Abstract
After the events of September 11, 2001, a decade of research on the development of medical countermeasures (MCMs) to treat victims of a radiological incident has yielded two FDA-approved agents to mitigate acute radiation syndrome. These licensed agents specifically target the mitigation of radiation-induced neutropenia and infection potential, while the ramifications of the exposure event in a public health emergency incident could include the entire body, causing additional acute and/or delayed organ/tissue injuries. Anecdotal data as well as recent findings from both radiation accident survivors and animal experiments implicate radiation-induced injury or dysfunction of the vascular endothelium leading to tissue and organ injuries. There are significant gaps in our understanding of the disease processes and progression, as well as the optimum approaches to develop medical countermeasures to mitigate radiation vascular injury. To address this issue, the Radiation and Nuclear Countermeasures Program of the National Institute of Allergy and Infectious Diseases (NIAID) organized a one-day workshop to examine the current state of the science in radiation-induced vascular injuries and organ dysfunction, the natural history of the pathophysiology and the product development maturity of potential medical countermeasures to treat these injuries. Meeting presentations were followed by a NIAID-led open discussion among academic investigators, industry researchers and government agency representatives. This article provides a summary of these presentations and subsequent discussion from the workshop.
INTRODUCTION
The current geopolitical environment has heightened fears of an imminent mass casualty event from the detonation of an improvised nuclear device (IND) or weaponized radiological material. Further, radiological accidents at Chernobyl (1986), Goiania (1987), Tokaimura (1997) and Fukushima-Daiichi (2011) underscore the pressing need for medical countermeasures (MCMs) to mitigate the complex injuries arising from unanticipated radiation exposure. To this end, the National Institute of Allergy and Infectious Diseases (NIAID) has been directed by the U.S. Department of Health and Human Services (HHS) to identify, characterize and develop appropriate MCMs to treat injured victims of a large-scale radiological/nuclear incident. NIAID implemented the Radiation and Nuclear Counter-measure Program (RNCP) in 2004 to accelerate research and product development of radiation MCMs, with the end goal of MCM licensure and purchase for the Strategic National Stockpile.
Licensure by the Food and Drug Administration (FDA) is feasible under the FDA’s Animal Rule, and relies on section 21 CFR, part 314, subpart I (“Approval of New Drugs when Human Efficacy Studies are not Ethical or Feasible”) and part 601, subpart H (“Approval of Biological Products when Human Efficacy Studies are not Ethical or Feasible”) (1). After a decade of intense effort, the FDA approved two MCMs for hematopoietic acute radiation syndrome (H-ARS); Neupogen® (granulocyte colony stimulating factor or G-CSF) was approved in March 2015 (2) followed by the approval of Neulasta® in November 2015 (3). However, several additional radiation-induced injury sequelae in individuals who survive H-ARS can result in multitissue/multiorgan dysfunction and failure, and late effects (4), for which there continues to be no specific treatment modalities. Delayed effects of acute radiation exposure (DEARE) are collectively characterized as a chronic condition manifested in multiple major organ systems of H-ARS survivors, including the gastrointestinal (GI) tract, bone marrow, lung, kidney, heart and brain. The vascular endothelium is an organ central to all tissues and may be important in acute radiation injuries and in DEAREs. Therefore, research focused on the mechanisms of these injury types and the development of MCMs to mitigate them is essential.
The RNCP conducted a one-day workshop on August 20, 2015 to address the current state of the research, MCM development and animal models used to assess and mitigate radiation injury to the vascular endothelium. Speakers included academicians and industry partners (Table 1). The objectives of this meeting were to: 1. To capture the current status of research in radiation-induced vascular injury; 2. Obtain scientific updates from researchers regarding MCM development; 3. Identify research gaps in this specific area; and 4. Provide a platform for an open, informal dialogue among the scientists with expertise in radiation-induced endothelial cell and vascular injuries, and representatives from U.S. government funding and regulatory agencies tasked with facilitating the development of MCMs for FDA licensure. Participating U.S. government panelists at the NIAID-led discussion included: RNCP program officers, the NIAID Office of Regulatory Affairs, the Biomedical Advanced Research and Development Authority (BARDA), the National Cancer Institute (NCI), the Department of Defense, and from the FDA, members of the Counterterrorism and Emergency Coordination Staff (CTECS), the Center for Drugs Evaluation and Research (CDER) and the Center for Biologics Evaluation and Research (CBER). Discussion topics centered on: 1. Identifying research gaps in the development of MCMs to treat vascular-endothelial injuries resulting from radiation exposure; 2. Understanding the complexity, progression, biomarkers and fate of the irradiated vasculature; and 3. Providing general guidance on NIAID requirements for investigators addressing radiation injury to the vascular endothelium, in the context of a radiation incident. This report summarizes the primary approaches discussed during the meeting.
TABLE 1.
Invited Presenters and Areas of Expertise
| Name | Affiliation | Expertise |
|---|---|---|
| Carney, Darrell | Chrysalis Biotherapeutics, Texas | Thrombin biology, wound healing |
| Chute, John | University of California, Los Angeles, California | Hematopoietic stem cell regeneration, vascular niche |
| Haimovitz-Friedman, Adriana |
Memorial Sloan Kettering Cancer Center, New York | Microvascular injury, apoptosis, signaling pathways |
| Hauer-Jensen, Martin | University of Arkansas for Medical Science, Arkansas | Thrombmodulin pathway, drug development |
| Hlatkey, Lynn | Tufts University School of Medicine, Massachusetts | Proton radiation, VEGF pathway, aging and radiation |
| Kennedy, Ann | University of Pennsylvania, Pennsylvania | Space irradiation, oxidative stress, radiation-induced coagulopathy |
| Kolesnick, Richard | Memorial Sloan Kettering Cancer Center, New York | Ceramide pathway, GI-ARS mitigation, platform development |
| Natarajan, Mohan | University of Texas Health Science Center, Texas | Cardiovascular injury, vascular homeostasis, eNOS pathway |
| Rafii, Shahin | Weill Cornell Medical College, New York | Stem cell differentiation, angiogenic factors, vascular niche |
| Sung, Anthony | Duke University, North Carolina | Nanotechnology, platelet biology |
| Zhou, Daohong | University of Arkansas for Medical Science, Arkansas | Senescence biology, targeting aging pathways, radiation ARS |
Background on Vasculature Biology
Radiation-induced damage to various organs/tissues, either in the context of a partial- or total-body irradiation (TBI), is accompanied by perturbations of the vascular endothelium. Four years after the discovery of X rays, Gassmann (5) published his findings on injury to the endothelium in irradiated skin. Historically, after irradiation, different tissues present with histological evidence of vascular/endothelial damage, lending credence to the pivotal role of vascular injury in tissue toxicity (6). Endothelial damage and subsequent progressive changes in the vasculature can contribute to chronic lesions in lung, liver, kidney (7, 8), heart and brain (9). Exposed populations that survive initial irradiation, whether during radiotherapy or a nuclear incident, are at risk of developing multiple organ dysfunction syndrome (MODS) associated with progression of vascular dysregulation. Therefore, the radiobiological response of the vascular network, which is still not completely understood, is of major importance for the medical management of radiation injury.
The endothelium is a monolayer of endothelial cells (ECs) lining the lumen of all blood vessels and is therefore present in every organ, with the exception of the lens and the cartilage. The endothelial surface area in an adult human is composed of approximately 1–6 × 1013 cells, weighs approximately 1 kg and covers a surface area of approximately 4,000–7,000 m2 (10). Initially regarded as an inert “tube” lining of the circulatory system, providing a conduit for transport of various substances within the bloodstream, the dynamic and tissue-specific nature of the vasculature endothelium continues to be of great interest, specifically in relationship to its role in the pathologies of radiation-induced injuries.
Juxtapositioned between the flowing blood and the vessel wall, the endothelium plays a key role in vasoregulation, homeostasis and in selectively controlling the traffic of choice hematopoietic cells and nutrients. Descriptions of heterogeneity of the EC phenotype, differences in function, interactions and cell communication are detailed in recent reviews (11, 12). As an active paracrine, endocrine and autocrine organ, the endothelium regulates vascular tone and blood flow, and maintains vascular homeostasis by releasing vasodilators such as nitric oxide (NO) and prostacyclin, as well as vasoconstrictors, including endothelin, thromboxane and platelet-activating factor (PAF). In addition to promoting vasodilatation, a healthy endothelium has antioxidant, anti-inflammatory, antiatherogenic, anticoagulant and fibrinolytic effects (13). Further, the EC surface consists of a layer of surface glycoprotein (glycocalyx) that provides not only a local charged barrier to the trans-endothelial migration of blood cells and plasma proteins under normal physiological conditions, but is also very metabolically active (14).
The Role of the Vasculature in Past Radiological Incidents
The vascular endothelium is intimately linked to both early and late radiation pathologies affecting several major organs. In the aftermath of the Hiroshima and Nagasaki atomic bombings, it is estimated that 60–100% of the casualties had evidence of hemorrhage at death (15). In survivors who developed late injury, the vascular tissue was involved in tissue damage, as is evident from studies on cerebrovascular disease (16), cardiovascular diseases (17, 18), chronic kidney disease (19) and gastrointestinal diseases (4). Of the 28 people who died within 98 days of the Chernobyl criticality incident, deaths were attributed to skin, gastrointestinal and lung reactions, but most deaths were characterized by circulatory problems with high incidence of edema and focal hemorrhages (20). After the Tokaimura incident, Akashi (21) discussed the possible role of inflammation and hemorrhage in multiple organ failure (MOF). In an elegant review of 110 case histories of radiation accidents spanning from 1945 through 2000, the authors analyzed MOF after TBI and stated, “It also became clear that the symptomatology of organ system involvement could be traced not only to the pathophysiology of the rapidly turning over cell renewal systems but—of equal or more importance—to the vascular system and specifically, to the endothelial components” (22). Although vascular injury is recognized as a key component in addressing radiation-mitigation strategies, the cellular, molecular and systemic mechanisms involved in the pathologies of MOF are still obscure, and therefore require tremendous efforts to elucidate the pathways and to develop MCM strategies for the vascular endothelium.
Pathophysiology of Radiation Injury to the Vascular Endothelium
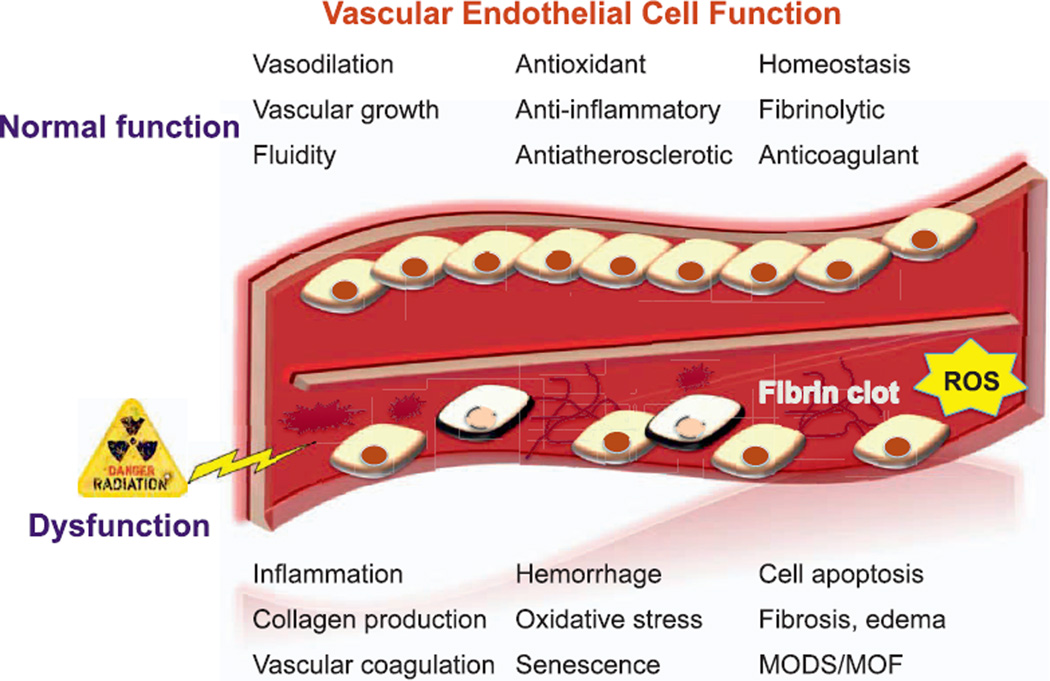
The microvasculature response to radiation can be classified according to acute, delayed and late effects, which contribute to the initiation, progression and maintenance of damage, both to the vasculature as well as the organs and tissues associated with it. The primary target of radiation injury to the vasculature is the endothelial cell. The acute phase of damage occurs within hours to weeks postirradiation, and is characterized by endothelial swelling, vascular permeability and edema, lymphocyte adhesion and infiltration and apoptosis (23). The denudation of ECs is followed by loss of barrier integrity and changes in permeability of the vessel. This phase is often accompanied by inflammation and migration of leukocytes and platelets as well as cellular debris with fibrin deposition, micro-thrombi formation and edema (Fig. 1). Later vascular effects occur weeks to months postirradiation and include capillary collapse, thickening of basement membranes, scarring and fibrosis, telangiectasia and a loss of clonogenic capacity (24).
FIG. 1.
Overview of radiation injuries to the vascular endothelial cells. ROS = reactive oxygen species; MODS = multiple organ dysfunction syndrome; MOF = multiple organ failure.
Pharmaceutical strategies to treat vascular injury often assume intervention in one or more of the pathways from onset of insult to fibrosis/major morbidities. Amid this complex multitude of cross-talk and interactions, key processes and pathways emerge as potential targets. Endothelial apoptosis is the heralding event in injury to the vasculature, accompanied by production of short and long-lived reactive oxygen species (ROS) and inflammation. The smaller vessels occlude due to swelling of the ECs, and activation of infiltrating granulocytes and platelets result in formation of microthrombi and fibrin deposition, all of which are accompanied by unique signatures of biochemical and/or signaling molecules (6). Although early endothelial and accompanying events precede late tissue damage such as fibrosis, the critical roles of key players and pathways involved in the late effects are as yet unclear.
Meeting Presentations Overview
The NIAID-sponsored, one-day workshop consisted of three sessions:
Understanding Radiation Injury to Endothelial Cells;
Identifying Pathways to Target Medical Countermeasures Development;
Identification of Medical Countermeasures Targeting Endothelial Injury.
This report summarizes the presented data, hypotheses and key discussion topics from the workshop. However, it is not intended to constitute a comprehensive review of radiation-induced vascular injury and all the possible mitigation strategies. Where prepublication data are mentioned below, the presenter’s name is provided in parentheses. Additional articles on the science presented at the meeting are included in this issue (Radiat Res, 186.2, 2016).
Session 1
In Session 1 of the workshop, speakers focused on describing: 1. Major pathways in radiation-induced endothelial injuries; 2. The role of vascular injury in delayed organ toxicities; and 3. Expression of cell surface markers as targets for MCMs.
Dr. Martin Hauer-Jensen described the role of radiation-induced injury to the ECs in gastrointestinal acute radiation syndrome (GI-ARS). GI-ARS, a sub-syndrome of radiation lethality, is attributed to the radiation sensitivity of the microvascular EC (23). The sensitivity of the enterocytic endothelium microvasculature is likely related to a stress-related surge of acid sphingomyelinase (ASMase) in ECs. Endothelial dysfunction, marked by a loss in thromboresistance and increased inflammatory markers, inhibits the recovery of the villus epithelium and leads to the breakdown of the epithelial barrier (25). These pathologies are mediated by thrombomodulin (TM), a transmembrane glycoprotein regulator of thrombin function, located on endothelial microvasculature cells. TM reduction appears to result from direct oxidative damage and a genetic downregulation of TM by inflammatory stimulus including, IL-1β, TNF-α and TGF-β (25–28). Reduction in TM levels causes over-activation of cellular thrombin receptors, and decreased activation of protein C, a plasma protein with anticoagulant, anti-inflammatory and cytoprotective functions. The presence of inflammatory proteases promotes ectodomain shedding and the ensuing breakdown of the vasculature (25, 29) (M. Hauer-Jensen).
Under normal conditions, TM removes thrombin from circulation by forming a binding complex; consequently, with thrombin unregulated, thrombin-dependent cellular receptors are hyperactivated. In addition, thrombin loses its ability to activate protein C (25, 30). Together these underlying mechanisms lead to a fibro-proliferative response and contribute to delayed intestinal dysfunction as well as the subsequent inactivation of endothelial cell membranes (25).
Altogether, TM and its related players in radiation enteropathy present a very attractive target for intervention strategies, such as administration of exogenous TM or activated protein C (APC), restoration of TM-protein C system or inhibition of downstream effectors of TM dysregulation. For instance, TM−/− mice demonstrated increased mortality after TBI. However, survival was significantly improved by administering gamma-tocotrienol (GT3), an unsaturated analogue of tocopherol, prior to irradiation. Tocols have antioxidant properties that may inhibit the enzyme hydroxyl-methyl-glutaryl-coenzyme A reductase (HMGCR). The inhibition of HMGCR leads to an upregulation of TM in vitro, protecting cells against radiation (31) (M. Hauer-Jensen). Furthermore, the GI tract can also be protected from radiation injury by endothelium survival factors such as vascular endothelial growth factor (VEGF), acidic and basic FGFs and interleukin 11(IL-11) (32–34).
Thrombomodulin and its modulation by irradiation are also involved in delayed response of intestinal radiation toxicity. Late vascular/endothelial tissue damage can occur several weeks to months postirradiation and fibrosis is considered an irreversible damage. These late effects are characterized by microvessel collapse, thickening of the basement membrane, and persistence of an activated, procoagulant endothelial phenotype, which is ultimately senescent and can result in fibrosis. Telangiectasia and sclerosis result from capillary dysfunction due to loss of endothelial and smooth muscle cells, and are accompanied by vessel wall thickening, and enlarged ECs. Fibrosis, a common feature in late tissue damage, has its origins in the primary damage to ECs. Endothelial cell death and denudation is followed by perivascular edema, changes in the vessel wall and fibrin deposition. A “Model of Mechanism of Self Perpetuation” for fibrosis in radiation-induced enteropathy has been proposed in which radiation injury to the ECs leads to increased levels of thrombin and inflammation mediated by TGF-β and TNF-α signaling (M. Hauer-Jensen).
Researchers at Tufts University School of Medicine have attempted to elucidate the differences in the mechanisms of damage to normal tissue, including the endothelium, in response to different qualities of radiation, protons and gamma rays. Radiation qualities elicit varying EC injury, vascular and remodeling responses. In fact, proton and gamma irradiation have produced contrasting effects with regards to angiogenesis (35). These studies present a very insightful overview of response, and a means to modulate vascular injury. Because there are considerable similarities between radiation-induced systemic response and the biological processes found in aging, such as angiogenesis and immunogenicity (L. Hlatky), the researchers identified a hub signaling molecule, TGF-β1, which is modulated with age and a prominent player in VEGF-mediated angiogenesis response.
Apart from the initial DNA damaging effect on the endothelium, radiation also promotes ROS, tissue and systemic senescence (36, 37). The accumulation of this damage leads to the dysfunction of stem cells and the onset of vascular endothelial cell senescence. The damage continues as senescent cells secrete inflammatory cytokines, proteases and other molecules that disrupt the normal tissue microenvironment (38, 39). Cell senescence has been associated with various delayed pathologies including atherosclerosis, osteoarthritis and chronic lung disease (40). In contrast, senescent cells have also been shown to secrete factors such as senescence-associated secretory phenotype (SASP) that mediate wound healing and tissue repair (40). While the roles of senescent cells are complex and conflicting, in vivo clearance of senescent cells has demonstrated a delay in aging-associated disorders (41). In fact, senolytic drugs were shown to significantly improve vascular endothelial function and improve cardiovascular function in mice (42). It is therefore possible that senolytic drugs (e.g., ABT263) represent a “fountain of youth” and can be effective MCMs in treating radiation injury (D. Zhou).
ABT263, an inhibitor of anti-apoptotic proteins BCL-2 and BCL-XL, is cytotoxic to senescent human fibroblasts, human renal epithelial cells and mouse embryo fibroblasts (D. Zhou). In addition, in vivo TBI mouse studies revealed that lung tissue expressed the greatest amount of senescent cells, followed by skeletal muscle and brain tissue, whereas liver and heart had a minimal increase in senescent cells. Senescent cells are the “new targets” for anti-aging therapy. Since radiation exposure may cause premature aging of the hematopoietic system, novel MCMs to prevent delayed effects via clearance of senescent cells are of significant interest.
In summary, this session noted differences in radiation injury due to radiation quality, identified signal-transduction specific, and highlighted systemic changes in the vasculature that involved inflammation, ROS and accelerated aging. These processes presented unique opportunities to disrupt the injury pathway using a variety of strategies.
Session 2
In Session 2 of the workshop, presenters discussed: 1. EC dysfunction and inflammation pathways after exposure with an emphasis on potential MCM targets against delayed cardiovascular disease; 2. The biology of high, single-dose radiation exposure on the microvasculature; and 3. Radiation responses of ECs that contribute to radiation-induced coagulopathies.
The primary lesion resulting from exposure to ionizing radiation is damage to the ECs, leading to cell death, apoptosis, faulty repair and/or dysfunction. Apoptosis or programmed cell death in ECs is mediated by the sphingomyelinase pathway (43, 44). This ubiquitous pathway links specific cell surface receptors to external stressors, such as radiation, to the nucleus. Sphingomyelin (SM) hydrolysis occurs rapidly after the initial insult via the action of sphingomyelin-specific forms of phospholipase C, termed sphingomyelinases, to generate ceramide. Ceramide then serves as a second messenger, leading to apoptotic DNA degradation (A. Haimovitz-Friedman). Bovine aortic endothelial cells are sensitive to radiation doses as low as 1– 2 Gy. The transfer of apoptotic signals from the irradiated cell membrane apparently employs the stress-activated protein kinase/Jun kinase cascade (44), which presents an attractive target for modulation of radiation injury. For instance, basic fibroblast growth factor (bFGF) reduced radiation-induced ASMase translocation and ceramide production, thus decreasing apoptosis. bFGF administered to mice, before and after whole-thorax irradiation, reduced endothelial apoptosis and lethality from radiation pneumonitis (45). Further, the investigators explored the role of VEGF in inhibiting ASMase activity, as well as an anti-ceramide antibody to prevent apoptosis (A. Haimovitz-Friedman). However, selection of appropriate model systems, radiation quality and schedule, dose, as well as timing of administration of anti-angiogenic therapies are crucial considerations to successful intervention strategies.
Work centering on the ASMase pathway has identified a causal link between apoptosis of ECs and radiation-induced GI death (46). The microvascular endothelium is the primary target for radiation injury to the GI tract with the secondary messenger, ceramide, central to injury (R. Kolesnick). The biology of this injury is ascribed to ceramide-driven, vascular dysfunction mediated by oxidative damage and alterations to the fidelity of the repair apparatus. Ceramide activity is facilitated by ceramide-rich platforms (CRPs), which are transient structures that form as lipid vesicles on the plasma membrane of cells in response to increase in ceramide (47). These CRPs afford a scaffold for protein polymerization. Targeting the ceramide pathway using sphingosine-1-phosphate (SIP), a metabolite of the ceramide pathway as well as a known inhibitor of ceramide-mediated apoptosis, has been shown to reduce cell death (43). A variety of other ceramide antagonists that are currently being investigated include anti-ceramide antibodies that inhibit apoptosis in cell culture, as well as murine gastrointestinal crypts, while mitigating GI-ARS lethality. Other modulators of ceramide, however, do not protect ASMase−/− mice, underscoring the participation of the ASMase pathway in radiation-induced vascular injury to the GI tract (R. Kolesnick).
Investigators from the University of Texas Health Science Center described the processes of vascular inflammation and potential target for radiation-mediated cardiovascular disease (M. Natarajan). Oxidative stress upregulates numerous pathways pertinent to vascular disease, including matrix metalloproteinases, adhesion molecules, pro-inflammatory cytokines and smooth muscle cell proliferation and apoptosis, while inactivating vasculo-protective nitric oxide (NO). Irradiated vascular cells suffer from persistent oxidative stress and inflammation subsequent to irradiation. Radiation negates EC migration and tube network formation (48), while simultaneously reducing the number of endothelial progenitor cells. Additionally, a fast interaction between NO and superoxide anions results in the formation of peroxynitrite (49). The accelerated degradation of NO and formation of peroxynitrite then reduces the availability of endothelium-derived NO by activation of the IKK/NF-κB pathway after irradiation, resulting in depression of several key aortic vessel functions. Radioprotectants that scavenge free radicals such as the aminothiols, and sulfhydryl containing ACE inhibitors such as captopril, attenuate radiation damage (50), while organic nitrates and sodium nitroprusside that can release NO in the body are severely limited due to their poor distribution and toxicity, and these prophylactics are impractical for use in the event of an unanticipated exposure. Enhancement of the endothelial nitric oxide synthase (eNOS)/NO pathway combined with sepiapterin and simultaneous inhibition of IKK-β represents a potential countermeasure approach for radiation-induced cardiovascular diseases (M. Natarajan).
Radiation-induced coagulopathy (RIC) manifests as a veno-occlusive disease in which the lumens of veins are obstructed, leading to hemorrhage, microthrombi formation and lethality due to changes in homeostasis. These events are particularly evident in irradiated lung (51), liver (52) and heart (53). Recently, it was hypothesized that RIC results in mortality from disseminated intravascular coagulopathy (DIC), especially in ferrets and pigs (54). The hallmark of DIC is an activated coagulation pathway, accompanied by simultaneous clotting and bleeding, with the presence of soluble fibrin in blood. Some researchers hypothesize that depletion of circulating end-cells of the myeloid, lymphoid and megakaryocytic lineages is not the primary cause of H-ARS mortality, which is instead due to the activation of the coagulation pathway and DIC (A. Kennedy). DIC is accompanied by coagulopathies, high levels of circulating nucleosomes/histones (cNH), which are highly toxic and associated with death, and the prevalence of hemorrhages. Treatment of radiation-induced coagulopathy requires a multipronged approach. Since ferrets exhibit highly significant reduction in clotting factors (55), treatment of ferrets with BeneFIX® (recombinant factor IX) can abolish the radiation-induced alteration in blood clotting times. This field is still in its infancy, and preliminary results suggest that clotting factors may serve as effective MCMs in radiation-induced DIC (A. Kennedy). Other interventions include the anticoagulants heparin, dicumarol and warfarin, which spare injuries to the lung, liver and heart, and steroids are also reported to decrease these injuries in the liver (56).
An increase in plasminogen activator inbibitor-1 (PAI-1) represses the activity of tissue plasminogen activator (tPA) and hence increases the deposition of fibrin in the injured organ (A. Kennedy). Further, ECs release von Willebrand factor (VWF), an acute phase protein that increases in systemic inflammation. VWF is produced constitutively in ECs, and is secreted as an ultra-large form of the molecule (ULVWF), which is rapidly cleaved into smaller pieces. Increased circulating levels of ULVWF increase pro-inflammatory states and are correlated with the degree of organ failure (A. Kennedy). There is evidence that treatment with dextran sulfate (a fibrinolytic stimulator) as well as actinomycin D and ACE inhibitors benefits the management of late tissue injury (56).
Session 3
In Session 3 of the meeting, presenters discussed approaches that show promise for the mitigation of radiation-induced injury to endothelial cells. These approaches included: 1. Targeting of pathways that are known to be activated by radiation exposure; 2. Cellular therapies to reconstitute the damaged bone marrow niche; and 3. Physical approaches to minimize leakiness of radiation-injured blood vessels.
As discussed previously (M. Hauer-Jensen), the TM molecule has an important role in mediating radiation injury to the vasculature. The thrombin molecule itself is a key initiator of tissue repair, activating both anti- and pro-inflammatory signaling. Radiation exposure leads to loss of EC-derived TM, which then limits repair. Accordingly, TP508, a single 23-amino acid natural thrombin breakdown product released after blood clot formation to initiate tissue repair, is being proposed as a novel therapeutic. TP508 has been shown to restore endothelial function through activation of eNOS (57), and to be safe and effective in human clinical trials (D. Carney). Licensure of this drug is being pursued and considered for other indications such cardiovascular repair (58–61), fracture healing (62) and diabetic foot ulcers (63). By increasing eNOS, TP508 minimizes inflammation and interferes with radiation-induced endothelial dysfunction, which leads to an overall significant increase in survival and mean survival time after lethal irradiation in mice (D. Carney). The drug has also has been shown to minimize radiation injury in the GI tract, brain and lungs, all of which are heavily vascularized.
Several laboratories are studying the role of the vascular niche and endothelial cells in radiation-induced injury and repair (S. Rafii). Because hematopoiesis occurs in the bone marrow niche (64), damage to this compartment can have a dramatic impact on the circulating immune cells. The niche involves a complex interplay of bone, immune cells, adipocytes and ECs, the latter of which are known to have a role in both angiogenesis as well as organ regeneration and repair. The vasculature that feeds different areas of the body is composed of ECs that have distinct geographical properties unique to the tissue that they perfuse, such as liver, bone marrow, lung and brain. Within specialized vascular niches such as the bone marrow, the vascular ECs interact with other cells via cell-to-cell signaling and provide additional signaling to other niche components. As a result, the blood vessels in each organ system can have differential radiation sensitivities (65). The vasculature can secrete specific factors at the sites of tissue injury that can influence tissue regeneration, as seen in the lung (66) and liver (67). This effect has also been noted in various tissues, including: pancreatic islet cells (68), adipose stem cells (69), bone (70, 71) and cardiac endothelium (72). Beginning in 2000, Rafii and other groups showed that it was possible to produce organ-specific endothelial cells (73–76). These endothelial cells expand hematopoietic stem and progenitor cells by deploying angiocrine growth factors (77) and restore hematopoietic recovery in myelosuppressed mice. Endothelial cells can also be generated from embryonic or induced pluripotent stem cells (iPS) (78). The problem with cells derived from these sources, however, is that they can still retain some of their fetal characteristics and can be unstable. To address this, mature amniotic cells have been reprogrammed into endothelial cells with success (79).
Radiation exposure can cause significant injury to the vasculature in the bone marrow (80). In previous studies, infusion of ECs into irradiated mice was reported to improve survival and lead to renewal of hematopoietic stem cells (81, 82). Mouse brain and fetal-derived ECs were both able to rescue the animals, however, mesenchymal cells were not (J. Chute). In addition, compounds that influence and support endothelial cells represent potential countermeasures to increase tissue survival after radiation injury. For example, pleiotrophin (PTN) administration yields a >25-fold increase in survival of hematopoietic stem cell (HSC)-supportive human ECs (83–85). Pleiotrophin is a 15 kD-secreted, heparin-binding growth factor that is produced by ECs as well as neurons and other cell types. The PTN molecule has been shown to have a role in angiogenesis and organ repair; additionally as an MCM it has demonstrated an increase in survival after radiation exposure in mice (86). Epidermal growth factor, a receptor expressed by hematopoietic stem cells, has also been shown to increase survival after radiation exposure and promote reconstitution of the bone marrow (87).
Other radiation mitigation approaches include targeting repair of the vascular leakiness that results from radiation injury (A. Sung). Breakdown of the EC wall can lead to bleeding in the tissues and edema. In an unirradiated, and otherwise uninjured vasculature, platelets roll along the vessels and interact with the ECs, leading to the release of soluble signaling molecules by both cell types. In turn, these molecules can modify the behavior of the cells. Radiation-induced platelet loss from the circulation leads to thrombocytopenia, and can play a major role in radiation-induced lethality (88). Fibrinogen-coated albumin nanospheres (FCN) have been shown to prevent bleeding from radiation-induced thrombocytopenia and vascular injury (A. Sung). These nanospheres interact with the endothelium to effectively seal up leaks in the vasculature. Even low levels of circulating platelets are able to bind to the FCN at the site of endothelial damage and create a plug. Mechanism-of-action studies, conducted in vitro with human umbilical vein endothelial cells (HUVECs), suggest that the FCNs bind only to activated ECs. In an irradiated mouse model, FCNs were shown to increase survival and reduce bleeding time, while having no effect on the level of circulating platelets (A. Sung). Studies were also undertaken using an anti-platelet antibody, (anti-CD42b) to achieve a more severe thrombocytopenia in the mouse model. In these experiments, FCNs also yielded a survival benefit when injected intravenously after irradiation (A. Sung).
Other MCMs (not discussed at the meeting), which have shown efficacy in mitigating injury to ECs and the vasculature include atorvastatin’s protection of HUVECs (89). This statin appears to exert its effect via upregulation of TM and APC levels. Another MCM that appears to have efficacy in reversing radiation-induced vascular changes is gamma-tocotrienol (GT3). While providing for hematopoietic progenitor cell mobilization as one of its mechanisms of action, the compound also induces VEGF, which is important for new vessel growth and endothelial progenitor mobilization (90). In summary, in addition to the approaches discussed during Session 3 of the meeting, anti-ceramide antibodies (R. Kolesnick), HMG-CoA reductase inhibitors, growth factors (bFGF, VEGF), exogenous APC or recombinant TM (rTM) administration, pharmacological upregulation of TM, the senolytic drug ABT263 (D. Zhou) and antioxidants represent interesting and novel approaches to the mitigation of vasculature radiation injury.
Biomarkers of Radiation Injury to the Vasculature
Radiation-induced damage can potentially be monitored in vivo by harnessing the signals produced by circulating ECs. Mature ECs are released into the circulation due to the normal turnover and renewal of the EC lining of the vasculature. These cells are heterogeneous in size (15–50 µm), carry the markers of ECs (e.g., VWF, CD144 and CD146), but not leukocyte markers (CD45), and serve as noninvasive markers of EC damage and dysfunction. Al-Massarani and colleagues demonstrated a reduction in circulating ECs after acute- and fractionated-dose gamma irradiation in rat peripheral blood, with an incomplete recovery 2 months postirradiaton (91), and in patients undergoing chemotherapy (92, 93). The presence of systemic signalers of processes, such as the oxidative stress pathway and inflammation, is attractive since there is vast potential for a noninvasive or minimally invasive source of biomarkers for early and delayed progression of injury.
Studies indicate that specific panels of growth factors, matrix metalloproteins and DNA damage markers are not only predictive of damage to a specific major organ, but in some cases are predictive of injury progression (lethality versus survival). For instance, IL-1, IL-6 and CXCL1 are markers of pulmonary damage (94), while TM downregulation after irradiation (M. Hauer-Jensen, D. Carney) in addition to inflammatory cytokines such as IL-1, TNF-α and TGF-β are noted in radiation enteropathy (25). Biomarkers may also function as predictors of radiation injury as well as the efficacy of the MCM in mitigating damage, ameliorating major morbidities and rescuing the organism from lethality. For instance, the presence of ULVWF (discussed earlier) is indicative of the degree of organ failure, while the presence of high levels of cNH is often associated with death (95). The presence of fibrin in blood is an indicator of delayed vascular injury. If fibrin decreases in the organ after antifibrin therapy, this indicates the treatment is efficacious. However, the kinetics of the biomarkers, as well as their multiphasic manifestation and intimate cross-talk with other signal transducers, pose a challenge.
Preclinical Models to Study Radiation Responses of the Vascular Endothelium
In vivo models
NIAID supports the development of appropriate animal models in several species, e.g., rodents (mice and rats), canines and non-human primates (NHPs) since the FDA Animal Rule licensure pathway requires that the MCM demonstrate proof of efficacy in two animal species that accurately model the expected human response (1). It is necessary that the mechanism of action of mitigation/therapy closely resembles the action of the MCM in humans. Similarly, selection of the primary end points must have relevance to the end user, and are usually mortality and major morbidities inherent to the vasculature. The selection of appropriate models must be dictated by the primary end point and the mechanism of action of the MCM. These models, as well as the pathophysiology of injury, should be clearly linked to the human experience.
The most studied model for evaluating radiation injury to the vasculature is the use of mouse strains such as C57BL/6, CD-1, CD2F1, C3H and BALB/c. These were used to evaluate both direct insult to the vasculature and late effect in major organs where the vascular/endothelial dysfunction mediates the delayed injury. NO-mediated EC dysfunction was studied in C57BL/6 wild-type and knockout strains (M. Natarajan). Others have studied the role of vascular dysfunction in different tissues after irradiation; C57BL/6 and CD-1 in gastrointestinal crypt protection and CNS injuries (M. Hauer-Jensen, R. Kolesnick, D. Carney). To study radiation-induced delayed cardiovascular diseases such as atherosclerosis in wild-type animals, Dr. Natarajan’s laboratory repurposed an in vivo model with interrupted blood flow in wild-type C57BL/6 mice for radiation response.
Knockout mice were another preferred model among the presenters and were mainly developed to understand the complexity of radiation response. The use of ASMase+/+ or ASMase−/− mice demonstrated the key role of sphingomyelin pathway and ceramide signaling in radiation-induced dysregulation of the vascular EC (R. Kolesnick). TM+/+ mice demonstrated higher survival than the TMPro/− (protein C deficient) strain, and B6.129P2-Nos (eNOS-deficient mice) were significantly more sensitive to radiation lethality than wild-type C57BL/6J mice (M. Hauer-Jensen). In addition to eNOS knockout mice, Dr. Natarajan’s group cross-bred mice to obtain vessel-specific eNOS-deficient mice (Tie2-eNOS−/−) and also constructed targeted deletion of IKK-β in the vascular endothelium using IKK-βflox/flox mice and Tie2-Cre transgene to elucidate the contribution of the IKK/NFκB pathway in progression of eNOS and radiation dysregulation in cardiovascular diseases. Since wild-type mice are rarely atherosclerotic, most rodent models of atherosclerosis are transgenic mice (96).
Knockout mice were also used to explain the mechanism of actions of PTN, which expands bone marrow hematopoietic stem cells (BM-HSC). While PTN+/+ mice survive lethal irradiation, PTN−/− mice with defective HSPC regeneration succumb to the insult (J. Chute). Mitigation of aortic endothelial function and maintenance of endothelial sprouting have also been demonstrated in irradiated CD-1 outbred mice after MCM administration (D. Carney).
Another group suggested that the mouse, with its LD50 far removed from the human LD50 and its distinctly different pathophysiology, is not the optimum model to study hemorrhage and coagulopathies that are hallmarks of radiation vascular injury (A. Kennedy). They noted that at the LD50, mouse mortality was predominantly due to infection, and this species did not exhibit hemorrhage, while fatalities of the atom-bomb attacks presented with widespread hemorrhage (97), as did patients from radiation accidents in Norway (98) and Brazil (99). Given these limitations in the rodent model, the investigators cited their work in ferrets and minipigs, and referenced publications by other researchers in dogs and cows, in which radiation response is more closely aligned with the human radiation experience.
To study radiation-induced cardiovascular disease, Dr. Natarajan’s laboratory developed an in vivo model with interrupted blood flow, since shear stress under normal flow conditions is considered atheroprotective. Since laminar flow shear that occurs in the long arteries is more atheroprotective, and the disturbed flow in the arterial bifurcation sites and curved arteries are more atherogenic, the group developed a technique to manipulate the arterial flow condition at a specific site and estimate the stimulatory effect of radiation on the development of vascular occlusions in a much shorter time in normal animals. In the GI-ARS model, others have utilized a two-tiered approach to spare hematopoietic deaths, which was comprised of TBI followed by a bone marrow cell transplant, or abdominal irradiation with a partial bone marrow shield (R. Kolesnick).
Few large mammals are currently being studied as models for radiation-induced vascular/endothelial injury. Researchers at the University of Pennsylvania have developed a ferret model to compare solar particle event ionizing radiation effects to gamma irradiation, with emesis as the primary consideration (100). This may represent a potential model for vascular injury, since the irradiated ferrets demonstrated hemorrhage and coagulation abnormalities, very similar to the human response at the LD50 dose. A study with the Yucatan minipig (101) indicated that minipigs respond very similarly to irradiated ferrets, while other large animal models, like irradiated canines and guinea pigs (54), provide some background information on hemorrhage for these species and underscore the need for robust research to develop appropriate models for MCM testing for vascular damage.
In vitro models
It is recognized that in vitro systems are highly attractive models for elucidating the radiation injury mechanism at the cellular level, to understand the contributions of the cell response to the tissue, as well as the signaling pathways. The investigators provided data generated from studies in HUVEC, bovine aortic ECs (BAEC), WI38 human diploid fibroblasts, renal epithelial cells and mouse embryonic cells. There were considerable differences in the culture conditions depending on the end points under study. For example, while most investigators used standard static culture conditions, one group described an in vitro system that simulated in vivo flow shear stress (M. Natarajan), while other laboratories used a serum- and growth factor-free culture condition (S. Rafii). At this preliminary stage, allowing the end point (apoptosis, signal pathways, transplantation assays) to drive culture conditions is recommended. Required guidance from regulatory bodies at this juncture can reduce the scope of research and limit the versatile approaches that can potentially uncover novel MCMs.
Irradiation Protocols to Enable Accurate Exposure and Interpretation of Results
As part of the current NIH-wide strategic plan objective to enhance scientific stewardship by ensuring rigor and reproducibility of scientific experiments, there is a renewed need for diligence in the development of irradiation protocols for the study of radiation effects in all organs and tissues (not just the vasculature). This effort requires greater details from the scientific community concerning radiation source, instruments, radiation field (size of the field, volume and uniformity), dose rate, source-to-surface distance, geometry of irradiation and animal orientation, details of most recent calibration and in-run dosimetry details. Animal details such as species, strain, sex, age, vendor source, health report and strategies for individual identification are also critical (102) This information helps to ensure that experiments performed in one laboratory can be repeated elsewhere, and that the data generated can be judged as accurate.
Apart from the volume of irradiated tissue/organ/organism, another consideration is the radiation quality used. Radiations of different quality are not merely the “insult”, but differ in modulating vascular response and can inform about the vastly different responses of radiation-induced vascular injury and ensuing unique response. The DNA damage that occurs at the molecular level in ECs is dependent not only on the radiation dose, but also on the radiation source (e.g. proton, gamma, etc.) (103, 104). Proton and gamma irradiation each result in unique changes in transcriptome regulation (105), gene methylation (106) and miRNA biogenesis (107, 108). Using protons and gamma sources, investigators at the Tufts University School of Medicine showed opposite angiogenic responses (L. Hlatky).
DISCUSSION
Panel Discussion Comments
During the meeting, oral presentations were followed by an open discussion among the speakers and a panel of other academicians, researchers and representatives from U.S. government funding and regulatory agencies. The discussions covered the global role of the vascular endothelium in radiation injury, potential targets for MCM intervention, biomarkers of radiation injury in the vasculature and the challenges of developing MCMs for these types of injuries.
Role of the ECs/Vasculature in Radiation Injury
The consensus among the panel was that radiation injury to the vascular endothelium is a complex, highly diverse and convoluted series of events. The panel emphasized that not all ECs are equal; there is significant heterogeneity found among ECs derived from different organs and tissue. Although ECs are present within all vessels, they display different structures and functions depending on the vessel types (arteries, veins, capillary and lymphatics). For example, ECs on the arteries and veins are continuous and robust compared to thinner capillary ECs. The response of ECs to radiation exposure also differs based on geographical location, proximity to organ–organ systems, and association with the vascular bed as well as the size of the vessel. The heterogeneity of ECs on arterioles, venules, microvasculature and capillaries is hypothesized to be due to intercellular differences, transcription factors, signals from major cells within the organs of immediacy and to interactions with the vessel bed. It is assumed that radiosensitive tissues have radiosensitive vasculature while the radioresistant organs (lung, kidney, brain) have radioresistant vasculature. Because the radiation sensitivity of ECs in specific organs is relatively unknown, a systematic approach is recommended to study this scientific gap. Since the normal, unstressed function of ECs in different organs is known to be different, it is not surprising that their radiation responses would also differ.
Differences in response are also dependent on the vascular bed associated with the endothelium, as well as the size, structure and function of the vessel. For example, the APC receptor presenting to TM-thrombin complex is found predominantly on the macrovasculature, but not in the microvasculature, a finding which could be attributed to different flow rates in vessels. The structure of the endothelium presents another challenge: while the micro-vasculature, with its single layer of endothelial cells and a few associated pericytes, is more sensitive to radiation damage, the microvasculature, with support structures such as smooth muscle, vascular bed and drainage, is more resistant. The ratio of pericytes to ECs may be another factor in radiation sensitivity within a specific organ. For example, the brain microvascular bed is known to have more pericytes, which could contribute to its radiosensitivity. Other aspects to consider are the inherent radiation sensitivity of cells surrounding/supporting the ECs, cycling rates of different ECs, oxygenation status of organs where ECs are located and how those ECs are grown in culture (e.g., use of serum in cell growth media) and the need for pure cellular populations for study. Therefore, the meeting participants supported the idea that very basic, mechanistic studies were still warranted in this area.
Early and Delayed Expression of Biomarkers of Vascular/Endothelial Injury
Although the pathobiology of vascular injury is not completely understood, an abundance of data from in vitro, in situ and in vivo studies suggest biomarkers that describe specific aspects of the damage, which can lead to a better understanding of the late effects of radiation. The biomarkers can be signal transducers (e.g., cytokines, chemokines, exosomes), cells and cellular products as well as signaling pathways (e.g., apoptosis, oxidative stress, inflammation, TM and senolytic). In addition, given that ECs appear to retain a memory of previous exposure, it is likely that there are also epigenetic signals involved. The pathophysiology of aging is thought to be extremely similar to radiation-induced late effects, and monitoring aging-related markers is one potential approach to quantify radiation-induced vascular injury. The panel also expressed concern about sectioning the injury into early and late effects; in particular, distinctions are needed between the early appearance of biomarkers and biokinetics in relationship to the time of exposure. Tissue damage to the vasculature is an evolving process that can take from seconds to years to manifest in humans. Thus, some of the processes can take more time than others to reach a level of detection.
Pathway to FDA Licensure
Ultimately, NIAID’s mission is to facilitate development and licensure of MCMs to ameliorate radiation mortality and reduce the consequences of vascular injury after irradiation. Although, there is an immediate need for very basic research in this field, it is still critical for investigators and organizations to meet with the FDA to consult on all aspects of MCM development. The licensing pathways under section 21 CFR Parts 314 and 601 of the FDA’s Animal Rule provide excellent guidance for forecasting appropriate animal models (1). In addition to the Animal Rule and selection of an appropriate animal model, considerable attention must be paid to the exposure protocols, the pathobiology of the MCM and its mechanism of action with bridging studies linking animal data to the human response (109), for successful pathways to licensure. The most optimal regulatory strategy will require continued, repeated and consistent discussions with the funding agency and the FDA to obtain feedback, affect midcourse corrections and achieve approval of MCMs for radiation-induced vascular dysfunction.
Identified Research Gaps and Meeting Conclusions
Radiation-induced vascular injury has been a recognized complication of radiation exposure since the discovery of radiation. However, research on the role of the vasculature in radiation injury and the development of MCMs specific to vascular/endothelial damage is inadequate. The topics discussed during this meeting demonstrate the need for early-research-stage investigations of radiation-induced injury to the vasculature. It is clear that research in this area is too premature to consider standardization of in vitro and in vivo models for studying the phenomenon. In addition, the role of TM in the thrombohemorrhagic imbalance after radiation injury requires additional study, as does distinguishing between responses in lymphatic ECs versus other vessel ECs. Tools continue to become available, including transgenic mouse models, which will simplify the kinds of studies that need to be performed. In addition, a better understanding of the cross-talk between ECs and other niche cells is required. More studies are necessary to understand the pathology of vascular damage, modulation of signaling pathways and the development of MCMs to treat the injury.
Acknowledgments
The authors are grateful to the participants for contributions to the workshop, especially to the speakers for their active engagement on topics of major interest in this research area and those who assisted with the preparation of this manuscript. The authors also thank NIAID colleagues David Cassatt, Carmen Rios and Bert Maidment for their careful review of the manuscript.
REFERENCES
- 1.Food and Drug Administration, HHS. New drug and biological drug products: evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Final rule. Fed Regist. 2002;67:37988–37998. [PubMed] [Google Scholar]
- 2.FDA approves radiation medical countermeasure. Silver Spring, MD: U.S. Food and Drug Administration; 2015. ( http://1.usa.gov/1Tpsbov) [Google Scholar]
- 3.Highlights of prescribing information for Neulasta. Silver Spring, MD: U.S. Food and Drug Administration; 2015. ( http://1.usa.gov/22Aj474) [Google Scholar]
- 4.Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009;29:A43–A59. doi: 10.1088/0952-4746/29/2A/S04. [DOI] [PubMed] [Google Scholar]
- 5.Gassmann A. Zur histologie der rontgenulcera. Fortschr a d Geb d Röntgenstrahlen. 1899;2:199. [Google Scholar]
- 6.Rubin DB, Griem ML. The histopathology of the irradiated endothelium. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton, FL: CRC Press LLC; 1998. pp. 13–38. [Google Scholar]
- 7.Jaenke RS, Robbins ME, Bywaters T, Whitehouse E, Rezvani M, Hopewell JW. Capillary endothelium. Target site of renal radiation injury. Lab Invest. 1993;68:396–405. [PubMed] [Google Scholar]
- 8.Juncos LI, Cornejo JC, Gomes J, Baigorria S, Juncos LA. Abnormal endothelium-dependent responses in early radiation nephropathy. Hypertension. 1997;30:672–676. doi: 10.1161/01.hyp.30.3.672. [DOI] [PubMed] [Google Scholar]
- 9.Lyubimova N, Hopewell JW. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br J Radiol. 2004;77:488–492. doi: 10.1259/bjr/15169876. [DOI] [PubMed] [Google Scholar]
- 10.Sumpio BE, Riley JT, Dardik A. Cells in focus: endothelial cell. Int J Biochem Cell B. 2002;34:1508–1512. doi: 10.1016/s1357-2725(02)00075-4. [DOI] [PubMed] [Google Scholar]
- 11.Sandoo A, van Zanten JJCSV, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Cardiovasc Med J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugsley MK, Tabrizchi R. The vascular system: An overview of structure and function. J Pharmacol Toxicol Method. 2000;44:333–340. doi: 10.1016/s1056-8719(00)00125-8. [DOI] [PubMed] [Google Scholar]
- 13.Pate M, Damarla V, Chi DS, Negi S, Krishnaswamy G. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem. 2010;52:109–130. [PubMed] [Google Scholar]
- 14.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69:777–784. doi: 10.1111/anae.12661. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy AR. Biological effects of space radiation and development of effective countermeasures. Adv Space Res. 2014;1:10–43. doi: 10.1016/j.lssr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson KG, Yano K, Kato H. Cerebral vascular disease in Hiroshima, Japan. J Chron Dis. 1967;20:545–559. doi: 10.1016/0021-9681(67)90085-9. [DOI] [PubMed] [Google Scholar]
- 17.Wong FL, Yamada M, Sasaki H, Kodama K, Akiba S, Shimaoka K, et al. Noncancer disease incidence in the atomic bomb survivors: 1958–1986. Radiat Res. 1993;135:418–430. [PubMed] [Google Scholar]
- 18.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, et al. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sera N, Hida A, Imaizumi M, Nakashima E, Akahoshi M. The association between chronic kidney disease and cardiovascular disease risk factors in atomic bomb survivors. Radiat Res. 2012;179:46–52. doi: 10.1667/RR2863.1. [DOI] [PubMed] [Google Scholar]
- 20.United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR Report to the General Assembly. Annex G: Early effects in man of high doses of radiation. 1988. pp. 545–565. [Google Scholar]
- 21.Akashi M. Role of infection and bleeding in multiple organ involvement and failure. Br J Radiol. 2005;27:69–74. [Google Scholar]
- 22.Fliedner TM, Dörr HD, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. Br J Radiol. 2005;27:1–8. [Google Scholar]
- 23.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 24.Milliat F, Sabourin JC, Tarlet G, Holler V, Deutsch E, Buard V, et al. Essential role of plasminogen activator inhibitor type-1 in radiation enteropathy. Am J Pathol. 2008;172:691–701. doi: 10.2353/ajpath.2008.070930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol. 2007;13:3047–3055. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter KK, Fink LM, Hughes BM, Shmaysani HM, Sung CC, Hauer-Jensen M. Differential effect of radiation on endothelial cell function in rectal cancer and normal rectum. Am J Surg. 1998;176:642–647. doi: 10.1016/s0002-9610(98)00280-3. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2072. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiler-Guettler H, Christie PD, Beeler DL, Healy AM, Hancock WW, Rayburn H, et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J Clin Invest. 1998;101:1983–1991. doi: 10.1172/JCI2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boehme MW, Deng Y, Raeth U, Bierhaus A, Ziegler R, Stremmel W, et al. Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: in vivo and in vitro studies. Immunology. 1996;87:134–140. [PMC free article] [PubMed] [Google Scholar]
- 30.Grinnell BW, Joyce D. Recombinant human activated protein C: a system modulator of vascular function for treatment of severe sepsis. Crit Care Med. 2001;29:S53–S61. doi: 10.1097/00003246-200107001-00020. [DOI] [PubMed] [Google Scholar]
- 31.Pathak R, Shao L, Ghosh SP, Zhou D, Boerma M, Weiler H, et al. Thrombomodulin contributes to gamma tocotrienol-mediated lethality protection and hematopoietic cell recovery in irradiated mice. PloS One. 2015;10:e0122511. doi: 10.1371/journal.pone.0122511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol. 1999;276:G249–G258. doi: 10.1152/ajpgi.1999.276.1.G249. [DOI] [PubMed] [Google Scholar]
- 33.Khan WB, Shui C, Ning S, Knox SJ. Enhancement of murine intestinal stem cell survival after irradiation by keratinocyte growth factor. Radiat Res. 1997;148:248–253. [PubMed] [Google Scholar]
- 34.Okunieff P, Mester M, Wang J, Maddox T, Gong X, Tang D, et al. In vivo radioprotective effects of angiogenic growth factors on the small bowel of C3H mice. Radiat Res. 1998;150:204–211. [PubMed] [Google Scholar]
- 35.Girdhani S, Sachs R, Hlatky L. Biological effects of proton radiation: what we know and don’t know. Radiat Res. 2013;179:257–272. doi: 10.1667/RR2839.1. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Yan X, Gao R, Mao L, Cotrim AP, Zheng C, et al. Effect of irradiation on microvascular endothelial cells of parotid glands in the miniature pig. Int J Radiat Oncol. 2010;78:897–903. doi: 10.1016/j.ijrobp.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panganiban RA, Day RM. Inhibition of IGF-1R prevents ionizing radiation-induced primary endothelial cell senescence. PloS One. 2013;8:e78589. doi: 10.1371/journal.pone.0078589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee M-O, Song S-H, Jung S, Hur S, Asahara T, Kim H, et al. Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscl Thromb Vas. 2012;32:343–352. doi: 10.1161/ATVBAHA.111.237651. [DOI] [PubMed] [Google Scholar]
- 39.Laberge RM, Adler D, DeMaria M, Mechtouf N, Teachenor R, Cardin GB, et al. Mitochondrial DNA damage induces apoptosis in senescent cells. Cell Death Dis. 2013;4:e727. doi: 10.1038/cddis.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolesnick R, Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- 44.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Differential inhibition of radiation-induced apoptosis. Stem Cells. 1997;15:43–47. doi: 10.1002/stem.5530150708. [DOI] [PubMed] [Google Scholar]
- 45.Fuks Z, Persaud RS, Alfieri A, McLoughlin M, Ehleiter D, Schwartz JL, et al. Basic fibroblast growth factor protects endothelial cells against radiation-induced programmed cell death in vitro and in vivo. Cancer Res. 1994;54:2582–2590. [PubMed] [Google Scholar]
- 46.Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 47.Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng X, Mohan S, Otto RA, Natarajan M. Endothelial cell migration was impaired by irradiation-induced inhibition of SHP-2 in radiotherapy: an in vitro study. J Radiat Res. 2011;52:320–328. doi: 10.1269/jrr.10071. [DOI] [PubMed] [Google Scholar]
- 49.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 50.Ward WF, Molteni A, Ts’ao CH, Kim YT, Hinz JM. Radiation pneumotoxicity in rats: modification by inhibitors of angiotensin converting enzyme. Int J Radiat Oncol. 1992;22:623–625. doi: 10.1016/0360-3016(92)90890-t. [DOI] [PubMed] [Google Scholar]
- 51.Maisin JR. The ultrastructure of the lung of mice exposed to a supra-lethal dose of ionizing radiation on the thorax. Radiat Res. 1970;44:545–564. [PubMed] [Google Scholar]
- 52.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation Hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 53.Fajardo LF, Berthrong M. Vascular lesions following radiation. Pathol Annu. 1988;23(Pt 1):297–330. [PubMed] [Google Scholar]
- 54.Krigsfeld GS, Kennedy AR. Is disseminated intravascular coagulation the major cause of mortality from radiation at relatively low whole body doses? Radiat Res. 2013;180:231–234. doi: 10.1667/RR3321.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krigsfeld GS, Sanzari JK, Kennedy AR. The effects of proton radiation on the prothrombin and partial thromboplastin times of irradiated ferrets. Int J Radiat Biol. 2012;88:327–334. doi: 10.3109/09553002.2012.652727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ward WF, Molteni A, Ts’ao Ch. Endothelial-oriented strategies to spare normal tissues. In: Rubin DB, editor. The radiation biology of the vascular endothelium. Boca Raton, FL: CRC Press LLC; 1998. pp. 185–208. [Google Scholar]
- 57.Olszewska-Pazdrak B, Hart-Vantassell A, Carney DH. Thrombin peptide TP508 stimulates rapid nitric oxide production in human endothelial cells. J Vasc Res. 2010;47:203–213. doi: 10.1159/000255963. [DOI] [PubMed] [Google Scholar]
- 58.Fossum TW, Olszewska-Pazdrak B, Mertens MM, Makarski LA, Miller MW, Hein TW, et al. TP508 (Chrysalin) reverses endothelial dysfunction and increases perfusion and myocardial function in hearts with chronic ischemia. J Cardiovasc Pharmacol Ther. 2008;13:214–225. doi: 10.1177/1074248408321468. [DOI] [PubMed] [Google Scholar]
- 59.Osipov RM, Robich MP, Feng J, Clements RT, Liu Y, Glazer HP, et al. Effect of thrombin fragment (TP508) on myocardial ischemia-reperfusion injury in hypercholesterolemic pigs. J Appl Physiol (1985) 2009;106:1993–2001. doi: 10.1152/japplphysiol.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu LM, Osipov RM, Robich MP, Feng J, Sheller MR, Sellke FW. Effect of thrombin fragment (TP508) on myocardial ischemia reperfusion injury in a model of type 1 diabetes mellitus. Circulation. 2010;122:S162–s169. doi: 10.1161/CIRCULATIONAHA.109.928374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Osipov RM, Bianchi C, Clements RT, Feng J, Liu Y, Xu SH, et al. Thrombin fragment (TP508) decreases myocardial infarction and apoptosis after ischemia reperfusion injury. Ann Thorac Surg. 2009;87:786–793. doi: 10.1016/j.athoracsur.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 62.Ryaby JT, Sheller MR, Levine BP, Bramlet DG, Ladd AL, Carney DH. Thrombin peptide TP508 stimulates cellular events leading to angiogenesis, revascularization, and repair of dermal and musculoskeletal tissues. J Bone Joint Surg Am. 2006;88:132–139. doi: 10.2106/JBJS.F.00892. [DOI] [PubMed] [Google Scholar]
- 63.Fife C, Mader JT, Stone J, Brill L, Satterfield K, Norfleet A, et al. Thrombin peptide Chrysalin stimulates healing of diabetic foot ulcers in a placebo-controlled phase I/II study. Wound Repair Regen. 2007;15:23–34. doi: 10.1111/j.1524-475X.2006.00181.x. [DOI] [PubMed] [Google Scholar]
- 64.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26:204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding BS, Cao Z, Lis R, Nolan DJ, Guo P, Simons M, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kao DI, Lacko LA, Ding BS, Huang C, Phung K, Gu G, et al. Endothelial cells control pancreatic cell fate at defined stages through EGFL7 signaling. Stem Cell Reports. 2015;4:181–189. doi: 10.1016/j.stemcr.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. 2011;123:2254–2262. doi: 10.1161/CIRCULATIONAHA.110.991125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rafii S, Dias S, Meeus S, Hattori K, Ramachandran R, Feuerback F, et al. Infection of endothelium with E1(−)E4(+), but not E1(−)E4(−), adenovirus gene transfer vectors enhances leukocyte adhesion and migration by modulation of ICAM-1, VCAM-1, CD34, and chemokine expression. Circ Res. 2001;88:903–910. doi: 10.1161/hh0901.089884. [DOI] [PubMed] [Google Scholar]
- 74.Zhang F, Cheng J, Hackett NR, Lam G, Shido K, Pergolizzi R, et al. Adenovirus E4 gene promotes selective endothelial cell survival and angiogenesis via activation of the vascular endothelial-cadherin/Akt signaling pathway. J Biol Chem. 2004;279:11760–11766. doi: 10.1074/jbc.M312221200. [DOI] [PubMed] [Google Scholar]
- 75.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Butler JM, Gars EJ, James DJ, Nolan DJ, Scandura JM, Rafii S. Development of a vascular niche platform for expansion of repopulating human cord blood stem and progenitor cells. Blood. 2012;120:1344–1347. doi: 10.1182/blood-2011-12-398115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–166. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chute JP, Saini AA, Chute DJ, Wells MR, Clark WB, Harlan DM, et al. Ex vivo culture with human brain endothelial cells increases the SCID-repopulating capacity of adult human bone marrow. Blood. 2002;100:4433–4439. doi: 10.1182/blood-2002-04-1238. [DOI] [PubMed] [Google Scholar]
- 84.Chute JP, Muramoto GG, Fung J, Oxford C. Soluble factors elaborated by human brain endothelial cells induce the concomitant expansion of purified human BM CD34+CD38− cells and SCID-repopulating cells. Blood. 2005;105:576–583. doi: 10.1182/blood-2004-04-1467. [DOI] [PubMed] [Google Scholar]
- 85.Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med. 2010;16:475–482. doi: 10.1038/nm.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Himburg HA, Yan X, Doan PL, Quarmyne M, Micewicz E, McBride W, et al. Pleiotrophin mediates hematopoietic regeneration via activation of RAS. J Clin Invest. 2014;124:4753–4758. doi: 10.1172/JCI76838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doan PL, Himburg HA, Helms K, Russell JL, Fixsen E, Quarmyne M, et al. Epidermal growth factor regulates hematopoietic regeneration after radiation injury. Nat Med. 2013;19:295–304. doi: 10.1038/nm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DiCarlo A, Kaminski JM, Hatchett RJ, Maidment BW. Role of thrombocytopenia in radiation-induced mortality and review of therapeutic approaches targeting platelet regeneration after radiation exposure. J Radiat Oncol. 2016;5:19–32. [Google Scholar]
- 89.Ran XZ, Ran X, Zong ZW, Liu DQ, Xiang GM, Su YP, et al. Protective effect of atorvastatin on radiation-induced vascular endothelial cell injury in vitro. J Radiat Res. 2010;51:527–533. doi: 10.1269/jrr.09119. [DOI] [PubMed] [Google Scholar]
- 90.Ray S, Kulkarni SS, Chakraborty K, Pessu R, Hauer-Jensen M, Kumar KS, et al. Mobilization of progenitor cells into peripheral blood by gamma-tocotrienol: a promising radiation countermeasure. Int Immunopharmacol. 2013;15:557–564. doi: 10.1016/j.intimp.2012.12.034. [DOI] [PubMed] [Google Scholar]
- 91.Al-Massarani G, Almohamad K. Evaluation of circulating endothelial cells in the rat after acute and gractionated whole-body gamma irradiation. Nukleonika. 2014;59:145–151. [Google Scholar]
- 92.Najjar F, Alammar M, Bachour M, Al-Massarani G. Circulating endothelial cells as a biomarker in non-small cell lung cancer patients: correlation with clinical outcome. Int J Biol Markers. 2014;29:e337–e344. doi: 10.5301/jbm.5000100. [DOI] [PubMed] [Google Scholar]
- 93.Najjar F, Alammar M, Bachour M, Almalla N, Altahan M, Alali A, et al. Predictive and prognostic value of circulating endothelial cells in non-small cell lung cancer patients treated with standard chemotherapy. J Cancer Res Clin Oncol. 2015;141:119–125. doi: 10.1007/s00432-014-1778-0. [DOI] [PubMed] [Google Scholar]
- 94.Johnston CJ, Hernady E, Reed C, Thurston SW, Finkelstein JN, Williams JP. Early alterations in cytokine expression in adult compared to developing lung in mice after radiation exposure. Radiat Res. 2010;173:522–535. doi: 10.1667/RR1882.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liebow AA, Warren S, De CE. Pathology of atomic bomb casualties. Am J Pathol. 1949;25:853–1027. [PMC free article] [PubMed] [Google Scholar]
- 98.Reitan JB. [Radiation accidents and radiation disasters] Tidsskr Nor Laegeforen. 1993;113:1583–1588. [PubMed] [Google Scholar]
- 99.The radiological accident in Goiania. IAEA Report No. STI/PUB/815. Vienna: International Atomic Energy Agency; 1988. ( http://bit.ly/1X0YD38) [Google Scholar]
- 100.Krigsfeld GS, Savage AR, Billings PC, Lin L, Kennedy AR. Evidence for radiation-induced disseminated intravascular coagulation as a major cause of radiation-induced death in ferrets. Int J Radiat Oncol. 2014;88:940–946. doi: 10.1016/j.ijrobp.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krigsfeld GS, Shah JB, Sanzari JK, Lin L, Kennedy AR. Evidence of disseminated intravascular coagulation in a porcine model following radiation exposure. Adv Space Res. 2014;3:1–9. doi: 10.1016/j.lssr.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.NIH-wide strategic plan. Fiscal years 2016–2020. Bethesda, MD: National Institutes of Health; 2016. ( http://1.usa.gov/1X0YOvb) [Google Scholar]
- 103.Leloup C, Garty G, Assaf G, Cristovao A, Breskin A, Chechik R, et al. Evaluation of lesion clustering in irradiated plasmid DNA. Int J Radiat Biol. 2005;81:41–54. doi: 10.1080/09553000400017895. [DOI] [PubMed] [Google Scholar]
- 104.Hada M, Sutherland BM. Spectrum of complex DNA damages depends on the incident radiation. Radiat Res. 2006;165:223–230. doi: 10.1667/rr3498.1. [DOI] [PubMed] [Google Scholar]
- 105.Finnberg N, Wambi C, Ware JH, Kennedy AR, El-Deiry WS. Gamma-radiation (GR) triggers a unique gene expression profile associated with cell death compared to proton radiation (PR) in mice in vivo. Cancer Biol Ther. 2008;7:2023–2033. doi: 10.4161/cbt.7.12.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goetz W, Morgan MN, Baulch JE. The effect of radiation quality on genomic DNA methylation profiles in irradiated human cell lines. Radiat Res. 2011;175:575–587. doi: 10.1667/RR2390.1. [DOI] [PubMed] [Google Scholar]
- 107.Kraemer A, Anastasov N, Angermeier M, Winkler K, Atkinson MJ, Moertl S. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat Res. 2011;176:575–586. doi: 10.1667/rr2638.1. [DOI] [PubMed] [Google Scholar]
- 108.Marta GN, Garicochea B, Carvalho AL, Real JM, Kowalski LP. MicroRNAs, cancer and ionizing radiation: Where are we? Rev Assoc Med Bras. 2015;61:275–281. doi: 10.1590/1806-9282.61.03.275. [DOI] [PubMed] [Google Scholar]
- 109.DiCarlo AL, Poncz M, Cassatt DR, Shah JR, Czarniecki CW, Maidment BW. Medical countermeasures for platelet regeneration after radiation exposure. Report of a workshop and guided discussion sponsored by the National Institute of Allergy and Infectious Diseases, Bethesda, MD, March 22–23, 2010. Radiat Res. 2011;176:e0001–e0015. doi: 10.1667/rrol01.1. [DOI] [PMC free article] [PubMed] [Google Scholar]