disease. Vaccination also protected against a decrease in counts of white blood cells (Supplementary Figure 2C) and platelets (Supplementary Figure 2D) after infection with EBOV-C07. Furthermore, none of the vaccinated animals showed substantial changes in their blood biochemistry parameters (Supplementary Figure 2E–2I).
Figure 3.
Survival, clinical scores, and viremia levels among adenovirus type 5 (Ad5) Ebola virus (EBOV) variant Makona glycoprotein (MakGP)–vaccinated nonhuman primates after challenge with EBOV-C07. Survival (A), clinical scores (B), and viremia levels measured by quantitative reverse transcription–polymerase chain reaction (C) and by live virus titration (D). Abbreviations: GEQ, genome equivalents; TCID50, 50% tissue culture infective dose.
Viremia and Virus Shedding Was Not Observed in Immunized NHPs
The viral load was measured by qRT-PCR and live virus titration. In control NHPs, EBOV RNA was detectable at approximately 103 genome equivalents (GEQ)/mL starting at 3 days after infection and increased to approximately 107 GEQ/mL by the time of death, at 7 days (Figure 3C). High titers of live EBOV (approximately 109 TCID50) were detected only on the day of death, at 7 days (Figure 3D). Shedding of infectious virus via the oral, nasal, and rectal routes was detected in the control group at 7 days by qRT-PCR, but live virus was not detected (Supplementary Figure 3A–3F). In contrast, EBOV was not detected in blood or swab specimens by qRT-PCR or TCID50 analyses in any animals vaccinated with Ad5-MakGP (Figure 3C and 3D and Supplementary Figure 3A–3F).
DISCUSSION
Ad5-vectored vaccines are well characterized owing to their past use against a variety of human conditions and cancers and are also being explored as potential countermeasures against other pathogens, such as Plasmodium falciparum [18] and Mycobacterium tuberculosis [19]. Preexisting immunity against Ad5 remains a concern, since approximately 40% of humans residing in the United States are positive for antibodies against Ad5, and this number increases to 90% for residents of some African countries [20]. However, past studies involving EBOV have shown that administration of Ad5-vectored vaccines via the intranasal/intratracheal routes can circumvent preexisting immunity in NHPs [21], resulting in sustained, long-term protection [22] and, if combined with an Ad5-vector expressing IFN α as an adjuvant, in postexposure protection [23].
Vaccination resulted in full protection for both the low- and high-dose Ad5-MakGP groups in guinea pigs. Furthermore, a single intramuscular dose of Ad5-MakGP provided sterile immunity and 100% protection from EBOV disease in NHPs when administered 28 days prior to infection. This vaccination induced a robust specific and neutralizing IgG response and T-cell immunity within 14 days and yielded similar findings to those for Ad5-KikGP [13, 24]. We also compared 2 different doses of the Ad5-MakGP vaccine to determine whether a low dose was effective and whether, to overcome expected problems of preexisting immunity in humans, an increased dose could be tolerated. Both doses induced similar levels of IgM (10–30 AU/mL) 4 weeks after vaccination, but the high Ad5-MakGP dose produced substantially higher levels of IgG by 4 weeks after vaccination (1000 AU/mL, compared with 100–300 AU/mL). Furthermore, levels of nAbs were observed to be slightly higher for the high-dose group. Although all immunized animals survived, higher IgG concentrations are likely more desirable because they have been shown to statistically correlate with survival from EBOV disease [17]. As such, a high dose of Ad5-MakGP (2 × 1011 viral particles per animal) should be further investigated in clinical trials.
In summary, this study contains Ad5-MakGP efficacy data generated from 2 different commonly used animal models of EBOV, which satisfies the 2-animal rule put forward by the Food and Drug Administration regarding preclinical testing of experimental compounds. When combined with safety data from our previous phase 1 clinical trials in China, the evidence warrants further clinical investigation of Ad5-MakGP in populations residing in West Africa, which may potentially result in a licensed prophylactic product to protect against future outbreaks of EBOV disease.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Acknowledgments. We thank Kevin Tierney for the animal care assistance.
L. H., W. C., and X. Q. conceived and designed the study. L. H., A. K., G. W., S. H., S. W., J. A., H. W., Z. Z., L. F., G. S., K. T., S. B., T. Z., X. Y., W. C., and X. Q. performed the experiments and analyzed the results. L. H., A. K., G. W., and X. Q. wrote the manuscript. All listed authors reviewed the manuscript.
Financial support. This work was supported by the National Science and Technology, the Beijing Institute of Biotechnology, Tianjin CanSino Biotechnology, the Public Health Agency of Canada, the Canadian Institutes of Health Research (Banting Postdoctoral Fellowship to G. W.), and the Chinese Academy of Sciences (President's International Fellowship to G. W.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. Ebola situation report—17 February 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-17-february-2016 Accessed 25 February 2016.
- 2.Bausch DG, Towner JS, Dowell SF et al. Assessment of the risk of Ebola virus transmission from bodily fluids and fomites. J Infect Dis 2007; 196(suppl 2):S142–7. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature 2000; 408:605–9. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan NJ, Geisbert TW, Geisbert JB et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 2003; 424:681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zahn R, Gillisen G, Roos A et al. Ad35 and ad26 vaccine vectors induce potent and cross-reactive antibody and T-cell responses to multiple filovirus species. PLoS One 2012; 7:e44115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley DA, Honko AN, Asiedu C et al. Chimpanzee adenovirus vaccine generates acute and durable protective immunity against ebolavirus challenge. Nat Med 2014; 20:1126–9. [DOI] [PubMed] [Google Scholar]
- 7.Jones SM, Feldmann H, Stroher U et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med 2005; 11:786–90. [DOI] [PubMed] [Google Scholar]
- 8.Bukreyev A, Rollin PE, Tate MK et al. Successful topical respiratory tract immunization of primates against Ebola virus. J Virol 2007; 81:6379–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herbert AS, Kuehne AI, Barth JF et al. Venezuelan equine encephalitis virus replicon particle vaccine protects nonhuman primates from intramuscular and aerosol challenge with ebolavirus. J Virol 2013; 87:4952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaney JE, Marzi A, Willet M et al. Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine. PLoS Pathogens 2013; 9:e1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rampling T, Ewer K, Bowyer G et al. A monovalent chimpanzee adenovirus Ebola vaccine - preliminary report. N Engl J Med 2015; 374:1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huttner A, Dayer JA, Yerly S et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis 2015; 15:1156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledgerwood JE, Costner P, Desai N et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine 2010; 29:304–13. [DOI] [PubMed] [Google Scholar]
- 14.Marzi A, Robertson SJ, Haddock E et al. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 2015; 349:739–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu FC, Hou LH, Li JX et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015; 385:2272–9. [DOI] [PubMed] [Google Scholar]
- 16.Marzi A, Falzarano D. An updated Ebola vaccine: immunogenic, but will it protect? Lancet 2015; 385:2229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong G, Richardson JS, Pillet S et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci Transl Med 2012; 4:158ra46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill AV, Reyes-Sandoval A, O'Hara G et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin 2010; 6:78–83. [DOI] [PubMed] [Google Scholar]
- 19.Radosevic K, Wieland CW, Rodriguez A et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun 2007; 75:4105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiang Z, Li Y, Cun A et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis 2006; 12:1596–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson JS, Pillet S, Bello AJ, Kobinger GP. Airway delivery of an adenovirus-based Ebola virus vaccine bypasses existing immunity to homologous adenovirus in nonhuman primates. J Virol 2013; 87:3668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JH, Jonsson-Schmunk K, Qiu X et al. A single dose respiratory recombinant adenovirus-based vaccine provides long-term protection for non-human primates from lethal Ebola infection. Mol Pharm 2015; 12:2712–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su S, Bi Y, Wong G, Gray GC, Gao GF, Li S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J Virol 2015; 89:8671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan NJ, Geisbert TW, Geisbert JB et al. Immune protection of nonhuman primates against Ebola virus with single low-dose adenovirus vectors encoding modified GPs. PLoS Med 2006; 3:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connolly BM, Steele KE, Davis KJ et al. Pathogenesis of experimental Ebola virus infection in guinea pigs. J Infect Dis 1999; 179(suppl 1):S203–17. [DOI] [PubMed] [Google Scholar]
- 26.Baize S, Pannetier D, Oestereich L et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 371:1418–25. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama E, Yokoyama A, Miyamoto H et al. Enzyme-linked immunosorbent assay for detection of filovirus species-specific antibodies. Clin Vaccine Immunol 2010; 17:1723–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods 1998; 221:35–41. [DOI] [PubMed] [Google Scholar]
- 29.Vu H, Shulenin S, Grolla A et al. Quantitative serology assays for determination of antibody responses to Ebola virus glycoprotein and matrix protein in nonhuman primates and humans. Antiviral Res 2016; 126:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stat.columbia.edu. Stan: A probabilistic programming language. http://www.stat.columbia.edu/~gelman/research/unpublished/stan-resubmit-JSS1293.pdf Accessed 28 February 2016. [DOI] [PMC free article] [PubMed]
- 31.Kruschke JK. Bayesian estimation supersedes the t test. J Exp Psychol Gen 2013; 142:573–603. [DOI] [PubMed] [Google Scholar]
- 32.Finak G, Frelinger J, Jiang W et al. OpenCyto: an open source infrastructure for scalable, robust, reproducible, and automated, end-to-end flow cytometry data analysis. PLoS Comput Biol 2014; 10:e1003806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.