Abstract
Microbial colonization of mucosal tissues during infancy plays an instrumental role in the development and education of the host mammalian immune system. These early-life events can have long-standing consequences: facilitating tolerance to environmental exposures or contributing to the development of disease in later life, including inflammatory bowel disease, allergy, and asthma. Recent studies have begun to define a critical period during early development in which disruption of optimal host-commensal interactions can lead to persistent and in some cases irreversible defects in the development and training of specific immune subsets. Here, we discuss the role of early-life education of the immune system during this “window of opportunity,” when microbial colonization has a potentially critical impact on human health and disease.
The commensal microbiota, which widely colonize the body, strongly influence the host immune system. Most of the internal and external surfaces of a mammals’ body during adult life—including the gastrointestinal tract, skin, and oral mucosa—are heavily colonized by microbiota, with the largest congregation of organisms contained within the colon. The human body was recently estimated to be composed of 3 × 1013 eukaryotic cells and 4 × 1013 colonizing bacteria (1). The exposure of the mammal to microbiota begins in utero and expands rapidly after birth. For example, maternal gut bacteria can be detected in the amniotic fluid of pregnant mice (2), and bacteria can be isolated in the meconium from preterm human babies (3). After birth, the composition of microbiota is initially derived from opportunistic colonization by the first types of bacteria to which a baby is exposed in his or her environment, which together with other environmental factors, such as diet, may substantially affect the entry of subsequent microbial species into the various mucosal niches. As such, the mode of delivery and subsequent environmental exposures greatly influence the composition of the microbiota in the infant (4). The microbiota composition of an adult may therefore in part reflect the history of exposure to microbes and environmental factors in early life. This concept is supported by experiments with mice (5, 6) and familial studies in humans (7) that indicate that the environment in early life may account for, at least in part, microbial composition in the adult. These events and their immune consequences are perhaps most important during the first year of life, when the microbial composition is highly variable (8), with progressive stabilization to an adult-like community structure after 3 years of age (9). The instability of the microbiota during early life makes the community structure of microbiota during this time more sensitive to environmental incursions (10). It is now increasingly clear that early-life colonization also coincides with a potentially time-limited period during which the immune system is permissive to microbial instruction. Further, emerging evidence suggests that the immune influences induced by the microbiota during this early period of life may be durable, creating a “window of opportunity” for proper (or improper) immune education to occur and resistance (or susceptibility) to disease in later life. This Review focuses on these issues and the recent evidence in support of this concept.
Evidence that the microbiota influences the immune system: Lessons from germ-free animal studies
The use of germ-free (GF) animals allowed for development of the concept that the microbiota influences the immune system. In 1885, Louis Pasteur first proposed the generation of animals deprived of common microorganisms to understand the relationship between microbes and their host (11). Ten years later, Nuttall and Thierfelder used guinea pigs to produce the first GF animals by means of cesarean section (12). It took around another 50 years, however, until enough was known about the nutrition of newborn animals to enable production of the first GF rats (13) and mice (14). The first comparisons of GF rodents with “microbiota-colonized animals” resulted in the observation that there were gross physiological differences between GF and the specific pathogen–free (SPF) state, including an enlarged cecum, principally due to an accumulation of undegraded mucus (15), and reduced gastrointestinal motility (16), due to the loss of the critical digestive functions of the inhabiting microbiota. In parallel, GF rodents display an aberrant intestinal epithelial cell (IEC) morphology that includes longer villi and shorter crypts in comparison with that observed in conventionalized mice (17) and reduced antimicrobial peptides (18, 19). Of most relevance, the absence of commensals has profound effects on the structural and functional development of the immune system, including but not limited to defects in lymphoid tissue development within the spleen, thymus, and lymph node (20, 21). Moreover, these structural abnormalities were most striking near the mucosal interface, suggesting that interactions with specific communities of microbes directly modulate development of these gut-associated lymphoid structures (GALTs). For instance, a type of lymphoid tissue called isolated lymphoid follicles (ILFs) are minimally present in the absence of microbiota in the small intestine but not the colon (22–24). In addition, GF animals contain smaller Peyer’s patches (PPs) and mesenteric lymph nodes (MLNs) (23). Collectively, these studies demonstrate that the microbiota participates in the maturation of the immune system and suggests that specific events in association with the process of microbial colonization may be important in the development of a normal immune system in a healthy individual.
Microbial colonization modulates early development of the immune system in mucosal tissues
Colonization of mucosal surfaces is characterized by fluctuating changes in microbial diversity during the first few years of life, until reaching a point of equilibrium that remains relatively stable throughout adulthood in the absence of environmental insults (25). In association with this, the early-life ecological succession of mucosal colonization occurs concomitantly with the development, expansion, and education of the mucosal immune system. It therefore follows that immune maturation is likely influenced directly and/or indirectly by the presence of commensal microbes (26, 27). The postnatal period thus represents a potentially critical time in which early-life microbial exposures can have profound influences on the morphological and functional development of the immune system. Neonatal immune cells differ in function from adult immune cells so that they preferentially develop tolerance in response to antigen exposure in a process that still remains to be defined but does not involve an intrinsic property of the T cell but rather the environment in which the T cell develops (28–30). Neonatal immune cells therefore learn to tolerate the new environment experienced after birth, such as the commensal microbiota. These concepts have been experimentally tested by varying the time of microbiota introduction into GF animals and observing the changes in immune development and/or function.
Although most abnormalities in GF animals are age-independent and can be corrected by introducing commensals at any age (31), the ability to restore a few cellular defects that occur in the absence of microbiota is restricted to a short time interval in early life and thus is age-dependent. Consistent with this, GF mice conventionalized during adult life possess a different transcriptional profile in the jejunum and colon in comparison with conventionally raised SPF mice, suggesting that if colonization does not occur during a critical “window of opportunity,” intestinal immune development cannot be fully achieved in the adult (32). This supports the notion that early exposure to microbes may have durable consequences for the host that may extend into adult life. As a corollary, in the absence of appropriate microbial exposures in early life, the immune consequences may elicit irreversible and potentially deleterious implications for the host.
Age-independent influences
T cell subsets
GF animals exhibit major defects in the development of primary and secondary lymphoid organs—including the GALT, spleen, and thymus—which is associated with a decreased frequency of CD4+ and CD8+ intestinal T cell subsets as well as reduced numbers of intraepithelial lymphocytes that express the αβ T cell receptor (TCR) (33). Normalization of these morphological and cellular defects in αβ T cells can be achieved by reintroducing standard mouse or human microbiota in adult mice (33–36). Furthermore, characterization of the TCR repertoire in intestinal tissues at the time of initial commensalization suggests an influx of polyclonal TCR-αβ+ T cells early in life that lose diversity and revert to an oligoclonal state in the adult (37). To address this, subsequent studies comparing colonized and monocolonized mice with GF animals have begun to clarify the role of distinct commensal bacterial communities in modulating specific T cell effects. GF mice are typically T helper 2 (TH2)–skewed. A balancing of systemic TH1 and TH2 cells is observed after monocolonization of GF mice with Bacteroides fragilis that is due to a single microbial molecule, polysaccharide A (PSA) (38). In another example, segmented filamentous bacteria (SFB) play a key role in intestinal TH cell responses and the induction of secretory immunoglobulin A (IgA) (39, 40). Colonization with rat microbiota or monocolonization with SFB, however, only partially restores CD4+ and CD8+ T cell numbers, suggesting that a diverse microbial population is necessary to restore a fully mature immune system in the adult (39). Particular bacterial populations have been linked to the development of specific T effector subsets, such as TH17 cells, a potent source of interleukin-17 (IL-17) that plays an important role in the maintenance of mucosal barrier integrity and clearance of pathogens in the tissues. TH17 development is dependent on microbiota and is absent in the small intestine of GF animals. TH17 cells can be restored in GF adult mice through colonization with standard mouse microbiota or SFB but not others (41). Consequently, SFB colonization confers enhanced protection compared with GF animals after infection with the bacterial pathogen Citrobacter rodentium, which is a direct result of enrichment of TH17 cells in the small bowel (41).
Regulatory T cells (Treg cells) are a major source of IL-10 and capable of recognizing commensal-derived antigens (42), which supports the maintenance of tolerance to intestinal microbes (43). GF mouse colon, but not small intestine, contains decreased Treg cells, which can be normalized through standard conventionalization and monocolonization with certain Clostridium species or a variety of intestinal microbes, regardless of age (44, 45).
B cells
B cells are essential to the immune system as a source of the five different isotypes of functionally distinct antibodies: IgA, IgE, IgG, IgD, and IgM. GF mice exhibit normal numbers and phenotypic maturation of B cells. However, they exhibit a general defect in the production of IgA and IgG1 antibodies in mucosal and nonmucosal organs that can be normalized after conventionalization by microbiota (31). Consistent with this, GF mice possess few IgA-expressing B cells in the small intestine. This decrease in IgA is likely due to ILF deficiency. Conventionalization of adult GF mice thus restores ILF numbers and rescues IgA production by B cells in the small intestine (31, 46). This may be through dendritic cells that do not migrate outside of intestinal tissues and retain live microorganisms for prolonged periods of time, which allows them the ability to induce IgA expression in the adult mouse intestine (47).
Innate lymphoid cells
Innate lymphoid cells (ILCs) represent lymphoid cells with innate immune function that lack a B or T cell receptor (48). Conflicting results surround our understanding of ILC regulation by microbiota. Natural killer (NK) cells (ILC group 1) exhibit reduced phenotypic evidence of activation in nonmucosal organs of GF mice, a process that is orchestrated by mononuclear phagocytes in a Toll-like receptor (TLR)–dependent manner and can be reversed through the colonization of adult mice with a standard microbiota (49). It has also been shown that GF mice display impaired development of Nkp46+ RAR-related orphan receptor–γt (RORγt)–dependent group 3 ILCs (50) and decreased expression of IL-22 by these cells, an important cytokine in epithelial responses to microbes in the small intestine (50, 51). However, another study has shown that GF mice exhibit no change in the numbers of Nkp46+ RORγt–dependent ILCs and increased IL-22 expression owing to IL-25 production by IECs (52, 53). In either case, dysregulation of IL-22 expression in GF mice can be normalized by means of standard microbiota conventionalization in adult mice (50, 52). Despite these inconsistencies, it is clear that microbiota regulate ILCs in an age-independent manner.
Epithelial cells
IECs are found at the interface between the lumenal microbiota and immune system in the mucosa and play an increasingly recognized role in a variety of immune functions (54). One of the functions of IECs is to produce antimicrobial peptides. RegIIIγ is a secreted C-type lectin that regulates the interaction between microbiota and the host through its antibacterial activity on Gram-positive microbes and is mainly expressed by Paneth cells at the base of the crypts of Lieberkuhn in the small intestine. In GF mice, IECs exhibit decreased expression of RegIIIγ that can be rescued after colonization with standard microbiota (55) or monocolonization with Bacteroides thetaiotaomicron in small intestine and colon (56). RegIIIγ expression is also regulated by IL-22 derived from Nkp46+ RORγt–dependent ILC3 (57). Resistin-like molecule β (RELMβ) is a secreted protein highly expressed in the intestinal tract and goblet cells in particular. In GF mice, RELMβ expression by goblet cells is strongly decreased when compared with that in SPF mice but can be rescued after colonization with standard microbiota (58). RELMβ can regulate the expression of inflammatory cytokines through intestinal macrophages influencing host responses to artificially induced colitis (59). Separately, GF mice exhibit a lower expression of major histocompatibility complex (MHC) class II on IECs that can be rescued through colonization with microbiota, an interferon-γ–dependent event (60). In parallel, microbial derived factors such as acetate from Bifidobacterium longum can enhance protective functions of IECs in response to infection (61). Current studies also suggest that conventionalization of GF adult animals leads to substantial changes in gene expression associated with IECs (62). In future studies, it will be interesting to examine the age dependency of these effects and the manner in which they alter epithelial expression of factors well known to play an important role in epithelial cell–immune interactions such as IL-25, transforming growth factor–β (TGF-β), B cell activating factor (BAFF), a proliferator-inducing ligand (APRIL), and thymic stromal lymphopoietin (TSLP).
Age-dependent influences
iNKT cells in colon and lung
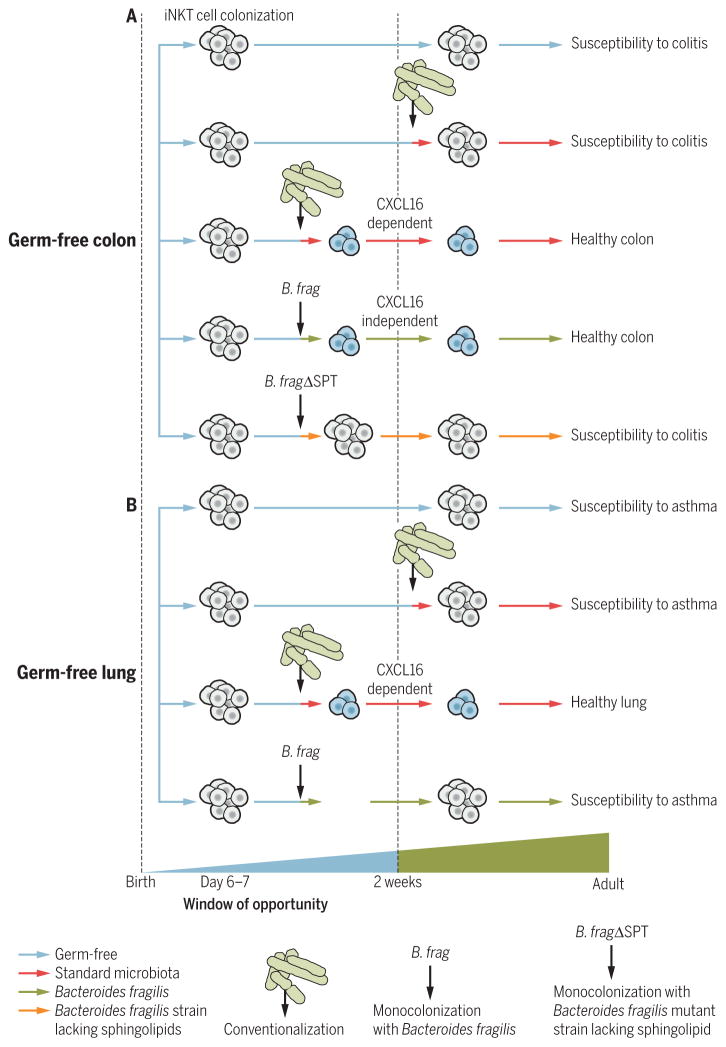
Invariant natural killer T (iNKT) cells express an invariant TCR-α chain and a diverse array of TCR-β chains and recognize endogenous and exogenous (bacterial) lipid antigens when presented by CD1d, a MHC class I–like molecule (63). In GF animals, iNKT cells are decreased in peripheral tissues such as the spleen and liver and are hyporesponsive to lipid antigen stimulation, which is normalized by monocolonization with bacteria expressing iNKT antigens in adult mice (64). In distinct contrast, mucosal tissues such as the lung and colon contain substantially increased quantities of iNKT cells when mice are GF in comparison with that observed in SPF mice in association with augmented inflammatory responses in experimental models of colitis and airway hyperresponsiveness (AHR) (65, 66). This exaggerated accumulation of iNKT cells and hyperresponsiveness to environmental triggers of iNKT cells that induce colitis can be normalized through colonization of GF mice with standard microbiota, Bacteroides fragilis monocolonization, or treatment with B. fragilis–derived sphingolipid antigens during the first 2 weeks of life but not thereafter (Fig. 1A) (65, 66). iNKT cell accumulation in the lung and susceptibility to asthma of GF mice can be rescued by colonization with standard microbiota but not by monocolonization with B. fragilis during the first 2 weeks of life (Fig. 1B).
Fig. 1. iNKT cell colonization is regulated by the microbiota during early life and influences susceptibility to colitis and asthma later in life.
(A) iNKT cells migrate from the thymus to the colon during the first weeks of life. Their abnormal accumulation in the colon of GF mice leads to later-life susceptibility to oxazolone-induced colitis. Conventionalization with a standard microbiota or monocolonization with B. fragilis (B. frag) but not B. fragilis deficient in sphingolipids (B. fragΔSPT) during the window of opportunity restores colonic iNKT cell numbers and abrogates the increased susceptibility to colitis. In contrast to conventionalization with a standard microbiota during the first 2 weeks of life that decrease iNKTcell number in a mechanism dependent on CXCL16, monocolonization with B. fragilis regulates colonic iNKT cell number in a CXCL16-independent manner that depends on inhibitory sphingolipids from B. fragilis that impede iNKT cell proliferation. (B) iNKT cell accumulation in lungs of GF mice lead to later-life susceptibility to asthma. Conventionalization with a standard microbiota but not monocolonization with B. fragilis during the window of opportunity restores colonic iNKT cell numbers in a CXCL16-dependent manner and abrogates the increased susceptibility to asthma.
These studies have revealed at least two non-mutually exclusive mechanisms by which the microbiota can modulate iNKT frequency in the colon and lung. Accumulation of iNKT cells in the colon in GF animals is associated with elevated levels of the chemokine ligand CXCL16, whose expression is normalized by conventionalization during this critical 2-week time period in a pathway dependent on microbiota-induced epigenetic changes of the Cxcl16 gene (65). In contrast, normalization of iNKT cell frequency by the human commensal B. fragilis appears to involve an inhibition of iNKT cell proliferation and expansion in early life through B. fragilis–derived sphingolipids that are inhibitory (66). It is particularly interesting that the effects of intestinal B. fragilis colonization is tissue-specific because it is restricted to the colon, strongly supporting the idea that specific microbes and potentially signals can regulate iNKT recruitment and proliferation in distinct organs.
B cells and IgE
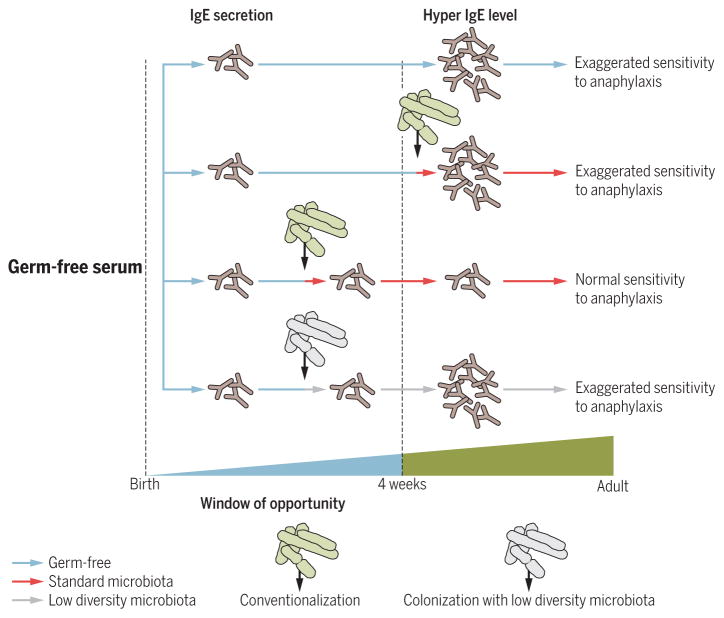
IgE are antibodies produced by B cells that play a pivotal role in response to allergens and immunity to pathogens. Whereas IgA and IgG1 levels are decreased in GF mice, a recent study showed that GF mice have elevated levels of serum IgE after weaning (~30 days) because of enhanced rates of B cell isotype class switching to IgE in PPs and MLNs (67). Elevated IgE levels in GF animals was associated with exaggerated responses to orally induced systemic anaphylaxis (67). Hyper-IgE levels in GF mice serum are associated with a concomitant increase in IL-4 production in PPs and MLNs and are dependent on CD4+ T cells. Colonization of GF mice with standard microbiota from birth to 4 weeks of age but not thereafter normalizes the IgE levels in adults, which is consistent with a regulation of IgE production by the microbiota that is specific to this time frame (Fig. 2). IgE production in adult mice is dependent on intestinal bacterial diversity in neonates rather than on colonization with specific bacterial species so that a low diversity of microbiota is not sufficient to normalize IgE levels during early life.
Fig. 2. Exposure to a microbiota during early life regulates IgE levels in serum of adult mice and their sensitivity to orally induced anaphylaxis.
IgE accumulates in the serum of GF mice 4 weeks after birth because of an isotype switch to IgE in mucosal B cells. “Hyper-IgE levels” lead to an exaggerated sensitivity to orally induced anaphylaxis that can be resolved through conventionalization with a standard microbiota during early life but not thereafter. Colonization of GF mice with low-diversity microbiota during the window of opportunity fails to normalize hyper-IgE levels in adult life.
Treg cells in lung and skin
Exposure to house dust mite (HDM) antigen—which possesses CD1d-restricted antigens for iNKT cells (68), during the first 2 weeks of life, but not thereafter—induces a helios-negative (presumably induced) subset of Treg cells from conventional CD4+ T cells in the lung (69). Consequently, initial HDM exposure during adult life results in increased AHR relative to that observed when HDM exposure first occurs in early life. Mechanistically, the allergen-induced development of the helios-negative Treg cells in early life is dependent on microbiota and involves the expression of the immune checkpoint molecule programmed cell death protein 1 (PD1) on the T cell and PD-L1 on dendritic cells so that early-life blockade of PD-L1 abrogates the expansion of the helios-negative Treg cell and increased asthma upon HDM exposure. Thus, a 2-week window of opportunity exists in which allergen exposure can induce a specific group of Treg cells and protection from asthma. Consistent with a prevalent role of microbiota in the regulation of lung allergic disease during early life, antibiotic treatment of mice during early life enhances susceptibility to allergic asthma (6).
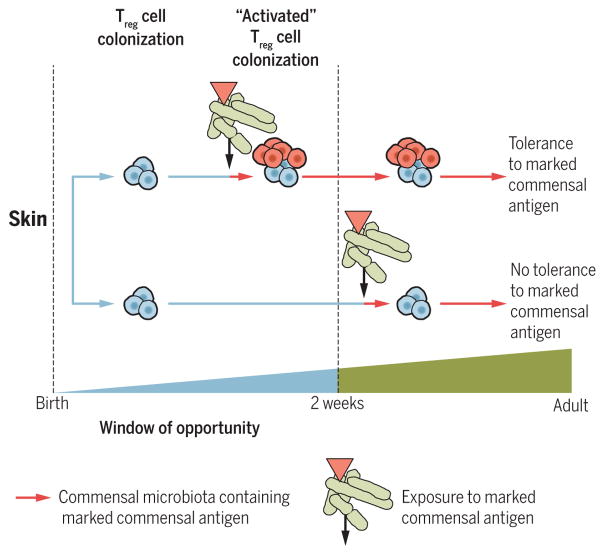
A similar age-restricted regulation of Treg cells also exists in skin. Colonization of mice with a commensal skin bacterial strain during the first 2 weeks of life, but not thereafter, induces accumulation of activated Treg cells in the skin that are derived from the thymus and which maintain tolerance to commensal derived antigens in the adult (70). When colonization is initiated in adult mice after the time of weaning, such commensal antigen-specific tolerance is not initiated (Fig. 3). Further, the accumulation of activated Treg cells during this early-life period appears to be specific to the skin and is not observed in the intestinal lamina propria. Antibiotic treatment in neonatal mice can induce long-term modification of microbial composition of the skin and susceptibility to psoriasis development in adulthood, which is associated with increased IL-22–producing γδ T cells (71). Regulation of thymically derived and induced Treg cells by microbiota during early life therefore appears to be tissue-specific, suggesting that even the same cell type (such as Treg) is under the influence of distinct organ-specific mechanisms.
Fig. 3. Treg cells colonize the skin of neonatal mice and induce tolerance to commensal bacteria.
Colonization of neonatal skin with a commensal microbiota leads to an accumulation of “activated” Treg cells (red cells) that specifically recognize an artificially marked antigen, with consequent tolerance to this same antigen in later life. Colonization of adult animals with the same microbiota containing the marked commensal microbiota (red triangle) does not lead to Treg cell accumulation in the skin or establish immune tolerance.
Epithelial cells
The establishment of tolerance during the neonatal period can be regulated by non-immune cells. As an example, TLR signaling is specifically down-regulated during the first 2 weeks of life in mouse IECs but not thereafter (72, 73). This renders epithelial cells hyporesponsive to TLR stimuli such as lipopolysaccharide derived from Gram-negative bacteria. This neonatal tolerance is dependent on TLR4 and the mode of birth delivery. Indeed, IECs from cesarean-born neonates do not down-regulate TLR signaling and are more prone to develop epithelial damage, suggesting an important role of specific types of microbial exposure in the development of epithelial tolerance. These studies emphasize the important potential role played by epithelial cells in the tolerance pathways that are established during early life.
Erythrocytes in spleen
A small subset of erythrocytes express the transferrin receptor CD71. CD71+ erythrocytes are enriched in spleen from birth to 2 weeks of age and diminished in adult life (74). Their development is independent of microbiota; similar numbers are observed in GF and SPF mice. However, they function in suppressing responses of myeloid cells to pathogens (for example, Listeria monocytogenes) or commensals. In the latter case, CD71 inhibition leads to increased basal expression levels of the inflammatory cytokine tumor neonates maintained under SPF conditions. These studies show that not only do microbial exposures regulate immune development during early life, but also that the host maintains critical and specific mechanistic pathways that regulate host response to microbes during this critical period of life.
Early-life perturbations of microbiota and their relationship to human disease
Because microbe-immune interactions appear to play an important role in early-life development of the immune system and when disrupted may result in potentially persistent immune abnormalities, it is worth considering whether early-life exposure to microbes may affect later-life susceptibility to disease. There is emerging evidence to support this hypothesis.
Children exposed to farm environments have a decreased risk for the development of allergic disease, possibly through mechanisms that involve the ubiquitin-modifying enzyme A20 (75, 76). Moreover, farming exposure during pregnancy modulates immune responses and protects against asthma in a mother’s offspring (77). This protective effect can be correlated with increased Treg cell activity in the infant. Although it is not firmly proven, one of the plausible explanations for the protective effect of early-life farm exposure is the role of microbiota because individuals exposed to a farm environment possess different microbial diversity compared with other lifestyles (78). In an opposite manner, exposure to antibiotics is associated with persistent changes in microbial composition in studies performed in human adults (79–81) that may potentially apply to early periods of life (82). As an example, antibiotic use within the first 6 months of life is associated with an increased susceptibility to allergy and asthma at 6 years of age (83). Another study has shown that antibiotic exposure during the first year of life is associated with the development of wheezing and eczema at 8 years of age, even after taking into account early-life respiratory infections (84). In parallel, exposure to antibiotics in childhood, and especially during the first year of life, is associated with an increased risk for development of inflammatory bowel disease (IBD) (85, 86). Antibiotic exposure during the first year of life is also associated with increased susceptibility to being overweight and developing central adiposity and may lead to increased development of type 2 diabetes mellitus later in life (87, 88). Moreover, a recent study has shown that microbiota composition shifts occur in the infant before development of type 1 diabetes mellitus (89). Consistent with this, early-life exposure of female NOD mice to microbiota from male NOD mice can confer protection against diabetes mellitus in a testosterone-dependent pathway (90). Children born by means of cesarean section have an altered microbiota composition and may be more susceptible to obesity, type 1 diabetes mellitus, allergy, and asthma during childhood and adult life (91–93). The mechanistic basis for this latter example is unclear and may involve numerous factors, including the exposure to antibiotics with this intervention.
Together, these studies support an indirect association between perturbations of microbial composition during early life and development of disease later in life. This potential association is investigated in a recent clinical study of children with eosinophilic esophagitis (EoE), which mouse models suggest is an iNKT cell–mediated disease (94). In this example, young children (<6 years of age) with EoE exhibited increased transcriptional levels of TCR Vα24 (the canonical TCR-α chain of iNKT cells), CD1d, and CXCL16 in the esophagi, which was normalized by a dietary intervention to treat the EoE, but not in those who did not respond to the therapy (95). Retrospective data suggested that those with increased iNKT cell–associated transcripts were exposed in early life to antibiotics (95).
Breast milk contains proteins, maternal bacteria, and nutrients important for the development of the newborn that may also participate in the establishment of the neonatal microenvironment (96, 97). This includes the neonatal immune system given the protective effects of breastfeeding on the development of obesity or IBD in adult life (98, 99). However, the specific maternal factors, including microbes and the mechanisms involved, remain to be identified.
Concluding remarks
The mucosal tissues of the host are a highly dynamic set of structures that reside at the interface between the external world and the internal immune and nonimmune tissues. These surfaces are also a critical site of microbial commensalism that plays a critical role in defining the morphology and function of the host and are, like the host that they serve, under the influence of a variety of environmental factors. It is becoming increasingly clear that the ordered establishment of this interface is critically important to the proper development of the immune system and likely the microbiota themselves. Moreover, there has been a recent recognition that a number of the critical immune mechanisms that determine the maintenance of homeostasis and tolerance to environmental exposures at this interface are determined by a set of poorly defined microbial-host interactions that occur during a narrow time frame contained within the earliest days of life. This specifically has lead to the notion of a “window of opportunity,” in which particular microbial exposures during early life determine specific immune events that are durably imprinted, or not. This time frame correlates with the suckling period of rodents, whose relation to human development remains to be defined. A recent study nicely demonstrates that such early-life influences by the microbiota may even be evident during gestation, in that microbial colonization during pregnancy regulates the number of specific innate immune cells and their activity in the offspring (100). This process is dependent on microbially induced, maternal antibodies specific for microbial-derived molecules that are present in the milk and transferred to the neonate during the period of suckling. In their totality, these insights have profound implications for human disease by suggesting that disease risk may begin at the earliest days of life, including the antenatal period. Defining the specific details of these events will therefore have great implications for intervention and understanding in the prevention of complex disease mechanisms.
Acknowledgments
D.L.K. is an inventor on patents licensed to Symbiotic Biotherapies by Brigham and Women’s Hospital and Harvard Medical School related to PSA of B. fragilis and its use as an immunomodulator for treating immune-mediated diseases. D.L.K. and R.S.B. are paid consultants for Symbiotic Biotherapies. R.S.B. is supported by NIH grant DK44319 and the Harvard Digestive Diseases Center (DK0034854). D.L.K is supported by NIH grant R21 AI090102 and the U.S. Department of Defense (W81XWH-15-1-0368).
REFERENCES AND NOTES
- 1.Sender R, Fuchs S, Milo R. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Jiménez E, et al. Curr Microbiol. 2005;51:270–274. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 3.Moles L, et al. PLOS ONE. 2013;8:e66986. doi: 10.1371/journal.pone.0066986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez-Bello MG, et al. Proc Natl Acad Sci USA. 2010;107:11971–11975. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson AK, et al. Proc Natl Acad Sci USA. 2010;107:18933–18938. doi: 10.1073/pnas.1007028107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell SL, et al. EMBO Rep. 2012;13:440–447. doi: 10.1038/embor.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, et al. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. PLOS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yatsunenko T, et al. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koenig JE, et al. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4578–4585. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasteur L. Compte Rendus Ge Acad Sci. 1885;100:68. [Google Scholar]
- 12.Nuttall GHF, Thierfelder H. Physiological Chem. 1896;21:109–121. [Google Scholar]
- 13.Reyniers JA, Trexler PC, Ervin RF. Lobund Rep. 1946;1:1–84. [PubMed] [Google Scholar]
- 14.Pleasants JR. Ann N Y Acad Sci. 1959;78:116–126. doi: 10.1111/j.1749-6632.1959.tb53099.x. [DOI] [PubMed] [Google Scholar]
- 15.Gustafsson BE, Midtvedt T, Strandberg K. Scand J Gastroenterol. 1970;5:309–314. [PubMed] [Google Scholar]
- 16.Abrams GD, Bishop JE. Proc Soc Exp Biol Med. 1967;126:301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- 17.Abrams GD, Bauer H, Sprinz H. Lab Invest. 1963;12:355–364. [PubMed] [Google Scholar]
- 18.Salzman NH, Ghosh D, Huttner KM, Paterson Y, Bevins CL. Nature. 2003;422:522–526. doi: 10.1038/nature01520. [DOI] [PubMed] [Google Scholar]
- 19.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Proc Natl Acad Sci USA. 2008;105:20858–20863. doi: 10.1073/pnas.0808723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauer H, Horowitz RE, Levenson SM, Popper H. Am J Pathol. 1963;42:471–483. [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon HA, Bruckner-Kardoss E, Staley TE, Wagner M, Wostmann BS. Cells Tissues Organs. 1966;64:367–389. [Google Scholar]
- 22.Mosconi I, et al. Mucosal Immunol. 2013;6:1157–1167. doi: 10.1038/mi.2013.12. [DOI] [PubMed] [Google Scholar]
- 23.Macpherson AJ, Harris NL. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 24.Baptista AP, et al. Mucosal Immunol. 2013;6:511–521. doi: 10.1038/mi.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spor A, Koren O, Ley R. Nat Rev Microbiol. 2011;9:279–290. doi: 10.1038/nrmicro2540. [DOI] [PubMed] [Google Scholar]
- 26.Geuking MB, et al. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 27.Rakoff-Nahoum S, Medzhitov R. Mucosal Immunol. 2008;1(suppl 1):S10–S14. doi: 10.1038/mi.2008.49. [DOI] [PubMed] [Google Scholar]
- 28.Ridge JP, Fuchs EJ, Matzinger P. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 29.Forsthuber T, Yip HC, Lehmann PV. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 30.Sarzotti M, Robbins DS, Hoffman PM. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 31.Crabbé PA, Nash DR, Bazin H, Eyssen H, Heremans JF. Lab Invest. 1970;22:448–457. [PubMed] [Google Scholar]
- 32.El Aidy S, Hooiveld G, Tremaroli V, Bäckhed F, Kleerebezem M. Gut Microbes. 2013;4:118–124. doi: 10.4161/gmic.23362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umesaki Y, Setoyama H, Matsumoto S, Okada Y. Immunology. 1993;79:32–37. [PMC free article] [PubMed] [Google Scholar]
- 34.Chung H, et al. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon HA, Bruckner-Kardoss E. Acta Anat (Basel) 1961;44:210–225. [Google Scholar]
- 36.Kawaguchi M, et al. Proc Natl Acad Sci USA. 1993;90:8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Probert CSJ, Saubermann LJ, Balk S, Blumberg RS. Immunol Rev. 2007;215:215–225. doi: 10.1111/j.1600-065X.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 38.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Gaboriau-Routhiau V, et al. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 40.Klaasen HL, et al. Infect Immun. 1993;61:303–306. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivanov II, et al. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lathrop SK, et al. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cebula A, et al. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atarashi K, et al. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sefik E, et al. Science. 2015;349:993–997. doi: 10.1126/science.aaa9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hapfelmeier S, et al. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macpherson AJ, Uhr T. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 48.Spits H, et al. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 49.Ganal SC, et al. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Sanos SL, et al. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satoh-Takayama N, et al. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Reynders A, et al. EMBO J. 2011;30:2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawa S, et al. Nat Immunol. 2011;12:320–326. doi: 10.1038/ni.2002. [DOI] [PubMed] [Google Scholar]
- 54.Peterson LW, Artis D. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 55.Cash HL, Whitham CV, Behrendt CL, Hooper LV. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Natividad JMM, et al. Appl Environ Microbiol. 2013;79:7745–7754. doi: 10.1128/AEM.02470-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Immunology. 2011;132:453–465. doi: 10.1111/j.1365-2567.2011.03410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He W, et al. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 59.McVay LD, et al. J Clin Invest. 2006;116:2914–2923. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsumoto S, Setoyama H, Umesaki Y. Gastroenterology. 1992;103:1777–1782. doi: 10.1016/0016-5085(92)91434-6. [DOI] [PubMed] [Google Scholar]
- 61.Fukuda S, et al. Nature. 2011;469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 62.Sommer F, Nookaew I, Sommer N, Fogelstrand P, Bäckhed F. Genome Biol. 2015;16:62. doi: 10.1186/s13059-015-0614-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dieren JM, et al. Inflamm Bowel Dis. 2007;13:1146–1152. doi: 10.1002/ibd.20164. [DOI] [PubMed] [Google Scholar]
- 64.Wingender G, et al. Gastroenterology. 2012;143:418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olszak T, et al. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.An D, et al. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Cell Host Microbe. 2013;14:559–570. doi: 10.1016/j.chom.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wingender G, et al. J Exp Med. 2011;208:1151–1162. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gollwitzer ES, et al. Nat Med. 2014;20:642–647. doi: 10.1038/nm.3568. [DOI] [PubMed] [Google Scholar]
- 70.Scharschmidt TC, et al. Immunity. 2015;43:1011–1021. doi: 10.1016/j.immuni.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanvit P, et al. Nat Commun. 2015;6:8424. doi: 10.1038/ncomms9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chassin C, et al. Cell Host Microbe. 2010;8:358–368. doi: 10.1016/j.chom.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Stockinger S, Hornef MW, Chassin C. Cell Mol Life Sci. 2011;68:3699–3712. doi: 10.1007/s00018-011-0831-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elahi S, et al. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Riedler J, et al. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 76.Schuijs MJ, et al. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 77.Schaub B, et al. J Allergy Clin Immunol. 2009;123:774–82.e5. doi: 10.1016/j.jaci.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 78.Dicksved J, et al. Appl Environ Microbiol. 2007;73:2284–2289. doi: 10.1128/AEM.02223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antonopoulos DA, et al. Infect Immun. 2009;77:2367–2375. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dethlefsen L, Huse S, Sogin ML, Relman DA. PLOS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dethlefsen L, Relman DA. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeissig S, Blumberg RS. Nat Immunol. 2014;15:307–310. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- 83.Risnes KR, Belanger K, Murk W, Bracken MB. Am J Epidemiol. 2011;173:310–318. doi: 10.1093/aje/kwq400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mai X-M, Kull I, Wickman M, Bergström A. Clin Exp Allergy. 2010;40:1230–1237. doi: 10.1111/j.1365-2222.2010.03532.x. [DOI] [PubMed] [Google Scholar]
- 85.Shaw SY, Blanchard JF, Bernstein CN. Am J Gastroenterol. 2010;105:2687–2692. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- 86.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Pediatrics. 2012;130:e794–e803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Int J Obes (Lond) 2014;38:1290–1298. doi: 10.1038/ijo.2014.119. [DOI] [PubMed] [Google Scholar]
- 88.Boursi B, Mamtani R, Haynes K, Yang Y-X. Eur J Endocrinol. 2015;172:639–648. doi: 10.1530/EJE-14-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kostic AD, et al. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Markle JGM, et al. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 91.Blustein J, et al. Int J Obes (Lond) 2013;37:900–906. doi: 10.1038/ijo.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Neu J, Rushing J. Clin Perinatol. 2011;38:321–331. doi: 10.1016/j.clp.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. Clin Exp Allergy. 2008;38:629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 94.Rajavelu P, Rayapudi M, Moffitt M, Mishra A, Mishra A. Am J Physiol Gastrointest Liver Physiol. 2012;302:G645–G654. doi: 10.1152/ajpgi.00223.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lexmond WS, et al. Am J Gastroenterol. 2014;109:646–657. doi: 10.1038/ajg.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andreas NJ, Kampmann B, Mehring Le-Doare K. Early Hum Dev. 2015;91:629–635. doi: 10.1016/j.earlhumdev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 97.Rogier EW, et al. Proc Natl Acad Sci USA. 2014;111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Pediatrics. 2005;115:1367–1377. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 99.Barclay AR, et al. J Pediatr. 2009;155:421–426. doi: 10.1016/j.jpeds.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 100.Gomez de Agüero M, et al. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]