Abstract
We present a hydrogel-based affinity microsensor for continuous glucose measurements. The microsensor is based on microelectromechanical systems (MEMS) technology, and incorporates a synthetic hydrogel that is attached to the device surface via in situ polymerization. Glucose molecules that diffuses into and out of the device binds reversibly with boronic acid groups in the hydrogel via affinity binding, and causes changes in the dielectric properties of the hydrogel, which can be measured using a MEMS capacitive transducer to determine the glucose concentration. The use of the in situ polymerized hydrogel eliminates mechanical moving parts found in other types of affinity microsensors, as well as mechanical barriers such as semipermeable membranes that are otherwise required to hold the glucose-sensitive material. This facilitates the miniaturization and robust operation of the microsensor, and can potentially improve the tolerance of the device, when implanted subcutaneously, to biofouling. Experimental results demonstrate that in a glucose concentration range of 0–500 mg/dL and with a resolution of 0.35 mg/dL or better, the microsensor exhibits a repeatable and reversible response, and can potentially be useful for continuous glucose monitoring in diabetes care.
Keywords: affinity sensor, continuous glucose monitoring, hydrogel
1. Introduction
Continuous glucose monitoring (CGM), which involves highly frequent and repetitive measurements of glucose, can detect abnormal glucose concentrations in diabetes patients in a timely manner. Existing CGM devices often rely on electrochemical detection of enzymatic reactions1–3. While commonly used for glucose sensing, these devices are typically hindered by large drift and insufficient accuracy because of the irreversible, consumptive nature of electrochemical reactions. Affinity sensors, which are based on non-reactive equilibrium binding of glucose with a specific receptor4, 5, can potentially overcome these limitations. Affinity glucose sensing can be implemented in microsensors, which have used measurements of affinity binding-induced changes in physical properties such as volume6, 7, viscosity8, 9, fluorescence10, 11 and electric conductivity12. However, these efforts have required the use of a semi-permeable membrane as a physical barrier or mechanically movable structures, which can increase the complexity and limit the reliability of the devices. In contrast, affinity dielectric sensors that detect the glucose-dependent dielectric properties can effectively address these limitations.
Affinity sensors that are based on dielectric measurements have been used in applications such as detecting or quantifying biochemical targets under excitations at various frequencies. Example of these applications include determination of protein concentration13, 14, detection of DNA15, 16, and monitoring of bacteria17, 18. Affinity glucose microsensors utilizing dielectric measurements have however not been widely explored. We have previously reported measurements of the permittivity of a polymer solution as the polymer binds to glucose microsensors19–21. While demonstrating the potential in sensitive and selective detection of the glucose through dielectric measurements both in vitro and in vivo, the polymer solution required sealing using a semi-permeable membrane that significantly increased the complexity, limited the level of miniaturization, and affected the reliability of the microsensor.
This paper presents an affinity microsensor that measures glucose concentrations via the dielectric response of a hydrogel embedded in a capacitive transducer. The microsensor is fabricated using microelectromechanical systems (MEMS) technology, and the hydrogel is synthetically prepared, non-toxic and polymerized in situ in the device. Reversible affinity binding of glucose with boronic acid groups in the hydrogel changes the dielectric properties of the hydrogel, which can be measured using a MEMS capacitive transducer to determine the glucose concentration. The design of the microsensor eliminates the use of mechanical moving parts found in other types of affinity microsensors that are not amenable to miniaturization7, 22. The hydrogel is directly immobilized onto the surface of the transducer and will be stable over time, allowing the device to eliminate the use of a semipermeable membrane that are otherwise required to hold the glucose-sensitive material20, and potentially offer improved tolerance to biofouling during implanted operation. Experimental results demonstrate that in a glucose concentration range of 0–500 mg/dL and with a resolution of 0.35 mg/dL or better, the hydrogel-based microsensor is capable of measuring glucose in a repeatable and reversible manner, and holds promise to enable CGM in a stable, accurate and rapid manner.
2. Method
2.1. Principle and design
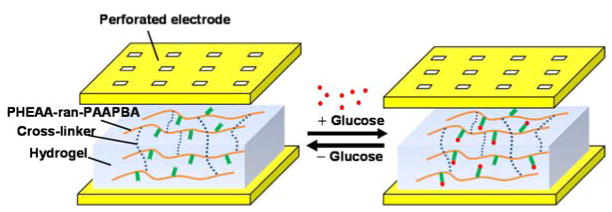
The affinity glucose microsensor utilizes a synthetic glucose-sensitive hydrogel, which consists of N-3-acrylamidophenylboronic acid (AAPBA) as the glucose-sensing component, and acryl N-Hydroxyethyl acrylamide (HEAA) as the hydrophilic component. The hydrogel uses tetraethyleneglycol diacrylate (TEGDA) as the cross-linker and 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) as the polymerization initiator. When glucose binds reversibly to the phenylboronic acid moieties in the AAPBA segments to form strong cyclic boronate ester bonds, a change in the dielectric properties of the hydrogel occurs and can be measured to determine the glucose concentration.
The dielectric properties of the hydrogel can be represented by the complex permittivity: ε* = ε′ – ε″, where the real permittivity ε′ represents the ability of the hydrogel to store electric energy, while the imaginary permittivity ε″ is related to dissipation of energy. When the gap between the electrodes of a parallel-plate transducer is filled with the hydrogel (Figure 1), the transducer can be represented by a capacitor (effective capacitance: Cx ) and resistor (effective resistance: Rx ) connected in series. Correspondingly, the real and imaginary parts of the complex permittivity are related to these parameters by ε′ = Cx/C0 and; ε″ = 1/(ωRxC0), where C0 is the capacitance when the electrode gap is in vacuum. The interactions of the hydrogel with glucose in general cause changes in its composition and conformation, and hence changes in its dielectric properties ε′ and ε″. Thus, the transducer’s effective capacitance and resistance will hence change and can be measured to determine the glucose concentration.
Figure 1.
Principle of hydrogel-based microsensor.
The transducer is enabled by MEMS technology and uses a pair of parallel electrodes sandwiching the hydrogel (Figure 2). The upper electrode is perforated to allow passage of glucose molecules, and is passivated within a perforated diaphragm to avoid direct contact with the hydrogel. The perforated electrode and diaphragm are supported by microposts so that they do not collapse onto the lower electrode on the substrate. Glucose molecules reversibly bind with the hydrogel, thereby changing the hydrogel’s complex permittivity. While changes in the real and imaginary parts of the complex permittivity can be used to determine the glucose concentration, we in the present work focus on the real permittivity, which can be interrogated via measurement of the capacitance between the electrodes, to determine the glucose concentration.
Figure 2.
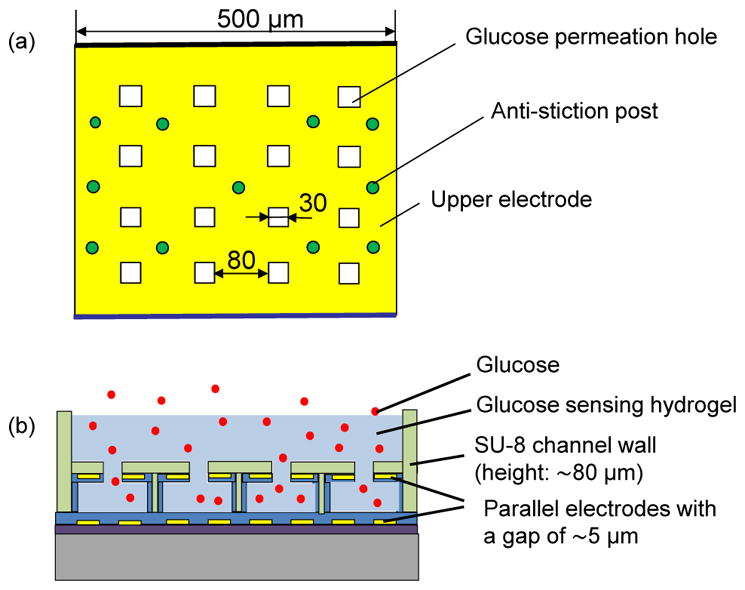
Schematics of the affinity microsensor: (a) top view and (b) side view.
2.2. Fabrication
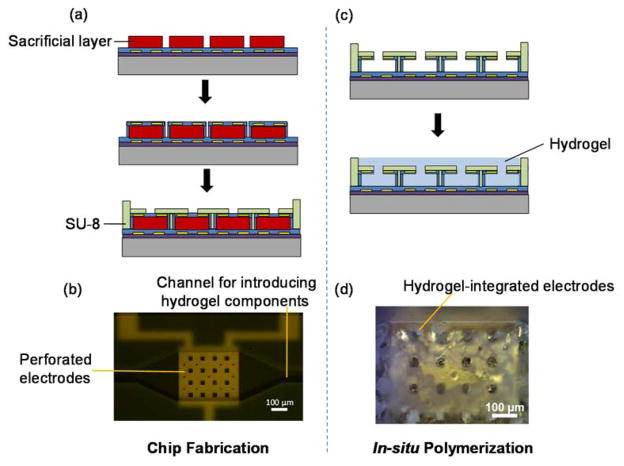
To fabricate the MEMS capacitive transducer, a chrome (Cr)/gold (Au) film (5/100 nm) was deposited by thermal evaporation and patterned to form the lower electrode (500 μm×500 μm) on a SiO2–coated wafer. The patterned gold electrode was then passivated with Parylene (1 μm). This was, followed by deposition of an S1818 sacrificial layer (5 μm) and an additional Parylene layer (1.5 μm). Another Cr/Au (5/100 nm) film was patterned to form the upper electrode and passivated by another Parylene layer. An SU-8 layer was then patterned to form a channel and anti-collapse microposts between the electrodes. The Parylene diaphragm was patterned with reactive ion etching (RIE) to form perforation holes that allow glucose permeation. The sacrificial photoresist layer was removed with acetone to release the diaphragm. The device fabrication process is shown in Figure 3(a).
Figure 3.
Chip fabrication: (a) standard fabrication procedures and (b) image of a fabricated capacitive transducer. In situ polymerization: (c) hydrogel integration in the transducer and (d) image of the hydrogel-integrated device.
The hydrogel was prepared in situ in the capacitive transducer. First, a mixture of the hydrogel components (AAPBA, HEAA, TEGDA, and AAPH) in solution was deoxygenated by nitrogen gas for 30 minutes, and was then injected into the device, filling the gap between the parallel electrodes. The device was placed in a nitrogen environment and heated for 4 hours at 70°C. The hydrogel was formed between the parallel electrodes, as shown in Figure 3(d). The hydrogel-integrated device was rinsed with water and ethanol to remove unreacted monomer and reagents.
2.3. Materials
The hydrogel was synthesized in house via free radical polymerization with AAPBA and HEAA monomers. An HEAA to AAPBA molar ratio of 9 (or approximately 10% AAPBA content among all monomers) was adopted. Then a solution consisting of AAPBA (1.1% w/v), HEAA (5.5% v/v), TEGDA (0.08% v/v), and AAPH (0.16% w/v) in distilled water was prepared for polymerization. A stock solution (0.1 M) of glucose was prepared by dissolving D-(+)-glucose (0.9 g) in distilled water to 50 mL. Glucose solution at varying concentrations (40, 70, 90, 180, 300, and 500 mg/dL) was prepared by diluting the stock solution.
2.4. Experimental setup
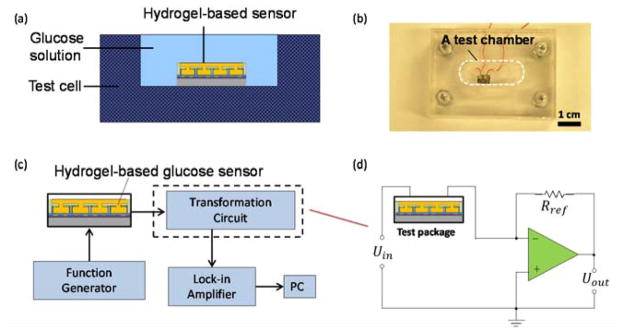
During testing, we placed the device in an acrylic test cell (2 mL in volume) filled with glucose solution (Figure 4). The device was connected to a capacitance/voltage transformation circuit driven by a sinusoidal input from a function generator (Agilent, 33220A), which imposes an AC electric field on the electrodes of the device to induce a glucose concentration-dependent change in the permittivity of the hydrogel. The resulting changes in the effective capacitance Cx of the capacitance/voltage transformation circuit are determined by measuring the output voltage (Uout) from a given input AC voltage (Uin). All experiments were conducted at frequencies in a range of 1 to 100 kHz as allowed by a lock-in amplifier (Stanford Research Systems, SR844) used in output voltage measurements.
Figure 4.
Experimental setup: (a) Schematics of a testing setup. (b) Image of the testing setup. (c) Experimental setup. (d) A capacitance/voltage transformation circuit.
3. Results and Discussion
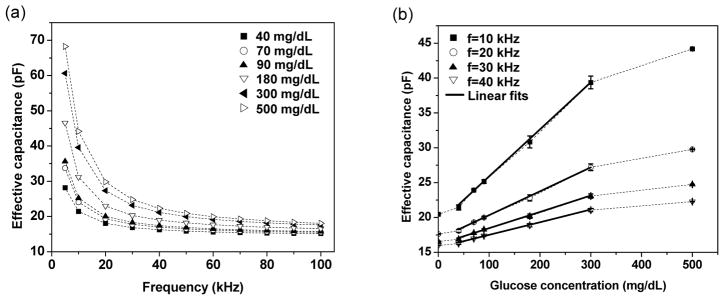
We first investigated the microsensor response to different glucose concentrations under bias voltages of different frequencies (Figure 5). First, we observed that, at each of a series of physiologically relevant glucose concentrations (0–500 mg/dL), the effective capacitance of the device, and hence the permittivity of the hydrogel, decreased with increasing frequency over the entire frequency range tested (1–100 kHz) (Figure 5a). This is consistent with the dielectric relaxation of the hydrogel, in which the dielectric properties of the hydrogel have a momentary delay with respect to a changing electric field23. The dielectric properties of the hydrogel in an electric field are in general influenced by a number of mechanisms of polarization (i.e., shift of electric charges from their equilibrium positions under the influence of an electric field24), such as electronic polarization, ionic polarization, dipolar polarization, counterion polarization, and interfacial polarization. Electronic polarization and ionic polarization involve the distortion of electron clouds with nucleus and the stretching of atomic bonds, while counterion polarization and dipolar polarization reflect redistribution of ions and reorientation of electrical dipoles25, 26.
Figure 5.
Effective capacitance of the microsensor averaged from triplicate measurements. (a) Dependence of effective capacitance on measurement frequency. (b) Dependence of effective capacitance on glucose concentration, where error bars reflect standard errors and linear fits (solid lines) have a coefficient of determination R2 ranging from 0.993 to 0.995. (Data points are connected by dashed lines to guide the eye.)
At a given frequency, the effective capacitance of the hydrogel increased consistently with glucose concentration in the entire range tested (0–500 mg/dL). This is clear from the device’s frequency response (Figure 5a), and can be more conveniently examined when the device’s response is plotted versus the glucose concentration (Figure 5b). For example, at 30 kHz, the effective capacitance increased from 16.2 pF to 24.8 pF as the glucose concentration increased from 0 mg/dL to 500 mg/dL. This reflected that the binding between the hydrogel and glucose significantly influences the polarization of the hydrogel, which may include changes in the hydrogel’s structural conformations, permanent dipole moments, elastic resistance to the dipole rearrangement in the electric field, and electric double layer characteristics. These effects, which are highly complex and require elucidation through further in-depth studies, combine to result in the glucose concentration dependence of the hydrogel’s dielectric properties, explaining the observed variation of the device’s effective capacitance with glucose concentration.
It can be seen from Figure 5b that the dependence of the effective capacitance on glucose concentration is in general nonlinear over the full glucose concentration range tested (0–500 mg/dL). Thus, in practical applications, a calibration curve represented by a lookup chart or nonlinear equation27 can be used to determine the glucose concentration from a measured effective capacitance value. Meanwhile, it is interesting to note that this dependence became considerably more linear in glucose concentration ranges that are moderately smaller but of strongest relevance to continuous glucose monitoring. For example, in a range of 40–300 mg/dL, the effective capacitance at a given frequency was approximately linear with glucose concentration as indicated by the linear fits. In such a range, a linear calibration equation may hence be adequate for the determination of glucose concentration from measurement results.
We conducted the above-mentioned experiments in triplicates to examine the ability of the microsensor to measure glucose concentrations in a repeatable manner and with adequate sensitivity (Figure 5b). At all glucose concentrations, the standard error in the effective capacitance was less than 0.91 pF (2.3%), indicating excellent repeatability. In addition, at all of the measurement frequencies used, the resolution and range of glucose measurement resolution were found to be appropriate for continuous glucose monitoring. Considering 30 kHz for example, the sensitivity of the microsensor was approximately 15 fF(mg/dL)−1 in the glucose concentration range of 0–40 mg/dL. With a capacitance measurement resolution of 3 fF as allowed by our measurement setup, the device’s resolution for glucose concentration measurement was correspondingly estimated to be 0.2 mg/dL. At a signal-to-noise ratio of 3, this yielded a detection limit of 0.6 mg/dL, well below the physiologically relevant glucose concentration range (typically greater than 40 mg/dL28). For glucose concentrations within 40–300 mg/dL, the sensitivity was approximately 23 fF(mg/dL)−1, corresponding to an estimated resolution of 0.12 mg/dL. At higher glucose concentrations (300–500 mg/dL), the nonlinear sensor response experienced a gradual declination in sensitivity and resolution (respectively to 8.4 fF(mg/dL)−1 and 0.35 mg/dL at 500 mg/dL) as an increasingly small number of binding sites remained available in the hydrogel. These sensor characteristics, appropriate for practical applications, are comparable to those of commercially available electrochemical sensors (e.g., 1 mg/dL over a glucose concentration range from 0 to 400 mg/dL29, 30 or 500 mg/dL31) as well as other research-stage boronic acid-based affinity sensors (e.g., 0.3 mg/dL32 over a range from 0 to 300 mg/dL33 or 540 mg/dL34) for continuous glucose monitoring.
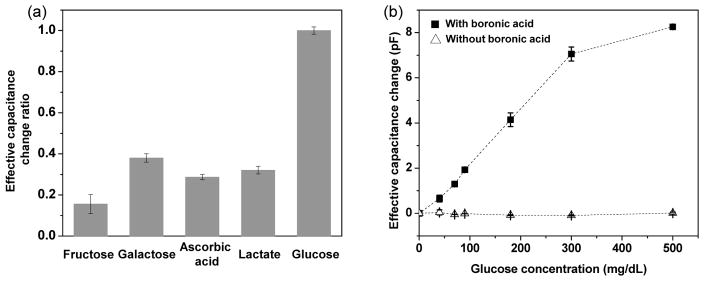
We investigated the response of the hydrogel-based microsensor to glucose as compared to its response to potential interferents. Nonspecific molecules exist in interstitial fluid and can interact with boronic acid, which is the glucose sensitive component of our hydrogel. These molecules include fructose (~1.8 mg/dL), galactose (~1.8 mg/dL), lactate (~9 mg/dL), and ascorbic acid (~1.32 mg/dL). We tested the hydrogel-based microsensor on these molecules and found that the resulting response was substantially lower than that to glucose. For example, at the same concentration of 90 mg/dL, the effective capacitance change (measured at 30 kHz) due to fructose, galactose, lactate and ascorbic acid was found to be 17%, 38%, 32% and 28% of that due to glucose, respectively (Figure 6a). Here, the effective capacitance change is calculated according to ΔC = C – C0 where C is the effective capacitance at a given glucose (or interferent) concentration, and C0 is the effective capacitance in the absence of glucose and interferents. Considering that the physiological concentrations of the potential interferents were about one order of magnitude lower than that of glucose, the microsensor was determine to be sufficiently selective for measurements of glucose in interstitial fluid for CGM applications.
Figure 6.
Selectivity and role of boronic acid for the microsensor: change of the effective capacitance calculated with reference to measurement in the absence of glucose or interferents (frequency: 30 kHz). (a) Ratio of the interferent-induced effective capacitance change to the glucose-induced effective capacitance change (concentration: 90 mg/dL for glucose and each of the interferents including fructose, galactose, ascorbic acid and lactate). (b) Effective capacitance change to glucose when boronic acid moieties were present (10% AAPBA content) or absent in the hydrogel (data points are connected by dashed lines to guide the eye).
While boronic acid binds to all diol-containing molecules, the selective response of the microsensor to glucose over the potential interferents could be attributed to the unique binding behavior between boronic acid and glucose. At a 1:1 ratio, boronic acid in fact binds more strongly to fructose than glucose. However, with a high concentration of boronic acid moieties (which was the case for our hydrogel), boronic acid can bind with glucose at a 2:1 ratio35–37. We exploited this property in previous work and developed solution-phase, viscometrically based affinity microsensors37, 38. In this work, we postulate that the existence of 2:1 binding between glucose and boronic acid moieties played a major role in the microsensor response, by causing additional crosslinking of the hydrogel that could lead to the augmentation of elastic resistance to electric field-induced dipole reorientation. The rather insignificant device response to the potential interferents (fructose, galactose, ascorbic acid and lactate) could, on the other hand, be attributed to a lack of this 2:1 binding mode.
We also studied the role of boronic acid in glucose recognition to gain further insight into the principle and operation of the microsensor. Using hydrogels containing with and without AAPBA content, we obtained the dependence of the effective capacitance on glucose concentration at a fixed measurement frequency (e.g., 30 kHz, Figure 6b). It also can be seen that when using an AAPBA-free hydrogel, the microsensor exhibited negligible changes in the effective capacitance in response to glucose concentration changes. This is in contrast to the strong glucose-induced response of the microsensor when it was equipped with the 10%-AAPBA hydrogel (shown in Figure 5b and reproduced in Figure 6b), indicating that boronic acid moieties in the hydrogel were critically responsible for the desired recognition of glucose by the microsensor.
Finally, we tested the device with time-resolved glucose concentration measurements to assess its ability to track glucose concentration changes in a consistent and reversible manner. The measured microsensor output at 30 kHz varied from 16.5 pF at 0 mg/dL to 24.8 pF at 500 mg/dL (Figure 7). In particular, when the device was exposed to a glucose concentration after experiencing another sample that was either higher or lower in concentration, virtually the same effective capacitance value was consistently obtained. For example, the effective capacitance at 40 mg/dL over the two periods (from 20 to 38 minutes, and from 321 to 341 minutes) were respectively 16.87 pF and 16.73 pF, agreeing within 0.8%. Similarly, the reversibility was within 3.4% and 1.3% for the measurement data at glucose concentrations of 180 and 300 mg/dL, respectively. This indicates that because the binding between glucose and boronic acid moieties in the hydrogel, the microsensor possesses excellent reversibility in response to glucose concentration changes. The time constant of the response (i.e., the time for the sensor to reach 63% of the steady state response) was approximately 16 min. This time constant was attributable to the relatively large thickness of the hydrogel in the proof-of-concept device (~200 μm), through which glucose molecules must diffuse to interact with the capacitive transducer. As the glucose diffusion time decreases with the square of the hydrogel thickness, it is expected that thinner hydrogels can be used to effectively obtain more rapid time responses.
Figure 7.
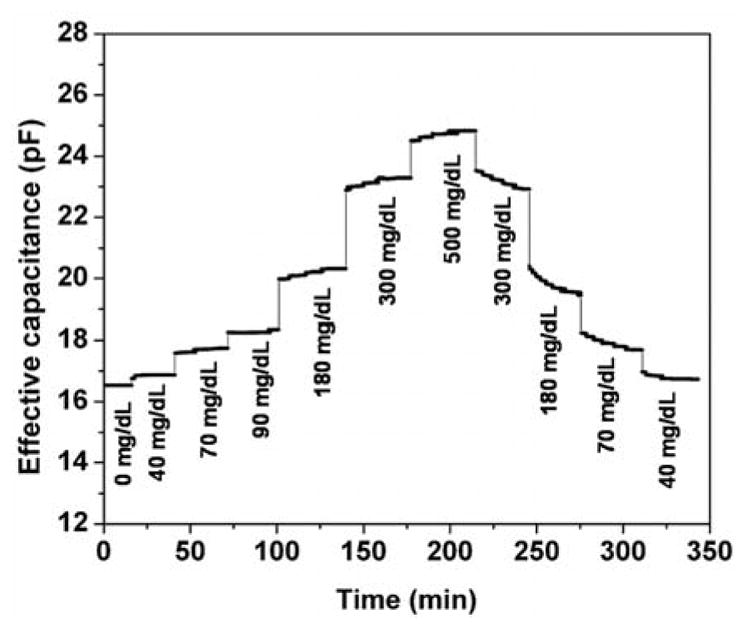
Time-resolved device response to time-varying glucose concentration. (Bias voltage frequency: 30 kHz.)
4. Conclusions
We have presented a hydrogel-based affinity glucose microsensor that measures glucose concentration through dielectric transduction. The device consists of a pair of thin-film parallel capacitive electrodes sandwiching a synthetic hydrogel. Glucose molecules permeate into the hydrogel through electrode perforations, and bind reversibly to boronic acid moieties of the hydrogel. This induces changes the dielectric polarization behavior, and hence the complex permittivity, of the hydrogel. Thus, the effective capacitance between the electrodes, which is directly related to the real part of the complex permittivity, can be measured to determine the glucose concentration. The use of an in situ polymerized hydrogel simplifies the design of the microsensor, facilitates its miniaturization and robust operation, and can potentially improve the tolerance of the device, when implanted subcutaneously, to biofouling. Testing results showed that the effective capacitance of the device, in a measurement frequency range of 1–100 kHz, responded consistently to glucose concentration changes ranging from 0 to 500 mg/dL. At a given frequency, the effective capacitance increased consistently with glucose concentration, suggesting that the affinity binding between glucose and boronic acid moieties caused the real permittivity of the hydrogel to increase. At 30 kHz, the measurement resolution of the microsensor was estimated to be 0.2, 0.12 and 0.35 mg/dL in the glucose concentration ranges of 0–40, 40–300, and 300–500 mg/dL, respectively. When subjected to time varying glucose concentration changes in the full 0–500 mg/dL range, the microsensor response was consistent and reversible. The time constant of this response was approximately 16 min, which can be readily improved by using thinner hydrogels for reduced glucose diffusion distances. These results have demonstrated that the microsensor can potentially allow accurate and consistent measurement of glucose concentration in interstitial fluid for continuous glucose monitoring.
Highlights.
An affinity microsensor is presented for continuous glucose monitoring.
The device surfaces are functionalized with a hydrogel via in situ polymerization
Glucose is detected by its affinity binding to the hydrogel’s boronic acid moieties.
Glucose detection has been made in 0–500 mg/dL range at 0.35 mg/dL resolution.
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health (NIH Grant No. 1DP3DK101085-01) and National Natural Science Foundation of China (Grant No. 61428402).
Biographies
Junyi Shang received the B.S. Degree in Thermal Engineering in 2011 from Sun Yat-sen University, Guangzhou, China, and the M.S. degree in Mechanical Engineeringin 2012 from Columbia University, New York, NY, where he is currently working toward the Ph.D. degree in BioMEMS and microfluidics. His research focuses on the development of implantable glucose sensor and integrated single cell analysis microchip.
Jing Yan received the B.S. Degree in Chemical Engineering and Technology in 2007 from Shandong Normal University, Ji’nan, China, and the Ph.D. degree in Applied Chemistry in 2012 from Jilin University, Ji’nan, China. She worked as Postdoctoral fellow in biomedical materials in the Queen University, Kingston, Canada and University of South Carolina, South Carolina, U.S during 2012 to 2015. She is currently working as Assistant Researcher in the Northwestern Polytechnical University, Xi’an, China. Her research focuses on the development of injectable biomaterials for medical application.
Zhixing Zhang received his B.S. degree in Automotive Engineering from Tsinghua University, Beijing, China in 2012. After that, he obtained his M.S. degree from Columbia University, New York, USA in 2014. His research interests include development of biosensors and micro-total-analysis-system using microfluidics and nanotechnology.
Xian Huang is an Assistant Professor of Mechanical Engineering at Missouri University of Science and Technology. He received his Ph.D. in Mechanical Engineering from Columbia University, New York, USA in 2011. His research interests are in the development of flexible and stretchable epidermal sensors, bioresorabable electronics, implantable multichannel affinity sensing devices, and biochips for body fluid analysis.
Panita Maturavongsadit received the B.S. Degree in Pharmaceutical Science in 2012 from Chulalongkorn University, Bangkok, Thailand. She is currently working toward the Ph.D. degree in Chemistry and Biochemistry from University of South Carolina, Columbia, SC. Her research focuses on the development of injectable hydrogels functionalized with rod-like nanoparticles for cartilage tissue engineering.
Yuan Jia received his B.S. degree in Mechanical Engineering from University of Rochester, Rochester, NY, USA in 2010. After that, he obtained his M.S. degree from Columbia University, New York, USA in 2012, where he is pursuing a Ph.D. degree in Mechanical Engineering. His research interests include the development of calorimetric sensors and biomedical applications of microelectromechanical systems (BioMEMS).
Bing Song is currently a Ph.D. student in Mechanical Engineering at Columbia University, New York, USA. She received her B.S. degree in measurement and control technology and instrument from Tianjin University, Tianjin, China, and M.S. degree in Mechanical Engineering from Columbia University, New York, NY, in 2011. Her research was about development of implantable affinity glucose sensors and she presently focuses on iterative learning control and repetitive control.
Tieying Ma is presently an Associate Professor in College of Optical and Electronic Technology, China Jiliang University, Hangzhou, China. She received her Ph.D. from Shanghai Institute of Microsystem and Information Technology, China Academy of Science. She was a visiting scholar in the Department of Mechanical Engineering at Columbia University, New York, USA, in 2014. Her research focuses on the development of micro/nano systems.
Dachao Li received the Ph.D. degree in Precision Instrument and Mechanics from Tianjin University, Tianjin, China, in 2001. From 2004 to 2006, he did postdoctoral research at Institute of Microelectronics, Peking University, Beijing, China. From 2006 to 2008, he was a research associate at Department of Electrical Engineering and Computer Science, Case Western Reserve University, Cleveland, USA. Presently, he is an associate professor in College of Precision Instrument and Optoelectronics Engineering, Tianjin University. His research specializations focus on micro sensor and optofluidics.
Kexin Xu received the Ph.D. degree in precision instrument engineering from Tianjin University, Tianjin, China, in 1988. From 1992 to 1999, he was a senior research fellow and team leader for non-invasive glucose measurement techniques in the Laboratory of Basic Technologies, KDK Corporation, Kyoto, Japan. He obtained his professorship from Tianjin University in 2000. Since 2002, he has been the Dean of the College of Precision Instrument and Opto-Electronic Engineering, Tianjin University. His research interest includes the design theory of acousto-optic tunable filter and spectroscopic technology, rapid detection of milk ingredients, monitoring of air and flue gas composition, non-invasive glucose monitoring, etc.
Qian Wang received the B.S. and Ph.D. degrees in chemistry from Tsinghua University, Beijing, China, in 1992 and 1997, respectively. After postdoctoral research with Prof. Manfred Schlosser at the University of Lausanne, Switzerland, and with Prof. M. G. Finn at the Scripps Research Institute, he started as an Assistant Professor at the University of South Carolina in 2003, where he has been a Full Professor since 2011 and currently is the Robert L. Sumwalt Chair of Chemistry and Carolina Distinguished Professor. He has published over 170 publications in peer reviewed journals and maintained an active research program which focuses on using chemical biology tools to probe intracellular activities and the development of hierarchically structured nanomaterials to study the cooperative response of cells to extracellular matrixes.
Qiao Lin received his Ph.D. in Mechanical Engineering from the California Institute of Technology in 1998 with thesis research in robotics. He conducted postdoctoral research in microelectromechanical systems (MEMS) at the Caltech Micromachining Laboratory from 1998 to 2000, and was an assistant professor of Mechanical Engineering at Carnegie Mellon University from 2000 to 2005. He has been an associate professor of Mechanical Engineering at Columbia University since 2005. His research interests are in designing and creating integrated micro/nano systems, in particular MEMS and microfluidic systems, for biomedical applications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY. Function of an implanted tissue glucose sensor for more than 1 year in animals. Science Translational Medicine. 2010;2 doi: 10.1126/scitranslmed.3001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricci F, Moscone D, Tuta CS, Palleschi G, Amine A, Poscia A, Valgimigli F, Messeri D. Novel planar glucose biosensors for continuous monitoring use. Biosensors & Bioelectronics. 2005;20:1993–2000. doi: 10.1016/j.bios.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki H, Tokuda T, Miyagishi T, Yoshida H, Honda N. A disposable on-line microsystem for continuous sampling and monitoring of glucose. Sensors and Actuators B-Chemical. 2004;97:90–97. [Google Scholar]
- 4.Shibata H, Heo YJ, Okitsu T, Matsunaga Y, Kawanishi T, Takeuchi S. Injectable hydrogel microbeads for fluorescence-based in vivo continuous glucose monitoring. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17894–17898. doi: 10.1073/pnas.1006911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegrist J, Kazarian T, Ensor C, Joel S, Madou M, Wang P, Daunert S. Continuous glucose sensor using novel genetically engineered binding polypeptides towards in vivo applications. Sensors and Actuators B-Chemical. 2010;149:51–58. [Google Scholar]
- 6.Siegel RA, Gu YD, Lei M, Baldi A, Nuxoll EE, Ziaie B. Hard and soft micro- and nanofabrication: An integrated approach to hydrogel-based biosensing and drug delivery. Journal of Controlled Release. 2010;141:303–313. doi: 10.1016/j.jconrel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei M, Baldi A, Nuxoll E, Siegel RA, Ziaie B. A hydrogel-based implantable micromachined transponder for wireless glucose measurement. Diabetes Technology & Therapeutics. 2006;8:112–122. doi: 10.1089/dia.2006.8.112. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Li SQ, Davis E, Leduc C, Ravussin Y, Cai HG, Song B, Li DC, Accili D, Leibel R, et al. A mems differential viscometric sensor for affinity glucose detection in continuous glucose monitoring. Journal of Micromechanics and Microengineering. 2013;23 doi: 10.1088/0960-1317/23/5/055020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao YJ, Li SQ, Davidson A, Yang BZ, Wang Q, Lin Q. A mems viscometric sensor for continuous glucose monitoring. Journal of Micromechanics and Microengineering. 2007;17:2528–2537. doi: 10.1088/0960-1317/23/5/055020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13399–13403. doi: 10.1073/pnas.1104954108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickup JC, Hussain F, Evans ND, Rolinski OJ, Birch DJS. Fluorescence-based glucose sensors. Biosensors & Bioelectronics. 2005;20:2555–2565. doi: 10.1016/j.bios.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Arnold FH, Zheng WG, Michaels AS. A membrane-moderated, conductimetric sensor for the detection and measurement of specific organic solutes in aqueous solutions. Journal of Membrane Science. 2000;167:227–239. [Google Scholar]
- 13.Zou ZW, Kai JH, Rust MJ, Han J, Ahn CH. Functionalized nano interdigitated electrodes arrays on polymer with integrated microfluidics for direct bio-affinity sensing using impedimetric measurement. Sensors and Actuators a-Physical. 2007;136:518–526. [Google Scholar]
- 14.Kara P, de la Escosura-Muniz A, Maltez-da Costa M, Guix M, Ozsoz M, Merkoci A. Aptamers based electrochemical biosensor for protein detection using carbon nanotubes platforms. Biosensors & Bioelectronics. 2010;26:1715–1718. doi: 10.1016/j.bios.2010.07.090. [DOI] [PubMed] [Google Scholar]
- 15.Kafka J, Panke O, Abendroth B, Lisdat F. A label-free DNA sensor based on impedance spectroscopy. Electrochimica Acta. 2008;53:7467–7474. [Google Scholar]
- 16.Baur J, Gondran C, Holzinger M, Defrancq E, Perrot H, Cosnier S. Label-free femtomolar detection of target DNA by impedimetric DNA sensor based on poly(pyrrole-nitrilotriacetic acid) film. Analytical Chemistry. 2010;82:1066–1072. doi: 10.1021/ac9024329. [DOI] [PubMed] [Google Scholar]
- 17.Labib M, Zamay AS, Koloyskaya OS, Reshetneva IT, Zamay GS, Kibbee RJ, Sattar SA, Zamay TN, Berezovski MV. Aptamer-based viability impedimetric sensor for bacteria. Analytical Chemistry. 2012;84:8966–8969. doi: 10.1021/ac302902s. [DOI] [PubMed] [Google Scholar]
- 18.Qi P, Wan Y, Zhang D. Impedimetric biosensor based on cell-mediated bioimprinted films for bacterial detection. Biosensors & Bioelectronics. 2013;39:282–288. doi: 10.1016/j.bios.2012.07.078. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Li S, Schultz JS, Wang Q, Lin Q. A dielectric affinity microbiosensor. Applied Physics Letters. 2010;96:033701. [Google Scholar]
- 20.Huang X, Leduc C, Ravussin Y, Li SQ, Davis E, Song B, Li DC, Xu KX, Accili D, Wang Q, et al. A differential dielectric affinity glucose sensor. Lab on a Chip. 2014;14:294–301. doi: 10.1039/c3lc51026c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X, Li SQ, Davis E, Li DC, Wang Q, Lin Q. A mems dielectric affinity glucose biosensor. Journal of Microelectromechanical Systems. 2014;23:14–20. doi: 10.1109/JMEMS.2013.2262603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuenzi S, Meurville E, Ryser P. Automated characterization of dextran/concanavalin a mixtures—a study of sensitivity and temperature dependence at low viscosity as basis for an implantable glucose sensor. Sensors and Actuators B: Chemical. 2010;146:1–7. [Google Scholar]
- 23.Woo HJ, Majid SR, Arof AK. Dielectric properties and morphology of polymer electrolyte based on poly(epsilon-caprolactone) and ammonium thiocyanate. Materials Chemistry and Physics. 2012;134:755–761. [Google Scholar]
- 24.Kremer F, Schönhals A. Broadband dielectric spectroscopy. New York, NY, USA: Springer; 2003. [Google Scholar]
- 25.Foster KR, Schwan HP. Dielectric-properties of tissues and biological-materials - a critical-review. Critical Reviews in Biomedical Engineering. 1989;17:25–104. [PubMed] [Google Scholar]
- 26.Feldman Y, Polygalov E, Ermolina I, Polevaya Y, Tsentsiper B. Electrode polarization correction in time domain dielectric spectroscopy. Measurement Science & Technology. 2001;12:1355–1364. [Google Scholar]
- 27.de Silva CW. Sensors and actuators: Control system instrumentation. Taylor & Francis; 2007. [Google Scholar]
- 28.Updike SJ, Shults MC, Gilligan BJ, Rhodes RK. A subcutaneous glucose sensor with improved longevity, dynamic range, and stability of calibration. Diabetes Care. 2000;23:208–214. doi: 10.2337/diacare.23.2.208. [DOI] [PubMed] [Google Scholar]
- 29.DexCom, Inc. DexCom™ G4 PLATINUM. http://www.dexcom.com/dexcom-g4-platinum.
- 30.Medtronic MiniMed. MiniMed Paradigm® REAL-Time Insulin Pump and Continuous Glucose Monitoring System. http://www.minimed.com/products/insulinpumps/index.html.
- 31.Abbott Diabetes Care. FreeStyle Navigator® Continuous Glucose Monitoring System. www.freestylenavigator.com.
- 32.Mesch M, Zhang CJ, Braun PV, Giessen H. Functionalized hydrogel on plasmonic nanoantennas for noninvasive glucose sensing. Acs Photonics. 2015;2:475–480. [Google Scholar]
- 33.Tokuda T, Takahashi M, Uejima K, Masuda K, Kawamura T, Ohta Y, Motoyama M, Noda T, Sasagawa K, Okitsu T, et al. Cmos image sensor-based implantable glucose sensor using glucose-responsive fluorescent hydrogel. Biomedical Optics Express. 2014;5:3859–3870. doi: 10.1364/BOE.5.003859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang CJ, Losego MD, Braun PV. Hydrogel-based glucose sensors: Effects of phenylboronic acid chemical structure on response. Chemistry of Materials. 2013;25:3239–3250. [Google Scholar]
- 35.Li S, Huang X, Davis EN, Lin Q, Wang Q. Development of novel glucose sensing fluids with potential application to microelectromechanical systems-based continuous glucose monitoring. Journal of diabetes science and technology. 2008;2:1066–1074. doi: 10.1177/193229680800200615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Davis EN, Anderson J, Lin Q, Wang Q. Development of boronic acid grafted random copolymer sensing fluid for continuous glucose monitoring. Biomacromolecules. 2008;10:113–118. doi: 10.1021/bm8009768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang X, Li S, Schultz JS, Wang Q, Lin Q. A mems affinity glucose sensor using a biocompatible glucose-responsive polymer. Sensors and Actuators B: Chemical. 2009;140:603–609. doi: 10.1016/j.snb.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang X, Li S, Schultz J, Wang Q, Lin Q. A capacitive mems viscometric sensor for affinity detection of glucose. Journal of Microelectromechanical Systems. 2009;18:1246–1254. doi: 10.1109/JMEMS.2009.2034869. [DOI] [PMC free article] [PubMed] [Google Scholar]