Abstract
Introduction
Data on laparoscopic gastrectomy in patients with gastric cancer in the Western hemisphere are lacking. This study aimed to compare outcomes following laparoscopic versus open gastrectomy for gastric adenocarcinoma at a Western center.
Methods
Eighty-seven consecutive patients who underwent laparoscopic gastrectomy from November 2005 to April 2013 were compared with 87 patients undergoing open resection during the same time period. Patients were matched for age, stage, body mass index, and procedure (distal subtotal vs. total gastrectomy). Endpoints were short- and long-term perioperative outcomes.
Results
Overall, 65 patients (37 %) had locally advanced disease, and 40 (23 %) had proximal tumors. The laparoscopic approach was associated with longer operative time (median 240 vs.165 min; p < 0.01), less blood loss (100 vs.150 mL; p < 0.01), higher rate of microscopic margin positivity (9 vs.1 %; p = 0.04), decreased duration of narcotic and epidural use (2 vs. 4 days, p = 0.04, and 3 vs. 4 days, p = 0.02, respectively), decreased minor complications in the early (27 vs. 16 %) and late (17 vs. 7 %) postoperative periods (p < 0.01), decreased length of stay (5 vs. 7 days; p = 0.01), and increased likelihood of receiving adjuvant therapy (82 vs. 51 %; p < 0.01). There was no difference in the number of lymph nodes retrieved (median 20 in both groups), major morbidity, or 30-day mortality.
Conclusions
Laparoscopic gastrectomy for gastric adenocarcinoma is safe and effective for select patients in the West.
Utilization of minimally invasive approaches for resection of gastric cancer has rapidly increased in recent years. Minimally invasive (MIS) gastrectomy for early-stage, distal gastric cancer is well established and routinely performed in Eastern countries where gastric cancer is highly prevalent and screening is performed. Six randomized, prospective trials have confirmed improvements in postoperative outcomes compared with open distal subtotal gastrectomy (DSG) for patients with early-stage disease.1–6 However, laparoscopic gastrectomy is not as well studied or widely performed in Western countries, where patients tend to have more advanced disease at presentation and more proximally-located tumors requiring total gastrectomy (TG).
We previously reported short-term postoperative outcomes for 30 patients who underwent laparoscopic DSG at our center.7 These patients were matched for age, disease stage, and sex to patients undergoing open DSG during the same time period. In this report, we found that the laparoscopic approach was associated with decreased postoperative pain, shorter length of hospitalization, and decreased complications in the late postoperative period. Margin status, lymph node retrieval, and short-term recurrence-free survival (RFS) were similar between the groups. This study was somewhat limited by the small number of patients and the relatively short follow-up. In addition, the majority of patients had early-stage disease.
Since the time of that publication, we have expanded our practice of laparoscopic gastrectomy to include more patients with advanced gastric cancer (AGC) and to those with proximally-located tumors requiring TG. The current study aims to report our mature experience with laparoscopic gastrectomy in a larger series of patients reflective of the Western gastric cancer profile.
METHODS
Patient Selection
All patients who underwent laparoscopic gastrectomy for gastric adenocarcinoma with curative intent from August 2005 to April 2013 were identified using a prospectively-maintained gastric cancer database. Patients undergoing palliative gastrectomy, or those with non-adenocarcinoma histology, were excluded. Patients who underwent attempted laparoscopic gastrectomy but who required conversion to an open procedure were similarly excluded (N = 42). The most common reasons for conversion in these patients were the inability to safely enter the lesser sac due to peritumoral adhesions (N = 17), direct invasion of adjacent organs (N = 14), or large amounts of intraabdominal fat (N = 7), and positive proximal margin on frozen section analysis (N = 4). A total of 87 patients who met these criteria were identified. These 87 patients were matched to 87 patients who underwent open gastrectomy for the same indication during the same time period. Matching variables were age, body mass index (BMI), American Joint Committee on Cancer (AJCC) stage, and procedure (DSG or TG).
Operative Technique for Laparoscopic Gastrectomy
Laparoscopic gastrectomy was performed as previously described.7 Briefly, the lesser sac was entered by dissecting the plane between the greater omentum and the transverse mesocolon. The gastrocolic ligament was divided up to the level of the short gastric vessels for DSG, and to the left crus of the diaphragm for TG using a laparoscopic ultrasonic scalpel (Ultracision-Harmonic Scalpel; Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). The dissection was then continued toward the pylorus, including the infrapyloric lymph nodes. The right gastroepiploic vessels were divided at their origins.
The lesser omentum was opened and the right gastric artery was identified and divided at its origin. The proximal duodenum was divided with a linear stapler with bioabsorbable reinforcement. A modified D2 lymphadenectomy was performed for all patients, with the exception of those with early stage (0 or IA) disease, including the proximal hepatic, splenic, and left gastric artery lymph nodes. The left gastric artery was divided at its base in all cases. For DSG, the lesser curve lymph nodes (stations 1 and 3) were peeled down off of the stomach to the level of transection using the ultrasonic scalpel (Ultracision-Harmonic Scalpel, Ethicon Endo-Surgery Inc.). The stomach, or for TG the distal esophagus, was then divided with a linear stapler. The proximal margin was sent for frozen section analysis. Intestinal continuity was restored with gastrojejunal or esophagojejunal anastomosis via a Roux-en-Y gastrojejunostomy or an antecolic Billroth-II gastrojejunostomy. Esophagojejunal anastomoses were created in an end-to-side fashion utilizing a circular stapler (3.5 mm open staple height) with a trans-oral anvil device (OrVil™; Covidien, Mansfield, MA, USA), as previously described.9 Gastrojejunal anastomoses were created in a side-to-side fashion utilizing a linear stapler (3.5–4.1 mm open staple height depending on tissue thickness). Open operations were performed in standard fashion, as previously reported by our center.
Clinical Data
Clinical and follow-up data were obtained from a prospectively maintained gastric cancer database. A complication was defined as any unintended event occurring within 30 days of surgery (early), or within 31 days–6 months of surgery (late). Complications were graded in severity from 1 to 5 based on a modified Clavien–Dindo system as follows: Grade 1: oral medication or bedside medical care required; Grade 2: intravenous medical therapy required; Grade 3: radiologic, endoscopic, or operative intervention required; Grade 4: chronic deficit or disability associated with the event; and Grade 5: death related to complication. Minor complications were defined as Grades 1–2 and major complications as Grades 3–5.
Statistical Analysis
Patients were matched one-to-one by age (within 5 years), procedure (DSG vs. TG), stage (pathologic), and BMI (≥30 vs.<30). Wilcoxon signed-rank, McNemar’s, and Stuart–Maxwell tests were used to examine the difference in covariates between the paired groups. Exact conditional logistic regression was used to examine major and minor complications. Overall survival (OS) and RFS were calculated from the date of surgery until the time of death (for OS) or until the first recurrence or death (for RFS). Patients who did not experience the event of interest by the end of the study were censored at the time of last available follow-up. OS and RFS were estimated using the Kaplan–Meier method, and were compared using a stratified log-rank test. All p values were based on two-tailed statistical analyses and a p value <0.05 was considered to indicate statistical significance. All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
RESULTS
Patient Demographics
Of the 174 case-matched patients evaluated, 83 patients (48 %) were female and the median age at the time of surgery was 64 years (range 20–87). The median BMI was 28 kg/m2 (range 16–44). Following the AJCC staging, 109 patients (63 %) had early-stage disease (0–Ib), 39 (22 %) had stage II disease, and 26 (15 %) had stage III disease. Fifty-two patients (30 %) received neoadjuvant therapy and 57 (33 %) received adjuvant treatment.
The open (N = 87) and laparoscopic (N = 87) groups were similar for the matched variables of age, BMI, procedure, and AJCC/UICC stage, as well as for race. More patients in the laparoscopic group were female (58 vs. 38 %; p = 0.01). The proportion of patients who received neoadjuvant therapy was similar between the groups (35 % open vs. 25 % laparoscopic; p = 0.22), but more patients in the laparoscopic group received adjuvant therapy (25 % open vs. 40 % laparoscopic; p < 0.01). In a subset analysis of 65 patients with stage II/III disease, laparoscopic resection was associated with a higher likelihood of receiving adjuvant therapy than open (82 vs. 51 %; p < 0.01). Demographic variables for all patients are summarized in Table 1.
TABLE 1.
Demographics for patients undergoing open and laparoscopic gastrectomy for adenocarcinoma
| Variable | All (N = 174) |
Open (N = 87) |
Laparoscopic (N = 87) |
p value |
|---|---|---|---|---|
| Age (years) | 64 (20–87) | 64 (26–86) | 64 (20–87) | NS |
| Female sex | 83 (48) | 33 (38) | 50 (58) | 0.01 |
| Race | 0.26 | |||
| White | 127 (73) | 62 (70) | 65 (74) | |
| Black | 18 (10) | 12 (14) | 6 (7) | |
| Asian | 24 (14) | 12 (14) | 12 (14) | |
| Unknown | 5 (3) | 1 (1) | 4 (5) | |
| BMI (kg/m2) | 28 (16–44) | 28 (16–40) | 27 (19–44) | NS |
| Pathologic stage | NS | |||
| 0 | 12 (7) | 6 (7) | 6 (7) | |
| I | 97 (56) | 48 (55) | 49 (56) | |
| II | 39 (22) | 19 (22) | 20 (23) | |
| III | 26 (15) | 14 (16) | 12 (14) | |
| Neoadjuvant treatment | 52 (30) | 30 (35) | 22 (25) | 0.22 |
| Adjuvant treatment | 57(33) | 22 (25) | 35 (40) | <0.01 |
Continuous variables are expressed as median (range) and categorical variables as N (%)
Bold values indicate statistical significance (p < 0.05)
NS non-significant, BMI body mass index
Operative Characteristics and Complications
Overall, 120 patients (68 %) underwent DSG and 54 (32 %) underwent TG. Median operative time was 204 min (range 62–478) and median estimated blood loss was 100 mL (range 5–1,500). Intravenous (IV) narcotics were required postoperatively for a median of 3 days (range 0–24). For patients with epidural anesthesia, the median duration of use was 3 days (range 2–6). Overall, 51 patients (34 %) experienced minor complications postoperatively [38 (22 %) in the early period and 23 (13 %) in the late period]. Thirty-six patients (20 %) experienced major complications postoperatively [23 (13 %) in the early period and 13 (7 %) in the late period]. One patient (<1 %) died within 30 days of surgery.
The open and laparoscopic groups were similar for type of gastrectomy as this was a matched variable. The laparoscopic approach was associated with longer operative time [median 240 min (range 109–445) vs. 165 min (range 26–478); p < 0.01], less blood loss [100 mL (range 5–500) vs. 150 mL (range 5–1,500); p < 0.01], less postoperative pain measured by decreased duration of IV narcotic use [2 days (range 0–24) vs. 4 days (range 0–12); p = 0.04], and decreased duration of epidural use [3 days (range 2–5) vs. 4 days (range 3–6); p = 0.02] for patients who had epidural anesthesia. Length of stay was shorter in the laparoscopic group [median 5 days (range 3–29) vs. 7 days (range 3–39); p = 0.01]. Minor complications were more frequent in the open group in both the early and late postoperative periods (27 vs. 16 %, p < 0.01, and 17 vs. 7 %, p = 0.03). Major complications and 30-day mortality were similar between the groups (Table 2). As more than one complication occurred in the same patient in some cases, Table 3 details the specific, individual complications that occurred in the early and late periods in both groups.
TABLE 2.
Operative characteristics and complications for patients undergoing open versus laparoscopic gastrectomy for adenocarcinoma
| Variable | All (N = 174) | Open (N = 87) | Laparoscopic (N = 87) | p value |
|---|---|---|---|---|
| Procedure | NS | |||
| Distal gastrectomy | 120 (68) | 60 (69) | 60 (69) | |
| Total gastrectomy | 54 (32) | 26 (31) | 26 (31) | |
| Operation time | 204 (62–478) | 165 (62–478) | 240 (109–445) | <0.01 |
| Blood loss (mL) | 100 (5–1,500) | 150 (5–1,500) | 100 (5–500) | <0.01 |
| Intravenous narcotic use (days) | 3 (0–24) | 4 (0–12) | 2 (0–24) | 0.04 |
| Epidural use (days) | 3 (2–6) | 4 (3–6) | 3 (2–5) | 0.02 |
| Length of stay (days) | 6 (3–39) | 7 (3–39) | 5 (3–29) | 0.01 |
| Minor complications | ||||
| Early, ≤30 days | 38 (22) | 24 (27) | 14 (16) | <0.01 |
| Late, 31 days–6 months | 21 (12) | 15 (17) | 6 (7) | 0.03 |
| Major complications | ||||
| Early, ≤30 days | 23 (13) | 11 (13) | 12 (14) | 0.57 |
| Late, 31 days–6 months | 13 (7) | 5 (6) | 8 (9) | 0.21 |
| 30-day mortality | 1 (<1) | 0 | 1 (1) | – |
Continuous variables are expressed as median (range) and categorical variables as N (%)
Bold values indicate statistical significance (p < 0.05)
TABLE 3.
Early and late postoperative complications following laparoscopic versus open gastrectomy
| Complication | Laparoscopic
|
Open
|
||
|---|---|---|---|---|
| Earlya | Lateb | Earlya | Lateb | |
| Minor (Grade 1–2) | ||||
| Acute kidney injury | 1 | |||
| Anemia | 3 | 4 | ||
| Arrhythmia | 2 | 2 | ||
| Atelectasis | 3 | |||
| Deep vein thrombosis | 1 | 1 | 2 | 1 |
| Dehydration | 1 | |||
| Delayed gastric emptying | 1 | 2 | ||
| Diarrhea | 1 | |||
| Fever | 1 | |||
| Gastrointestinal bleeding | 1 | 1 | ||
| Hematoma | 1 | |||
| Hypertension | 1 | |||
| Ileus | 1 | 4 | 1 | |
| Incisional hernia | 1 | 4 | ||
| Malnutrition | 1 | |||
| Pancreatitis | 1 | |||
| Superficial site infection | 2 | 5 | ||
| Urinary tract infection | 3 | |||
| Total | 11 | 5 | 26 | 11 |
| Major (Grade 3–4) | ||||
| Anastomotic leak | 4 | 4 | ||
| Anastomotic stricture | 3 | |||
| Deep space infection | 1 | 1 | 1 | |
| Dehiscence | 2 | |||
| Duodenal stump leak | 6 | 4 | ||
| Incisional hernia | 5 | |||
| Internal hernia | 1 | 2 | ||
| Myocardial infarction | 1 | |||
| Pancreatic leak/fistula | 1 | |||
| Pleural effusion | 1 | 2 | ||
| Pulmonary embolism | 2 | 1 | 1 | |
| Small bowel obstruction | 1 | |||
| Total | 16 | 6 | 16 | 7 |
| Mortality (Grade 5) | ||||
| Gastrointestinal bleeding | 1 | |||
Early = ≤ 30 days of surgery
Late = 31 days–6 months following surgery
Pathologic Characteristics
Tumors were located at the gastroesophageal junction in three patients (2 %), the proximal stomach in 32 patients (18 %), the distal stomach in 134 patients (77 %), and involved the whole stomach (linnitus) in 5 patients (3 %). Lauren type was intestinal in 80 patients (48 %), diffuse in 63 patients (38 %), and mixed in 25 patients (15 %). In patients undergoing modified D2 lymphadenectomy, the median number of lymph nodes removed was 20 (range 10–54). Margins were microscopically negative (R0) in 165 patients (95 %) and microscopically positive (R1) in 9 patients (5 %). No patients had grossly positive margins.
Tumor location, Lauren type, and lymph node retrieval were similar between the two groups. The laparoscopic approach was associated with a higher rate of microscopic margin positivity (9 vs. 1 %; p = 0.04). Pathologic variables for all patients are summarized in Table 4.
TABLE 4.
Pathologic characteristics of gastric cancer patients undergoing open versus laparoscopic gastrectomy
| Variable | All (N = 174) |
Open (N = 87) |
Laparoscopic (N = 87) |
p value |
|---|---|---|---|---|
| Tumor location | 0.66 | |||
| GE junction | 3 (2) | 1 (1) | 2 (2) | |
| Proximal | 32 (18) | 14 (16) | 18 (21) | |
| Distal | 134 (77) | 69 (79) | 65 (75) | |
| Linnitis | 5 (3) | 3 (3) | 2 (2) | |
| Lauren type | 0.93 | |||
| Intestinal | 80 (48) | 42 (48) | 38 (47) | |
| Diffuse | 63 (38) | 33 (38) | 30 (37) | |
| Mixed | 25 (15) | 12 (14) | 13 (16) | |
| Margin status | 0.04 | |||
| R0 | 165 (95) | 86 (99) | 79 (91) | |
| R1 | 9 (5) | 1 (1) | 8 (9) | |
| Lymph nodes removeda | 20 (10–54) | 20 (12–46) | 20 (10–54) | 0.47 |
Continuous variables are expressed as median (range) and categorical variables as N (%)
Bold value indicates statistical significance (p < 0.05)
GE gastroesophageal
In patients who underwent modified D2 lymphadenectomy
Oncologic Outcomes
Median follow-up for survivors was 19.2 months (31.1 for open, 11.0 for laparoscopic). Within this period, 16 patients experienced recurrence and 13 patients died of disease. Median RFS was not reached. The estimated 5-year RFS and OS were 84 and 81 % (p = 0.25) in the laparoscopic group, and 78 % and 80 % (p = 0.40) in the open group, respectively (Fig. 1).
FIG. 1.
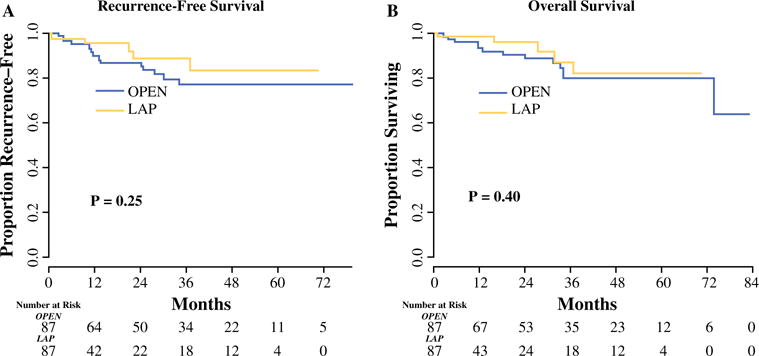
a Recurrence-free survival and b overall survival for patients undergoing laparoscopic versus open gastrectomy for gastric adenocarcinoma. The median follow-up for survivors was 19.2 months (31.1 for open, 11.0 for laparoscopic). LAP laparoscopic
DISCUSSION
Laparoscopic gastrectomy for early-stage gastric adenocarcinoma offers several advantages for patients compared with the open approach, including decreased blood loss and need for transfusion, decreased stress of surgery, decreased postoperative pain, speedier recovery, and decreased morbidity.1,6,10 More recently, the application of minimally invasive approaches for AGC and proximal tumors has been investigated in Eastern centers.11,12 These studies provide evidence that the laparoscopic approach is safe and feasible for AGC in Eastern patients, but it is not clear whether these data can be applied to patients in the Western hemisphere. Western gastric cancer patients more often have proximally-located tumors and tend to have more advanced disease at presentation than Eastern patients.13 There is also evidence to suggest that tumor biology is different in Eastern and Western patients, with the incidence of Lauren’s diffuse-type gastric cancer with signet ring cells increasing in the US.14 Lastly, surgeon and hospital volume of minimally invasive gastrectomy are markedly different in the East and West. More than 400 minimally invasive gastrectomies are performed per year at high-volume Eastern centers. Conversely, a center with 20 minimally invasive gastrectomies per year is considered high-volume in the West. Data confirming the safety and oncologic efficacy of laparoscopic gastrectomy in Western patients with AGC are lacking.
The current study contributes to the Western experience by providing data on the safety and oncologic efficacy of laparoscopic gastrectomy in a large series of patients in the US. Consistent with Eastern studies, the laparoscopic approach was associated with longer operating time (median 240 vs. 165 min for open). However, this is an improvement from our previous report of our initial 30 patients, where the median operating time for laparoscopic DSG was 270 min, despite the inclusion of more complex patients and laparoscopic TG in the current report.7 This observation suggests that operative time can be improved with increased surgeon experience.
The laparoscopic approach was also associated with decreased blood loss, decreased postoperative pain (as measured by duration of IV narcotic and epidural use), decreased hospital length of stay, and decreased minor complications in both the early and late postoperative periods compared with the open approach. The most common minor complications were wound infection and delayed gastric emptying (DGE), requiring IV therapy in the form of antibiotics and parenteral nutrition, respectively. Minimization of blood loss and morbidity is critically important as postoperative complications and blood transfusions have been shown to be associated with poor oncologic outcomes in multiple gastrointestinal cancers, including gastric adenocarcinoma.15–22
Perhaps most importantly, the laparoscopic approach was associated with a higher likelihood of receiving adjuvant systemic therapy when indicated. Adjuvant therapy is critically important for patients with AGC as surgery alone is not sufficient for cure, and some patients are not eligible for neoadjuvant treatment. Furthermore, there are no validated, solely neoadjuvant regimens available for Western patients aside from those with tumors of the gastroesophageal junction. Gastrectomy is a highly morbid procedure, with serious morbidity rates of 20–30 %.23 Many patients are therefore not fit to complete, or in some cases to receive any, planned adjuvant treatment. In modern, prospective trials for AGC, adjuvant therapy was only able to be delivered in 48–67 % of patients.8,24,25 In the current study, the laparoscopic approach was associated with decreased minor morbidity in both the early and late postoperative periods, and with increased likelihood of receiving adjuvant therapy as patients recovered faster. While definitive conclusions cannot be drawn from this retrospective study, the observations of both decreased postoperative morbidity and increased delivery of adjuvant therapy in the laparoscopic group suggest an important association.
For operative variables, lymph node retrieval was similar in the laparoscopic and open groups, but the laparoscopic approach was associated with a higher incidence of microscopic margin positivity. Interestingly, the majority of cases of R1 margin in the laparoscopic group (75 %) occurred in patients with Lauren’s diffuse-type disease. This association suggests that a high degree of suspicion for proximal margin positivity should be maintained in patients with diffuse-type disease, and rigorous frozen section assessment of the proximal margin should be performed in all cases. Furthermore, such findings suggest that perhaps for some diffuse gastric cancers, particularly more advanced tumors, an open approach to palpate the stomach may be helpful. Additionally, techniques that allow palpation of the gastric remnant such as placement of a hand-port or creation of a small upper midline laparotomy should be considered for these patients. R1 margins should be managed with reoperation and excision to achieve an R0 margin in patients with node-negative disease, or adjuvant chemotherapy and/or radiation in patients with node-positive disease.
Limitations of this study include its retrospective design and limited follow-up. Despite efforts to control for baseline factors by matching, this was not a prospective, randomized trial and the two groups of patients were not the same. Furthermore, the median follow-up of <20 months is immature to make conclusions regarding long-term oncologic outcomes in this disease.
In summary, laparoscopic gastrectomy was associated with improvements in several short-term perioperative outcomes, including decreased blood loss, pain, length of stay, minor morbidity, and increased likelihood of receiving adjuvant treatment when indicated. The laparoscopic approach was also associated with longer operative time and a higher rate of microscopic (R1) margin positivity. Rigorous frozen section analysis should be performed for patients with diffuse-type gastric cancer.
CONCLUSIONS
Laparoscopic gastrectomy is safe and technically feasible in select Western patients with early and advanced gastric cancer and is associated with improvements in several short-term outcomes, but most importantly associated with decreased morbidity and increased likelihood of receiving adjuvant therapy. Longer follow-up is needed to determine if these short-term benefits may translate into improvements in long-term survival.
References
- 1.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–6. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 2.Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–7. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial) Ann Surg. 2010;251:417–20. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 4.Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–7. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–73. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 6.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131:S306–11. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 7.Strong VE, Devaud N, Allen PJ, Gonen M, Brennan MF, Coit D. Laparoscopic versus open subtotal gastrectomy for adenocarcinoma: a case-control study. Ann Surg Oncol. 2009;16:1507–13. doi: 10.1245/s10434-009-0386-8. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 9.LaFemina J, Vinuela EF, Schattner MA, Gerdes H, Strong VE. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol. 2013;20:2975–83. doi: 10.1245/s10434-013-2978-6. [DOI] [PubMed] [Google Scholar]
- 10.Vinuela EF, Gonen M, Brennan MF, Coit DG, Strong VE. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg. 2012;255:446–56. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 11.Cai J, Wei D, Gao CF, Zhang CS, Zhang H, Zhao T. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg. 2011;28:331–7. doi: 10.1159/000330782. [DOI] [PubMed] [Google Scholar]
- 12.Fang C, Hua J, Li J, et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am J Surg. 2014;208(3):391–6. doi: 10.1016/j.amjsurg.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010;251:640–6. doi: 10.1097/SLA.0b013e3181d3d29b. [DOI] [PubMed] [Google Scholar]
- 14.Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765–70. doi: 10.5858/2004-128-765-DTITIA. [DOI] [PubMed] [Google Scholar]
- 15.Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum. 2014;57:858–68. doi: 10.1097/DCR.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 16.Ito H, Are C, Gonen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]
- 17.Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–66. doi: 10.1245/s10434-007-9434-4. [DOI] [PubMed] [Google Scholar]
- 18.Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol. 2013;19:4060–5. doi: 10.3748/wjg.v19.i25.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mrak K, Eberl T, Laske A, Jagoditsch M, Fritz J, Tschmelitsch J. Impact of postoperative complications on long-term survival after resection for rectal cancer. Dis Colon Rectum. 2013;56:20–8. doi: 10.1097/DCR.0b013e31826f2672. [DOI] [PubMed] [Google Scholar]
- 20.Sutton JM, Kooby DA, Wilson GC, et al. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg. 2014;18(9):1575–87. doi: 10.1007/s11605-014-2567-4. [DOI] [PubMed] [Google Scholar]
- 21.Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83. doi: 10.1245/s10434-012-2720-9. [DOI] [PubMed] [Google Scholar]
- 22.van der Gaag NA, Harmsen K, Eshuis WJ, Busch OR, van Gulik TM, Gouma DJ. Pancreatoduodenectomy associated complications influence cancer recurrence and time interval to death. Eur J Surg Oncol. 2014;40:551–8. doi: 10.1016/j.ejso.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014;21(9):3008–14. doi: 10.1245/s10434-014-3664-z. [DOI] [PubMed] [Google Scholar]
- 24.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 25.Bouche O, Ychou M, Burtin P, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801) Ann Oncol. 2005;16:1488–97. doi: 10.1093/annonc/mdi270. [DOI] [PubMed] [Google Scholar]


