Abstract
Background. A unit of the European Mobile Laboratory (EMLab) consortium was deployed to the Ebola virus disease (EVD) treatment unit in Guéckédou, Guinea, from March 2014 through March 2015.
Methods. The unit diagnosed EVD and malaria, using the RealStar Filovirus Screen reverse transcription–polymerase chain reaction (RT-PCR) kit and a malaria rapid diagnostic test, respectively.
Results. The cleaned EMLab database comprised 4719 samples from 2741 cases of suspected EVD from Guinea. EVD was diagnosed in 1231 of 2178 hospitalized patients (57%) and in 281 of 563 who died in the community (50%). Children aged <15 years had the highest proportion of Ebola virus–malaria parasite coinfections. The case-fatality ratio was high in patients aged <5 years (80%) and those aged >74 years (90%) and low in patients aged 10–19 years (40%). On admission, RT-PCR analysis of blood specimens from patients who died in the hospital yielded a lower median cycle threshold (Ct) than analysis of blood specimens from survivors (18.1 vs 23.2). Individuals who died in the community had a median Ct of 21.5 for throat swabs. Multivariate logistic regression on 1047 data sets revealed that low Ct values, ages of <5 and ≥45 years, and, among children aged 5–14 years, malaria parasite coinfection were independent determinants of a poor EVD outcome.
Conclusions. Virus load, age, and malaria parasite coinfection play a role in the outcome of EVD.
Keywords: Filovirus, Ebola virus disease, malaria, Guinea, epidemic, mobile laboratory
Since its discovery in 1976, Ebola virus (EBOV) has caused several outbreaks of Ebola virus disease (EVD) in sub-Saharan Africa, with case-fatality ratios (CFRs) of up to 90% [1]. The largest EVD outbreak in history occurred from 2014 to 2016 in West Africa and primarily affected the countries of Guinea, Liberia, and Sierra Leone [2]. As of March 2016, 28 639 confirmed cases with 11 316 deaths had been reported by the World Health Organization (WHO) [3]. Outbreak control mainly relied on preventing transmission through isolation of individuals with suspected EVD and patients with confirmed EVD, community engagement, contact tracing, and rapid laboratory diagnostic tests [4]. On request of the WHO Global Outbreak Alert and Response Network (GOARN) and Emerging Dangerous Pathogens Laboratory Network, a laboratory unit of the European Mobile Laboratory (EMLab) consortium was rapidly deployed to the EVD treatment unit (ETU) in Guéckédou, Guinea, immediately after the causative agent of the outbreak had been identified [2, 5]. The laboratory unit departed from Europe on 26 March 2014, and the first patient samples were tested on-site by EBOV reverse transcription–polymerase chain reaction (RT-PCR) on 30 March 2014. It was operational until March 2015, when it was relocated to the ETU in Coyah. Here, we report the analysis of the laboratory data generated by EMLab in Guéckédou from March 2014 through March 2015, in conjunction with epidemiological data collected by the WHO country office in Guinea.
METHODS
Patients and Specimens
A blood specimen was collected from live patients with suspected EVD to establish the diagnosis. The WHO case definition for a suspected case was as follows: “any person, alive or dead, suffering or having suffered from a sudden onset of high fever and having had contact with: a suspected, probable or confirmed Ebola or Marburg case; a dead or sick animal (for Ebola); a mine (for Marburg); OR any person with sudden onset of high fever and at least three of the following symptoms: headaches, lethargy, anorexia/loss of appetite, aching muscles or joints, stomach pain, difficulty swallowing, vomiting, difficulty breathing, diarrhea, hiccups; OR any person with inexplicable bleeding; OR any sudden, inexplicable death” [6p2]. The vast majority of suspected EVD cases were managed at the ETUs in Guéckédou and Macenta, both of which were operated by Médecins Sans Frontières. A few samples originated from patients who were managed in other places in Guinea. The EMLab unit also tested suspected EVD cases being managed in Liberia and Sierra Leone. Laboratory-confirmed EVD cases were admitted at the ETU. Patients with suspected EVD were retested 1–2 days later if the result of their first test, performed on a sample collected within the first 3 days after onset of symptoms, was negative. During this time, they were kept in the area of the ETU reserved for individuals with suspected EVD. Another blood sample was collected for analysis from patients who were scheduled for discharge from the ETU. In rare cases, specimens of other body fluids, such as urine, were collected from live patients with EVD and tested. No further samples were collected from patients who died of EVD in the ETU. In addition, EMLab tested oral swab specimens from patients who were found dead in their communities (hereafter, “community deaths”).
Diagnostic Assays
Viral RNA was extracted from 50 µL of whole blood collected in ethylenediaminetetraacetic acid (EDTA)–lined tubes (hereafter, “EDTA–whole blood”) or 140 µL of cell-free fluid (plasma, urine, amniotic fluid, or saliva), using the QIAamp Viral RNA Mini Kit (Qiagen). Comparison in the field of cycle threshold (Ct) values, using RT-PCR analysis of 50 µL of EDTA–whole blood or 140 µL of plasma, revealed equivalent results (mean Ct difference [±SD] between whole blood and plasma,−0.08 ± 2.46; n = 12). Material on dry swabs was released in 200 µL of water by agitation, of which 50 µL was processed. Noninactivated specimens were manipulated in a glove box. After addition of AVL buffer and incubation, the tubes were decontaminated and moved out of the glove box for RNA extraction. EBOV RNA was detected using the RealStar Filovirus Screen RT-PCR Kit 1.0 (Altona Diagnostics) on a SmartCycler (Cepheid) [7]. The internal control template of the kit was added to the sample before RNA extraction, and only results with a valid run control were communicated. Ct values were reported to the clinicians as a quantitative measure of viral load. EDTA–whole blood was evaluated for the presence of Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale antigens by using the BinaxNow Malaria (Alere) rapid diagnostic test (RDT) in the glove box according to the manufacturers instructions [8]. This test has an analytical sensitivity of 99.5% for a parasitemia level of >1000 parasites/µL blood for P. falciparum [8], the predominant Plasmodium species in Guinea [9]. This threshold provides a reasonable compromise between sensitivity and specificity in detecting true severe malaria, rather than severe disease with incidental parasitemia, in areas with moderate-to-high transmission [10, 11].
Data Management and Statistical Analysis
Demographic data for patients were provided by Médecins Sans Frontières, the Red Cross, the WHO, national authorities, contact tracing teams, and other partners in field on the laboratory request forms accompanying the sample. Name, age, sex, residence, ETU patient identifier, sample identifier, sample type, collection date, date of symptom onset, EBOV RT-PCR result with corresponding Ct value, and malaria RDT results were captured in the EMLab database (Excel, Microsoft) and reported on a daily basis to the WHO and national authorities. The operational EMLab sample database was the basis for further analysis. To facilitate allocation of various samples to individual patients, validate the demographic information, and document outcome, the EMLab database was merged manually with the Guinean EVD patient database maintained at the WHO country office in Conakry. Patient name and sample identifier recorded in both databases were used as primary identifiers for merging; additional variables were used to verify the match. Inconsistencies between the 2 databases and between sample entries for the same patient were resolved, and the data were cleaned as much as possible, using Stata 14 (StataCorp). On the basis of specific criteria, patients were classified into 3 main categories for analysis: (1) suspected cases of EVD not confirmed by PCR testing (noncases), (2) patients with PCR-confirmed EVD (EVD cases), and (3) community deaths. Database entries for patients who could not be assigned to one of these categories because of missing or conflicting data (n = 200 samples) and cases managed in Liberia and Sierra Leone (n = 1083 samples lacking any epidemiological data) were excluded from the analysis. The final database comprised 2741 patients with 4719 samples collected between 17 March 2014 and 29 March 2015. In hospitalized patients, only samples collected at admission were included in the analysis.
Statistical analysis was performed with Stata 14. Categorical variables were described as percentages. The denominator varied between variables because of missing data. Continuous variables were described by medians and interquartile ranges (IQRs). Associations of independent variables with the dichotomous outcome (survival or death) were displayed with crude (unadjusted) odds ratios (ORs). Multivariate logistic regression models were used to account for confounding factors. Categorical variables were contrasted against a reference value (dummy coding). In the final model, an interaction term (the product of 2 interacting categorical variables) was included to assess outcome associations of one independent variable within levels of another independent variable. To describe the interaction effect, ORs were calculated for each level of the second independent variable. The corresponding effect estimates of the interaction term, used to derive the ORs, are provided as well.
Ethics
The National Committee of Ethics in Medical Research of Guinea and the Ethics Committee of the Medical Association of Hamburg approved the use of diagnostic leftover samples and corresponding patient data for this study (permits 11/CNERS/14 and PV4910).
RESULTS
The cleaned EMLab database contained 2178 cases of suspected EVD (79%) who attended a hospital/ETU and 563 community deaths (21%). EVD was confirmed by PCR in 1231 suspected cases (57%) and 281 community deaths (50%). The CFR of hospitalized cases with confirmed EVD was 60%. Demographic and laboratory data are summarized in Table 1. Most patients originated from the regions of N'Zérékoré (1955 [77%]), Kankan (374 [15%]), and Faranah (196 [8%]).
Table 1.
Characteristics of Individuals Included in the Analysis
| Characteristic | EVD Suspected Cases in Hospital |
Community Deaths |
||||
|---|---|---|---|---|---|---|
| Overall | EBOV RT-PCR Positive | EBOV RT-PCR Negative | Overall | EBOV RT-PCR Positive | EBOV RT-PCR Negative | |
| Individuals | 2178/2178 (100) | 1231/2178 (57) | 947/2178 (43) | 563 | 281/563 (50) | 282/563 (50) |
| Female sex | 1135/2157 (53) | 645/1228 (53) | 490/929 (53) | 260/545 (48) | 136/271 (50) | 124/274 (45) |
| Age, y, median (IQR) | 30 (18–44)a | 30 (19–45)b | 30 (18–42)c | 37 (25–55)d | 35 (23–53)e | 40 (25–56)f |
| Malaria RDT positive | 541/1937 (28) | 261/1091 (24) | 280/846 (33) | Not tested | Not tested | Not tested |
| Fatal outcome | 769/2049 (38) | 719/1205 (60) | 50/844 (6) | 563 (100) | 281 (100) | 282 (100) |
Data are proportion of individuals with the characteristic/no. evaluated (%), unless otherwise indicated.
Abbreviations: EBOV, Ebola virus; EVD, Ebola virus disease; IQR, interquartile range; RDT, rapid diagnostic test; RT-PCR, reverse transcription–polymerase chain reaction.
a Data are for 2153 observations.
b Data are for 1225 observations.
c Data are for 928 observations.
d Data are for 521 observations.
e Data are for 252 observations.
f Data are for 269 observations.
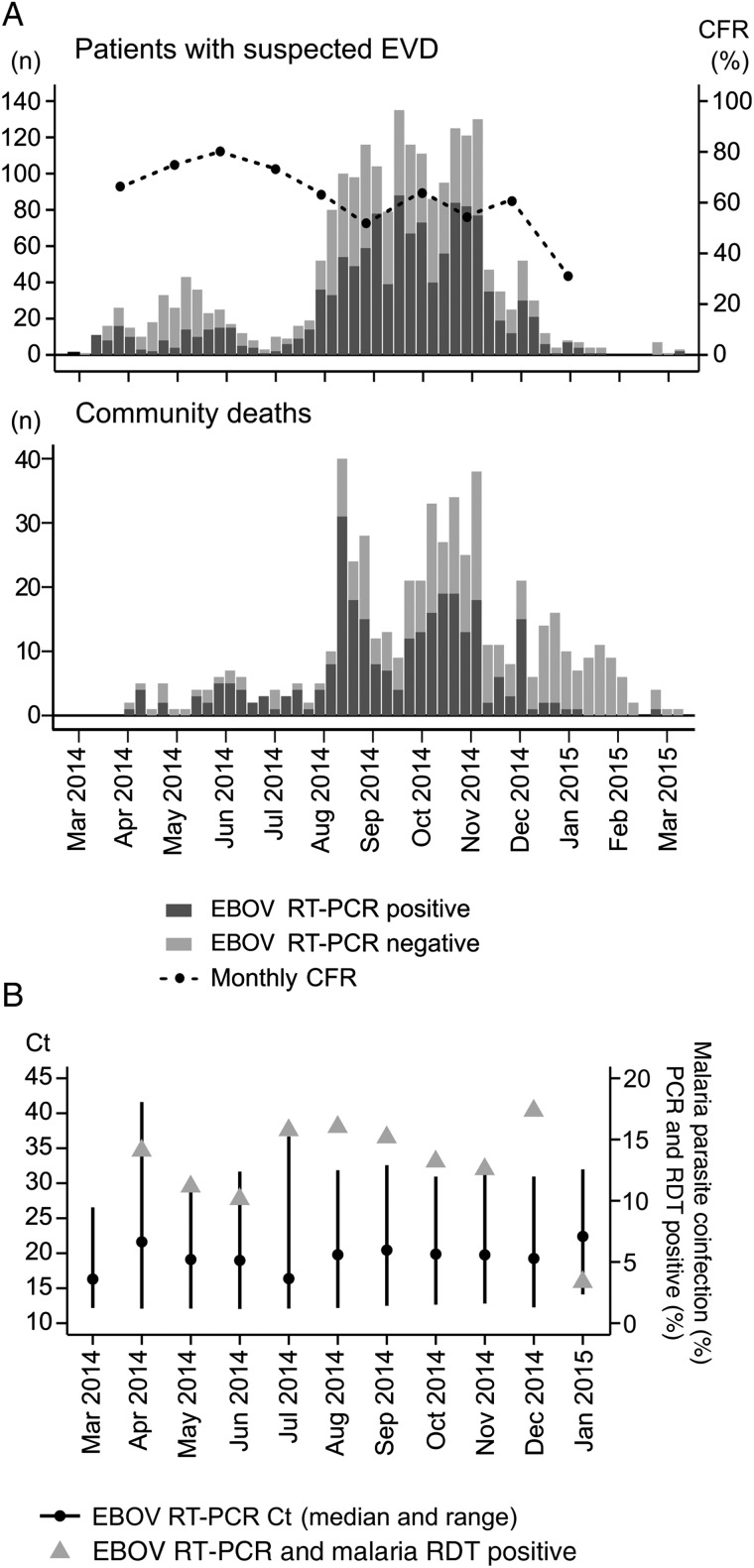
The weekly incidence of EBOV RT-PCR–positive cases in the hospital and community shows that the outbreak in Guéckédou progressed in 2 major waves (March–July and August–January; Figure 1A). However, specifically the community data suggest that the 2 major waves actually consisted of 5 subwaves: March–April, May–July, August–September, October–November, and December–January. The median of the weekly EVD confirmation rate among hospital attendees was 52% (IQR, 31%–66%). The median weekly CFR for confirmed cases of EVD was 66% (IQR, 54%–79%), with a decreasing trend during the outbreak period (Figure 1A). Among community deaths, the median weekly EVD confirmation rate was 50% (IQR, 13%–71%). The median Ct for patients with EVD on admission to the hospital showed no trend over time (Figure 1B). The coinfection rate with malaria parasites among hospitalized patients with EVD also remained at a similar level during the epidemic, with the notable exception of a drop in January 2015 (Figure 1B).
Figure 1.
Frequency of patients tested by Ebola virus (EBOV) reverse transcription–polymerase chain reaction (RT-PCR), case-fatality ratios (CFRs), cycle threshold (Ct) values, and malaria parasite coinfection rate over time. A, EBOV RT-PCR results are shown for 2178 patients attending an Ebola virus disease (EVD) treatment unit (ETU; upper panel) and 563 patients who died in their communities (lower panel), by week of the deployment period. For patients with EVD who were treated at an ETU, the CFR is shown in the upper panel. B, Ct values on admission and malaria parasite coinfection rate for patients with EVD who were treated at an ETU. Abbreviation: RDT, rapid diagnostic test.
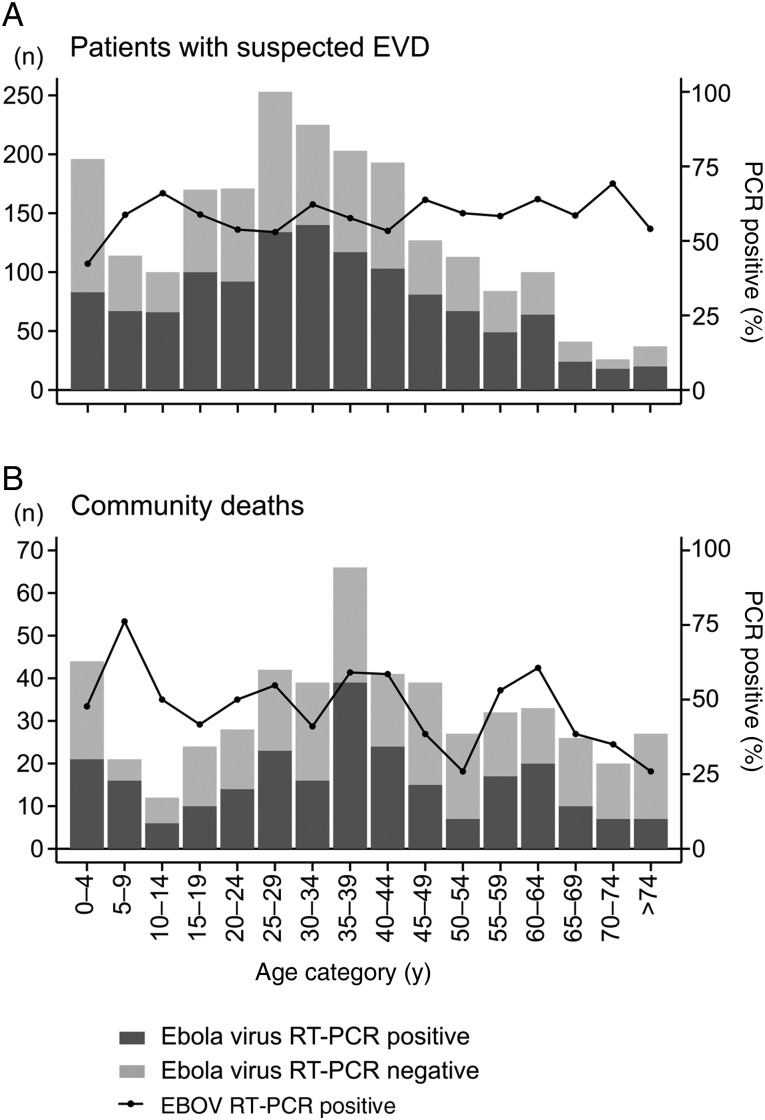
Figure 2 shows the age distributions among hospitalized patients with EVD and community deaths. Essentially, both distributions show 3 peaks—young children, young adults aged 15–50 years, and elderly persons—although this structure was more pronounced among community deaths. EVD confirmation rates in the hospital were comparable among age groups, with a median of 58% (IQR, 54%–62%). In the communities, the EVD confirmation rate varied more among the age groups (median, 50%; IQR, 38%–59%) but had an overall decreasing trend toward higher age.
Figure 2.
Age distribution for patients tested by Ebola virus (EBOV) reverse transcription–polymerase chain reaction (RT-PCR). Results are shown for 2153 patients attending an Ebola virus disease (EVD) treatment unit (A) and 521 patients who died in their communities (B), by age category.
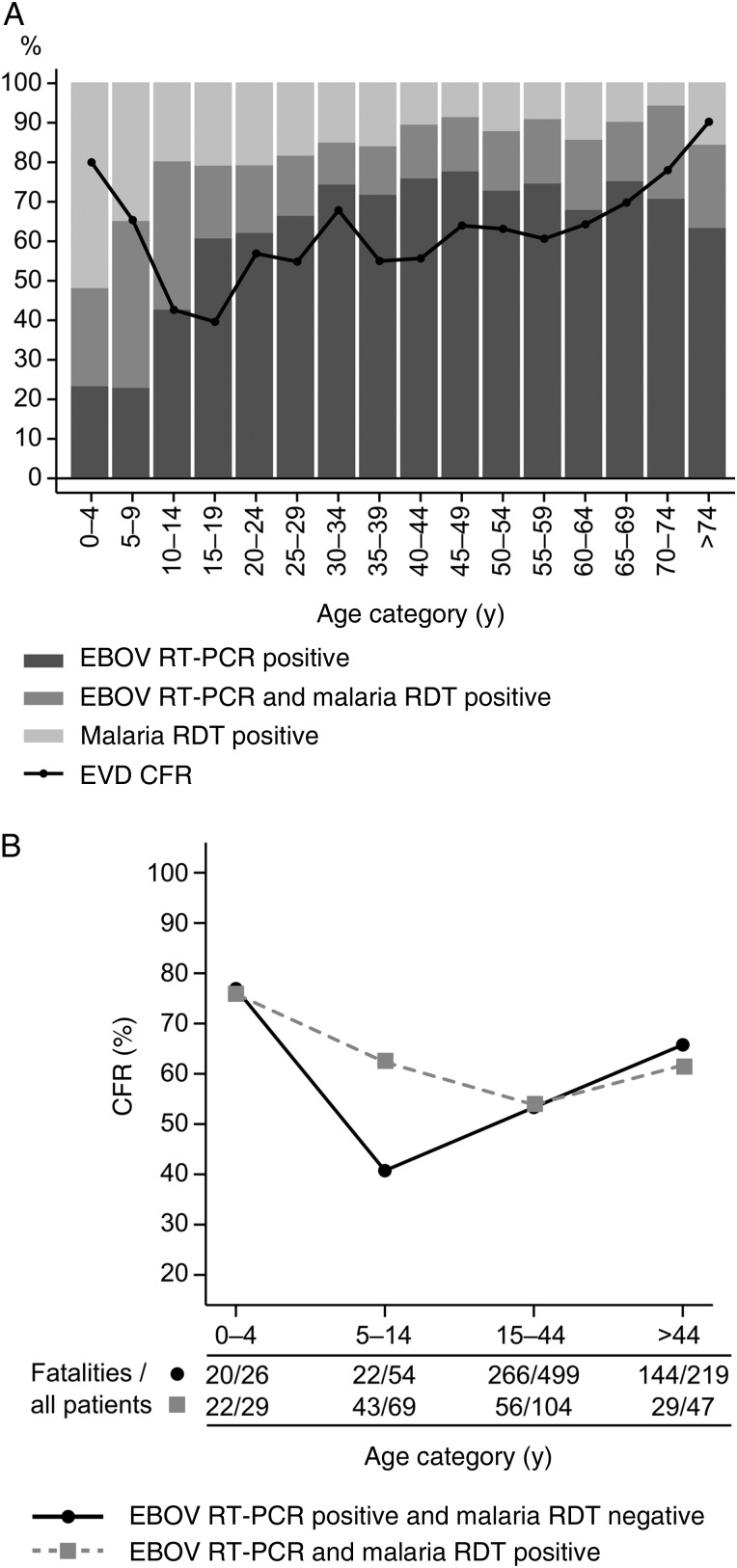
A malaria RDT was performed for 1937 hospital attendees (89%), of whom 541 (28%) tested positive. Malaria RDT–positive patients had a median age of 20 years (IQR, 7–35 years) and thus were younger than malaria RDT–negative patients (median age, 33 years; IQR, 24–45 years). The highest malaria prevalence was observed among children aged <15 years, of whom 220 (59%) had a positive test result. The proportion of malaria RDT–positive patients decreased relative to that of EVD-positive patients toward the higher age groups (Figure 3A). In total, 261 (24%) EVD cases had a malaria parasite coinfection. The highest proportion of coinfections was found in children aged <15 years. The CFR for EVD showed an age-related effect with 2 maxima and a minimum (Figure 3A). Maximum CFRs were observed in young children aged <5 years (80% [63]) and elderly patients aged >74 years (90% [18]). The lowest CFR was observed in 15–19-year-old young adults (39% [39]). Malaria parasite coinfection increased the CFR in 5–14-year-old children by >20% (Figure 3B).
Figure 3.
Proportion of patients with Ebola virus disease (EVD) and/or malaria, as well as case-fatality ratios (CFRs) for EVD, according to age and malaria parasite coinfection status. A, The relative frequencies of hospitalized patients with positive results of Ebola virus (EBOV) reverse transcription–polymerase chain reaction (RT-PCR) analysis and/or malaria rapid diagnostic tests (RDTs) are shown. The CFR refers to EVD cases irrespective of malaria parasite coinfection. B, CFR depending on age group and malaria parasite coinfection status. The number of fatalities and total number of patients per age group are shown below the graph. The data set used to generate the graph (for 1047 patients) corresponds to the data set used to calculate the regression models in Tables 2 and 3.
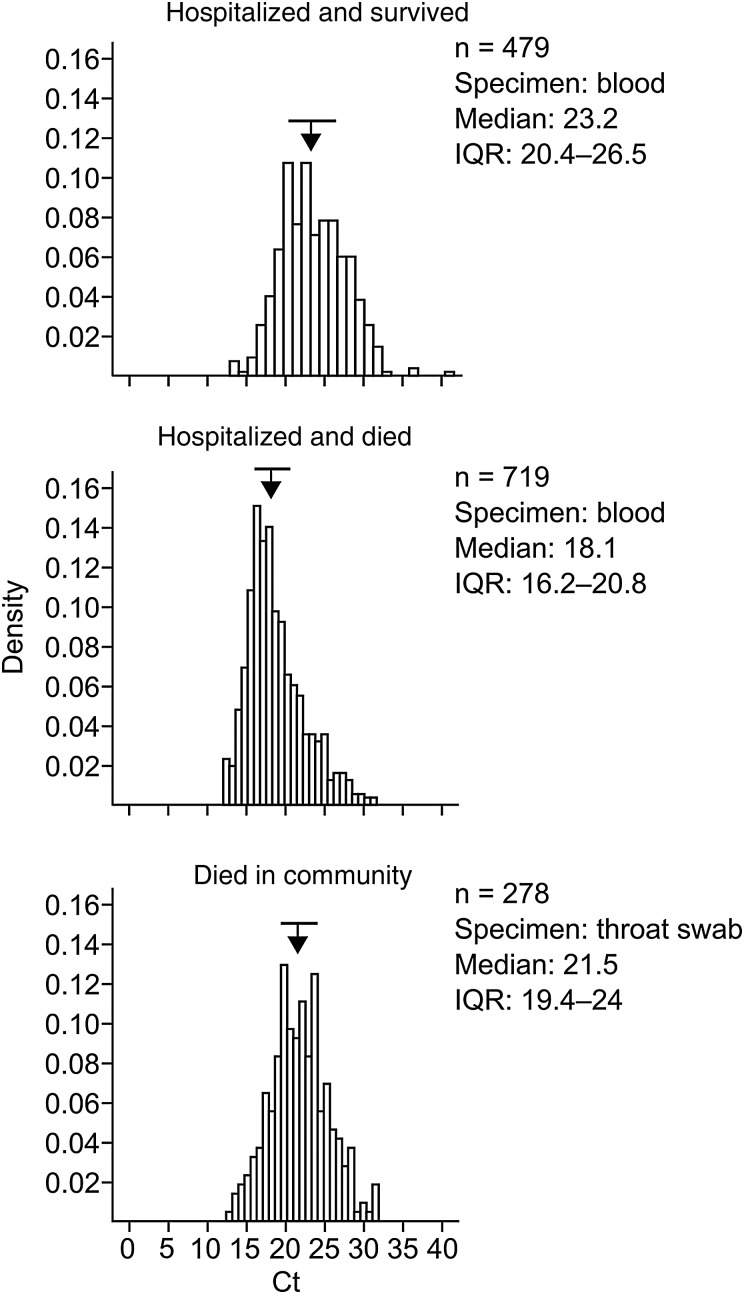
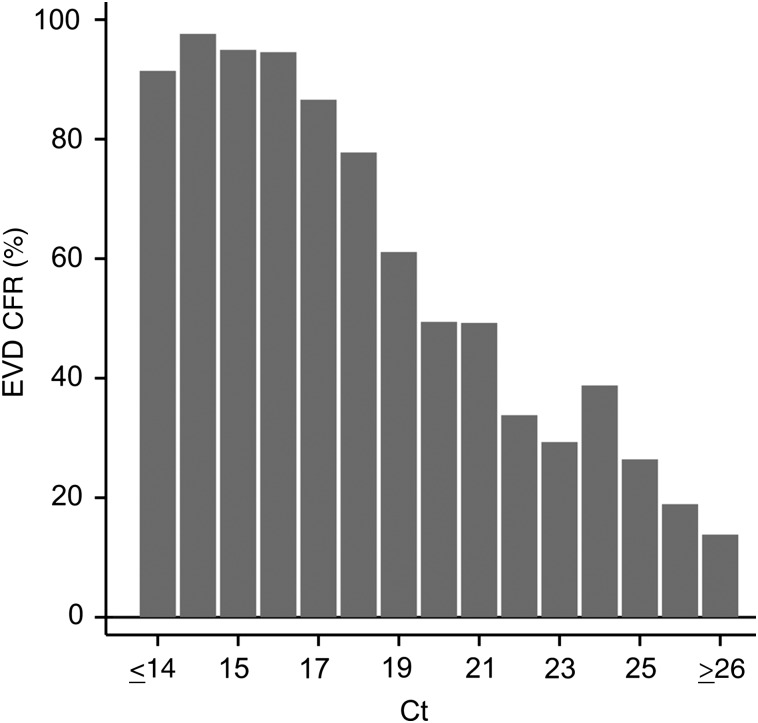
Figure 4 shows the distributions of Ct values for the first blood sample collected from hospitalized patients who died of or survived EVD and for the throat swab collected from community deaths. Patients who died in the hospital had a lower median Ct on admission, indicating a higher virus load, than survivors (18.1 vs 23.2). The median Ct for community deaths (21.5) was 3.4 Ct units higher than for patients who died while hospitalized, which may be related to the different clinical material tested. Given the difference in Ct between people who survived and those who died of EVD, we have plotted the CFR versus Ct categories to evaluate the relationship between virus load and outcome in more detail (Figure 5). The data show a clear inverse correlation between Ct and CFR, indicating that the Ct on admission has a strong prognostic value.
Figure 4.
Distribution of cycle threshold (Ct) values on admission to hospital for patients who died of or survived Ebola virus disease (EVD) and for individuals who died of EVD in the community. Arrows and horizontal bars above the histograms indicate medians and interquartile ranges (IQRs), respectively.
Figure 5.
Case-fatality ratios (CFRs) among hospitalized patients with Ebola virus disease (EVD), according to cycle threshold (Ct) category. The Ct values for the first Ebola virus reverse transcription–polymerase chain reaction–positive blood sample from 2527 patients were included in the analysis.
The analysis of individual factors indicated that age, malaria parasite coinfection, and virus load may be outcome determinants. Therefore, we assessed their influence, using logistic regression models. Variables were Ct of the first EBOV-positive blood sample, age category (ie, 0–4, 5–14, 15–45, and >45 years), and malaria RDT result, stratified within the established age groups. Complete data sets for 1047 EVD patients were available for analysis. In the crude analysis, patients with EVD who had lower Ct values on admission and an age of ≤4 years or ≥45 years had a higher chance of death (Table 2). Malaria had no effect on outcome in the crude analysis. However, as 5–14-year-old children had the highest malaria parasite coinfection rate and an increased CFR if coinfected with malaria parasites (Figure 3B), we assumed an effect of malaria on outcome in this specific age group, which is obliterated in the crude analysis. Therefore, an interaction term (the combination of age category and malaria RDT positivity) was included in the full regression to model an interaction of age and malaria. In agreement with the crude analysis, the full model revealed a higher chance of fatal outcome in particular within the age categories ≤4 years and ≥45 years, irrespective of malaria parasite coinfection (Table 3). Consistent with the data shown in Figure 3B, an effect of malaria RDT positivity was only seen in children 5–14 years of age, who had a higher chance of dying if coinfected with malaria parasites. There was no evidence of an impact of malaria parasite coinfection on outcome in the other age groups. The Ct was not confounded by these variables and showed a similar effect estimate as in the crude analysis.
Table 2.
Crude (Unadjusted) Logistic Regression Analysis of the Association Between a Fatal Outcome and Both Age and Malaria Rapid Diagnostic Test (RDT) Result Among 1047 Patients With Ebola Virus Disease
| Variable | Fatal Cases/Total Cases (%) | Crude Model, OR for Fatal Outcome (95% CI) | P Value |
|---|---|---|---|
| Ct of EBOV RT-PCR (increasing, continuous) | 602/1047 (57.5) | 0.7 (.7–.7) | <.001 |
| Age category, y | |||
| 0–4 | 42/55 (76.4) | 2.9 (1.4–5.9) | .004 |
| 5–14 | 65/123 (52.8) | 1 (Reference) | |
| 15–44 | 322/603 (53.4) | 1.0 (.7–1.5) | .91 |
| ≥45 | 173/266 (65.0) | 1.6 (1.1–2.6) | .02 |
| Malaria RDT result | |||
| Negative | 452/798 (56.6) | 1 (Reference) | |
| Positive | 150/249 (60.2) | 1.2 (.9–1.5) | .32 |
Abbreviations: CI, confidence interval; Ct, cycle threshold; EBOV, Ebola virus; OR, odds ratio; RT-PCR, reverse transcription–polymerase chain reaction.
Table 3.
Multivariate Logistic Regression Analysis of the Association Between Age and Fatal Outcome, by Malaria Rapid Diagnostic Test (RDT) Result, and the Effect of Malaria per Age Group (Interaction) Among 1047 Patients With Ebola Virus Disease
| Variable | Malaria RDT Negative |
Malaria RDT Positive |
Interaction |
|||
|---|---|---|---|---|---|---|
| Full Model, OR for Fatal Outcome (95% CI) | P Value | Full Model, OR for Fatal Outcome (95% CI) | P Value | Full Model, OR for Fatal Outcome (95% CI) | P Value | |
| Ct of EBOV RT-PCR (increasing, continuous) | 0.7 (.7–.7) | <.001 | 0.7 (.7–.7) | <.001 | 0.7 (.7–.7) | <.001 |
| Age category, y | ||||||
| 0–4 | 14.3 (3.5–58.5) | <.001 | 12.3 (3.2–47.7) | <.001 | 0.9 (.2–4.8)a | .86 |
| 5–14 | 1 (Reference) | 4.2 (1.7–10.1) | .002 | 4.2 (1.7–10.1)a | .002 | |
| 15–44 | 3.0 (1.5–5.9) | .002 | 2.6 (1.2–5.9) | .02 | 0.9 (.5–1.5)a | .63 |
| ≥45 | 5.0 (2.4–10.5) | <.001 | 3.9 (1.5–10.2) | .006 | 0.8 (.3–1.7)a | .52 |
Abbreviations: CI, confidence interval; Ct, cycle threshold; EBOV, Ebola virus; OR, odds ratio; RT-PCR, reverse transcription–polymerase chain reaction.
a Estimates of the corresponding interaction terms are as follows: age 0–4 years: OR, 0.2 (95% CI, .1–1.4; P = .11); age 5–14 years: OR, 1 (reference); age 15–44 years: OR, 0.2 (95% CI, .1–.6; P = .003); and age ≥45 years: OR, 0.2 (95% CI, .1–.6; P = .006).
DISCUSSION
During the stay in Guéckédou, EMLab tested specimens from 2741 patients with suspected EVD from Guinea who either attended a hospital or died in their community. EVD was confirmed in 1512 cases, representing 44% of all EVD cases reported from the entire country during that period [12]. Irrespective of whether patients attended a hospital or died in the community, EVD was diagnosed in about 50% of all suspected cases. This high incidence suggests that EVD was a major cause of mortality and morbidity in the affected area during the epidemic. Nearly 20% of all EVD cases died in the community and were diagnosed on the basis of analysis of swabs. The median Ct for swabs was 3.4 Ct units higher than the admission Ct for blood from fatal hospital cases, which roughly corresponds to a 1 log unit difference in virus load. Nevertheless, the Ct distribution curve for swabs lies well within the detection range of the EBOV RT-PCR assay. As the Ct values appear to be largely normally distributed, the observed curve suggests that the vast majority of throat swabs contain a virus load that can easily be detected in that assay. Thus, a throat swab is a suitable clinical specimen for postmortem EVD diagnostic testing. In addition, the epidemic curve for EVD community deaths corresponds quite well to the epidemic curve for people hospitalized with EVD. Both imply that testing of community deaths is a reliable and sensitive method for surveillance. Indeed, it has been successfully used in the affected countries in the postoutbreak phase.
The CFR remained largely constant or slightly decreased during the epidemic, until December 2014, when it dropped considerable. The reason for this drop is not clear but may be related to the higher median Ct value (23) and lower malaria parasite coinfection rate (3%), compared with previous months, and to the initiation of the JIKI trial in Guéckédou during this period, which showed a trend toward efficacy of favipiravir in patients with a Ct of ≥20 [13]. The EMLab data have not been collected for scientific purposes, and therefore our results should be interpreted with caution. Patients attending the treatment centers are not a random sample from the hospital's catchment area. Attendance at the ETU may be influenced by campaigns, reputation of the center, perceived individual disease severity, willingness to be tested, distance to the center, or availability of alternative treatment options. All of these factors may change and explain the variation in CFR over time [14].
Virus load—in the field, usually represented by the Ct—is closely correlated with outcome, as has been observed in previous outbreaks [15] as well as in the West African outbreak [14, 16–24]. We found a difference between the median Ct values of EVD fatalities and survivors of 5.1 Ct units, roughly corresponding to a difference in virus RNA concentration of 1.5 log units. Moreover, the Ct on admission has strong prognostic value, providing a quantitative estimate of outcome. Patients with EVD who have a Ct of <17 have a CFR of 95%, and those with a Ct of >26 have a CFR of 15%. Between these 2 extremes, the Ct is nearly perfectly (negatively) correlated with the CFR.
Malaria parasite coinfections occur in a significant fraction of patients with EVD and seem to codetermine the outcome. The overall prevalence of coinfection was comparable to findings in studies from Liberia [25]. As expected, we found the highest incidence of malaria in children <15 years of age. Consistent with this finding, the coinfection rate of Ebola virus and malaria parasites was highest in this age group. However, the interaction between malaria and EVD and their effect on outcome seems to be complex. The CFR has a first maximum in children aged <5 years, followed by a minimum among individuals aged 10–19 years and a second maximum among patients aged >74 years. Similar distributions have been observed in other studies from the West African outbreak [20, 23, 26]. It may be that both an immature immune system in conjunction with malaria parasite coinfection leads to the increased CFR in young children, while the high CFR in elderly individuals may be due to comorbidities and a generally reduced health and immune status. The shape of the EVD CFR curve by age resembles the “U” or “W” shape of the mortality and CFR curves for severe influenza [27], suggesting that similar host determinants might underlie both distributions. The uneven malaria distribution among the patients with EVD and the age dependency of the effect of malaria parasite coinfection has been taken into account by our full regression model. It revealed that both young age (≤4 years) and malaria parasite coinfection in children aged 5–14 years are independent risk factors for a fatal outcome. The lack of significant contribution of malaria parasite coinfection in most age groups may be the result of treatment with antimalarials in the ETU (irrespective of age, all patients received artemisinin-based combination therapy). In addition, the regression analysis confirmed the clear association between Ct and outcome.
Notes
Acknowledgments. We thank the World Health Organization (WHO) field teams and the Guinean health authorities for their commitment and excellent cooperation.
The European Mobile Laboratory is a technical partner of the WHO Emerging and Dangerous Pathogens Laboratory Network and Global Outbreak Alert and Response Network (GOARN), and the deployments in West Africa have been coordinated and supported by the GOARN Operational Support Team at WHO headquarters.
Financial support. This work was supported by the European Union's Horizon 2020 research and Innovation Program (grant agreement 666100 to project EVIDENT [Ebola virus disease: correlates of protection, determinants of outcome, and clinical management]) and the Directorate-General for International Cooperation and Development (service contract IFS/2011/272-372).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baize S, Pannetier D, Oestereich L et al. Emergence of Zaire Ebola virus disease in Guinea. N Engl J Med 2014; 371:1418–25. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Ebola situation report—16 March 2016. http://apps.who.int/ebola/current-situation/ebola-situation-report-16-march-2016 Accessed 30 March 2016.
- 4.Lever RA, Whitty CJ. Ebola virus disease: emergence, outbreak and future directions. Br Med Bull 2016; 117:95–106. [DOI] [PubMed] [Google Scholar]
- 5.Wolfel R, Stoecker K, Fleischmann E et al. Mobile diagnostics in outbreak response, not only for Ebola: a blueprint for a modular and robust field laboratory. Euro Surveill 2015; 20:pii:30055. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Interim guideline: case definition recommendations for Ebola or Marburg virus diseases, 9 August 2014. http://apps.who.int/iris/bitstream/10665/146397/1/WHO_EVD_CaseDef_14.1_eng.pdf?ua=1&ua=1 Accessed 25 June 2016.
- 7.Rieger T, Kerber R, El Halas H et al. Evaluation of RealStar RT-PCR kits for filovirus detection in the laboratory and field. J Infect Dis 2016; doi:10.1093/infdis/jiw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alere. Test kit product instructions—BinaxNOW malaria test kit. IN665000 Rev. 6, 2015/04. http://www.alere.com/en/home/product-details/binaxnow-malaria.html Accessed 25 June 2016.
- 9.World Health Organization (WHO). Guinea In: World malaria report. Geneva, Switzerland: WHO, 2015:121 http://www.who.int/entity/malaria/publications/country-profiles/profile_gin_en.pdf?ua=1 Accessed 25 June 2016. [Google Scholar]
- 10.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis 2005; 191:1932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bejon P, Berkley JA, Mwangi T et al. Defining childhood severe falciparum malaria for intervention studies. PLoS Med 2007; 4:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. Ebola situation report—25 March 2015. http://apps.who.int/ebola/current-situation/ebola-situation-report-25-march-2015. Accessed 13 March 2016.
- 13.Sissoko D, Laouenan C, Folkesson E et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick G, Vogt F, Moi Gbabai OB et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Medecins Sans Frontieres Ebola case management centre, Kailahun, Sierra Leone, June-October 2014. J Infect Dis 2015; 212:1752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Towner JS, Rollin PE, Bausch DG et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 2004; 78:4330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schieffelin JS, Shaffer JG, Goba A et al. Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan T, Mu J, Qin E et al. Clinical characteristics of 154 patients suspected of having Ebola virus disease in the Ebola holding center of Jui Government Hospital in Sierra Leone during the 2014 Ebola outbreak. Eur J Clin Microbiol Infect Dis 2015; 34:2089–95. [DOI] [PubMed] [Google Scholar]
- 18.Hunt L, Gupta-Wright A, Simms V et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis 2015; 15:1292–9. [DOI] [PubMed] [Google Scholar]
- 19.Lanini S, Portella G, Vairo F et al. Blood kinetics of Ebola virus in survivors and nonsurvivors. J Clin Invest 2015; 125:4692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faye O, Andronico A, Faye O et al. Use of viremia to evaluate the baseline case fatality ratio of Ebola virus disease and inform treatment studies: a retrospective cohort study. PLoS Med 2015; 12:e1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bah EI, Lamah MC, Fletcher T et al. Clinical presentation of patients with Ebola virus disease in Conakry, Guinea. N Engl J Med 2015; 372:40–7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Rong Y, Sun L et al. Prognostic analysis of patients with Ebola virus disease. PLoS Negl Trop Dis 2015; 9:e0004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu HJ, Qian J, Kargbo D et al. Ebola virus outbreak investigation, Sierra Leone, September 28-November 11, 2014. Emerg Infect Dis 2015; 21:1921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe SJ, Maenner MJ, Kuah S et al. Prognostic indicators for Ebola patient survival. Emerg Infect Dis 2016; 22:217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Wit E, Falzarano D, Onyango C et al. The merits of malaria diagnostics during an Ebola virus disease outbreak. Emerg Infect Dis 2016; 22:323–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agua-Agum J, Ariyarajah A, Blake IM et al. Ebola virus disease among children in West Africa. N Engl J Med 2015; 372:1274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis 2006; 12:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]