Abstract
In vivo and in vitro studies were conducted to determine whether testosterone-producing Leydig cells are able to develop from cells associated with rat seminiferous tubules, interstitium, or both. Adult rat seminiferous tubules and interstitium were isolated, encapsulated separately in alginate, and implanted subcutaneously into castrated rats. With implanted tubules, serum testosterone increased through two months. Tubules removed from the implanted rats and incubated with LH produced testosterone, and cells on the tubule surfaces expressed steroidogenic enzymes. With implanted interstitial tissue, serum levels of testosterone remained undetectable. However, co-culture of interstitium plus tubules in vitro resulted in the formation of Leydig cells by both compartments. These results indicate that seminiferous tubules contain both cellular and paracrine factors necessary for the differentiation of Leydig cells, and that the interstitial compartment contains precursor cells capable of forming testosterone-producing Leydig cells but requires stimulation by paracrine factors from the seminiferous tubules to do so.
Keywords: Stem cells, Leydig cells, alginate, transplantation, testosterone
1. INTRODUCTION
Testosterone, produced by Leydig cells of the mammalian testis, plays an essential role in the development and maintenance of the male reproductive system, as well as in metabolism, muscle mass, and bone mineral density (Mooradian et al., 1987; Tuck and Francis, 2009). Previous studies have shown that in both the human and rat, testosterone formation gradually increases from the peripubertal period through the adult, coincident with the development of adult Leydig cells (Habert et al., 2001; Svechnikov et al., 2010; Teerds and Huhtaniemi, 2015). In mice and rats, the adult Leydig cells develop from stem cells (stem Leydig cells, referred to herein as SLCs) which, in postnatal day 7 testes, express the stem cell markers nestin (Davidoff et al., 2004; Jiang et al., 2014), COUP-TFII (Qin et al., 2008; Kilcoyne et al., 2014), Arx (Miyabayashi et al., 2013), CD51 (Jiang et al., 2014), p75NTR (Jiang et al., 2014), and platelet-derived growth factor receptor PDGFRα (Ge et al., 2006; Landreh et al., 2013). These cells do not express Leydig cell lineage markers (Ge et al., 2006; Landreh et al., 2013; Davidoff et al., 2004; Jiang et al., 2014; Kilcoyne et al., 2014). By day 11 postpartum, some of the SLCs commit to a differentiation pathway, forming progenitor Leydig cells (PLCs) that express the Leydig cell lineage markers 3β-hydroxysteroid dehydrogenase (3βHSD), cholesterol side-chain cleavage (P450scc or CYP11A1) and luteinizing hormone receptor (Benton et al., 1995; Chen et al., 2010). The PLCs differentiate into immature Leydig cells (ILCs) from day 21 to day 35, and the latter into adult Leydig cells (ALCs) from day 28 to 56 (Benton et al., 1995; Chen et al., 2009; Chen et al., 2010; Teerds and Huhtaniemi, 2015).
Numerous studies have shown that the elimination of the Leydig cells from the adult rat testis by treating rats with the alkylating agent ethane dimethanesulfonate (EDS) is followed by the formation of a new generation of ALCs (Jackson et al., 1986; Kerr et al., 1987). The new cells arise from stem cells that proliferate and then differentiate (Jackson et al., 1986; Kerr et al., 1987; Davidoff et al., 2004; Stanley et al., 2012; Li et al., 2016). The location(s) of the stem cells, and the nature and origin of regulatory factors involved in their proliferation and differentiation to ALCs, remain uncertain (Davidoff et al., 2004; O’Shaughnessy et al., 2008; Chen et al., 2010; Stanley et al., 2012; Li et al., 2016)
Recently, we reported that PDGFRα-expressing cells isolated from the testes of EDS-treated rats had the ability to proliferate for extended periods of time in vitro, or to differentiate into testosterone-producing cells (Stanley et al., 2012). These are properties expected of stem cells. When isolated rat seminiferous tubules were cultured in vitro, functional Leydig cells were generated on their surfaces (Stanley et al., 2012; Zhang et al., 2013; Odeh et al., 2014; Li et al., 2016). However, under similar culture conditions, the interstitium failed to form functional Leydig cells (Stanley et al., 2012), indicating that the interstitium may lack stem cells, regulatory (niche) factors, or both. Previous studies, however, provided evidence that various cells of the testicular interstitium might be precursors of Leydig cells, including peritubular myoid cells (O’Shaughnessy et al., 2008; Stanley et al., 2012), blood vessel-associated pericytes (Davidoff et al., 2004), mesenchymal cells (Hardy et al., 1989), or combinations of fibroblasts, lymphatic endothelial cells and pericytes (Jackson et al., 1986).
In the present study, we pursued an in vivo approach to ask whether testosterone-producing Leydig cells would be generated from rat seminiferous tubules and/or from interstitial tissue in the absence of paracrine influences of one on the other. A number of previous studies in the rodent, dog, and human had shown that encapsulation in alginate-poly-L-lysine can effectively maintain the integrity of implanted tissue and cells and prevent immunorejection (Barsoum et al., 2003; de Vos et al., 2006; Orive et al., 2006). This approach has been used for applications that include insulin delivery by pancreatic islet cells, therapeutic gene product delivery by recombinant cells, and testosterone delivery by adult Leydig cells (Barsoum et al., 2003; Machluf et al., 2003; de Vos et al., 2006). Using alginate encapsulated seminiferous tubules and interstitium, we show in the present studies that: 1) cells associated with seminiferous tubules are able to develop into testosterone-producing Leydig cells in vivo outside the testis and therefore in the absence of paracrine factors from the interstitial compartment; 2) in contrast, interstitial tissue failed to form functional Leydig cells in vivo; but 3) in vitro co-culture of interstitium with seminiferous tubules resulted in the formation of testosterone-producing cells by the interstitium, We conclude that the interstitial compartment contains Leydig cell precursor cells, but lacks required paracrine factors from the seminiferous tubules to differentiate into testosterone-producing Leydig cells.
2. MATERIALS AND METHODS
2.1. Chemicals
Tissue culture supplements ITS (insulin/transferrin/selenite), fetal bovine serum (FBS), sodium alginate, alginate lyase, poly-L-lysine (21 kDa), and β-actin antibody were from (Sigma-Aldrich) (St. Louis, MO). M199 and DMEM/F12 medium and Hank’s Balanced Salt Solution (HBSS) were from Invitrogen (Carlsbad, CA). [1,2,6,7,16,17-3H(N)]-Testosterone (115.3 Ci/mmol) was from Perkin Elmer Life Sciences, Inc (Boston, MA). Testosterone antibody was from ICN (Costa Mesa, CA). CYP11A1 antibody was from Chemicon International (Temecula, CA). Bovine LH (USDA-bLH-B-6) was provided by the USDA Animal Hormone Program (Beltsville, MD). Testosterone was from Steraloids (Newport, RI). Reagents for morphological studies were from Electron Microscopy Sciences (Hatfield, PA).
2.2. Animals
Adult male Sprague-Dawley rats (3 month-old) were obtained from Harlan Sprague-Dawley Inc. (Indianapolis, IN). Rats were housed in the animal facilities of the Johns Hopkins School of Public Health, under conditions of controlled light (14h light: 10h dark) and temperature (22°C) and with free access to rat chow and water. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals, according to protocols approved by the Johns Hopkins Animal Care and Use Committee.
2.3. Isolation and encapsulation of seminiferous tubules and interstitial tissue
Rats received a single, intraperitoneal injection of ethane dimethanesulfonate (EDS; 85 mg/kg BW) to eliminate Leydig cells, and were sacrificed by decapitation 4 days later. The testes were perfused with DMEM/F12 medium via the testicular artery to eliminate blood. After decapsulation, testes were separated into 8 equal-sized pieces. The seminiferous tubules were mechanically separated from the interstitium using fine forceps (Toppari et al., 1991; Stanley et al., 2012). Tubules and interstitium were encapsulated separately in alginate gel as reported previously (Machluf et al., 2003), but with minor modifications as follows: Tubules or interstitial tissue were placed briefly into 1.2% sodium alginate in Ca2+- and Mg2+-free HBSS solution, and then incubated (5 min) in calcium solution (1.5% CaCl2 in HBSS) to crosslink the alginate. After washing with HBSS to remove free calcium ions, the capsules were coated with 0.1% poly-L-lysine in HBSS (12 min). Unreacted poly-L-lysine was removed by washing the capsules 3 times with HBSS, and the capsules were additionally coated with 0.125% sodium alginate in HBSS (10 min). The encapsulated tissues were either cultured in vitro or transplanted into animals. For in vitro studies, 5 cm lengths of tubules were encapsulated in alginate and cultured in 24 well plates. For in vivo experiments, multiple tubule fragments were encapsulated together. A total of 8 capsules of tubules and of interstitium were prepared from each testis.
2.4. Culture of seminiferous tubules and interstitium in vitro
Dual-chamber co-culture experiments were conducted. Seminiferous tubules (10 cm total length per testis) were incubated in the inner chamber of the Millicell Hanging Inserts (6 well, 1.0 μm pore; MilliporeSigma, Billerica, MA). Interstitium (isolated from 1/8 of each testis) was placed in the outer chamber. Previous studies showed that proliferation of SLCs associated with rat seminiferous tubules occurs when the tubules are cultured with LH for one week, and that differentiation occurs during weeks 2–3 (Odeh et al., 2014; Li et al., 2016). To examine the effects of co-culture on proliferation, interstitium and tubules were cultured together in the presence of LH in the medium during week 1, and then were separated and cultured with LH alone for 3 additional weeks. To examine the effects of co-culture on differentiation, the interstitium was cultured with LH for the first week, co-cultured with tubules in the presence of LH for 2 additional weeks, and then separated from the tubules and cultured alone for another week with LH. To assess the ability of the interstitium to produce testosterone after these treatments, tissue was incubated with maximally stimulating LH (10 ng/ml) for 24 hours, and testosterone was assayed in the medium. Additionally, interstitial cells were stained for 3β-HSD after dispersed the cells with collagenase (1 mg/ml; 20 min) and attached to culture plate for 24 hours (see section 2.6).
2.5. Implantation of encapsulated tissues in vivo
We first examined the effects of encapsulation itself on the seminiferous tubules and interestitium by comparing Leydig cell development by encapsulated and non-encapsulated tissue in vitro. Encapsulated seminiferous tubules and intersitium were cultured in 24 well plates with M199 medium containing ITS, LH (10 ng/ml), and FBS (0–10%) for up to 4 weeks. Each well contained either tubules or interstitium. As controls, tubules and interstitium were cultured that were not alginate-encapsulated. The media were replaced every 3–4 days, and ultimately collected for assay of testosterone. On the last day of culture, some tubules and interstitium were freed from the alginate by digesting the capsules with 10 units/ml of alginate lyase for 30 min at 37C. Tissues were then used for immunochemical staining of CYP11A1, cytochemical staining of 3β-HSD (Klinefelter et al., 1987), and morphological studies.
For in vivo studies, tubules and interstitial tissue were encapsulated as above and implanted subcutaneously in the backs of adult rats (N=8) that had been castrated one week earlier. Total tubules or intersitium from one testis were implanted into each animal. Alginate capsules without encapsulated tubules or interstitium were implanted as controls. At days 1 and 30 after implantation, blood was collected from the rats by tail bleeding and the serum was frozen at −70 C until testosterone measurement. At day 60, the rats were sacrificed and implanted capsules were removed. Some of the capsules were fixed for light and electron microscopic analysis of the encapsulated tissues. Others were treated with 10 units/ml of alginate lyase for 30 min at 37 C to degrade the alginate coating. The freed tubules and interstitial tissue were washed and transferred into serum-free medium containing maximally stimulating LH (100 ng/ml) for 24 hours, and testosterone production was measured in the medium by RIA. Tissue was also used for cytochemical staining of 3β-HSD or for Western blots analysis of CYP11A1.
2.6. CYP11A1 immunofluorescence and 3βHSD activity staining
Seminiferous tubules and interstitium were washed with Ca2+- and Mg2+-free HBSS solution and fixed in neutral buffered formalin. After washing with HBSS solution, the tissues were incubated with CYP11A1 primary antibody (1:100) overnight. Tissue then was treated with fluorescent secondary antibodies (Alexa-conjugated anti-rabbit IgG, 1:1000) for 1h and, after washing, was examined using a Nikon Eclipse 800 microscope. Staining for 3β-HSD was conducted as previously reported (Klinefelter et al., 1987). In brief, tubules or cells were washed in HBSS solution and then dried on slides at room temperature for 30 minutes. Slides were stained for 40 mins with a solution containing 0.4 mM 5β-androstan-3β-ol-17-one steroid substrate, 1 mg/ml NAD, and 0.2mg/ml tetranitro blue tetrazolium. After washing in HBSS solution, the stained tissue was fixed with 10% formalin in HBSS containing 5% sucrose (15 min).
2.7. Light and electron microscopy
For light and electron microscopic analyses, tissues were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, post-fixed in cacodylate-buffered 1% osmium tetroxide, and embedded in Epon. Sections of 600–800Å were mounted on 200-mesh grids, stained with uranyl acetate and lead citrate, and examined with a Hitachi HU12F electron microscope. For light microscopy, sections (1μm) were mounted on glass slides and stained with toluidine blue.
2.8. Western blot analysis
Tissues were lysed with Tris-HCl buffer (100 mM Tris, 1% Triton X-100, 50 mM dithiothreitol, 1X Sigma protease inhibitor cocktail, pH 6.8). After sonication and centrifugation (18,000g, 10 min), the supernatant was mixed with 3X SDS loading buffer (New England BioLab, Ipswich, MA). Equal amounts of total protein (about 30 μg) from each sample were separated by 10% SDS-PAGE, and then transferred onto a nitrocellulose membrane. After incubation with primary CYP11A1 antibody (1:1000) and horseradish peroxidase (HRP)-conjugated secondary antibody (1:5,000), signal was detected by the enhanced chemiluminescence Western blot kit from Pierce (Rockford, IL). The bound antibodies on the membranes were stripped by Restore Western Blot Stripping Buffer (Pierce, Rockford, IL), and the membranes were re-probed with antibody to β-actin. CYP11A1 protein was quantified by digitally scanning the Western blot bands of 4 animals. The results were processed by NIH Image (ImageJ) software.
2.9. Statistical analyses
One-way analysis of variance (ANOVA) was used for comparisons of multiple group means. If group differences were revealed by ANOVA (P<0.05), differences between individual groups were determined with the Student-Neuman-Kuels test, using SigmaStat software (Systat Software Inc., Richmond, CA). Values were considered significant at P<0.05.
3. RESULTS
3.1. Differentiation of testosterone-producing cells by alginate-encapsulated tissues in vitro
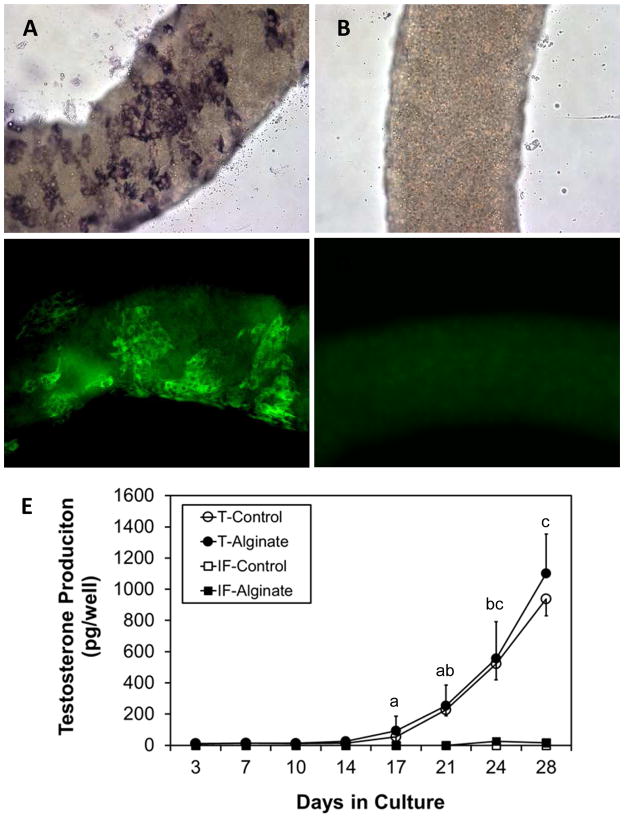
Seminiferous tubules (Figs. 1A, 1C) and interstitium (Figs. 1B, 1D) were dissected from the testes of EDS-treated rats and separated from each other. When encapsulated in alginate (Fig. 1E), the tubules maintained their original morphology for at least 4 weeks in culture even in the presence of 10% FBS. Without encapsulation, cells migrated away from the tubules (Fig. 1F).
Figure 1.
Effect of alginate encapsulation on the maintenance of tissue integrity in vitro. Freshly isolated seminiferous tubules (A, C) and interstitium (B, D) were cultured in vitro for 4 weeks (E, F) in the presence of 10% FBS.
Cytochemical staining for 3βHSD identified Leydig cell clusters that formed on the surfaces of the encapsulated tubules after 4 weeks in culture (Fig. 2A), whereas freshly isolated tubules lacked 3βHSD-positive cells (Fig. 2B). Immunofluorescent staining of CYP11A1 was consistent with that of 3βHSD; CYP11A1-positive cells formed in clusters on the tubule surfaces by 4 weeks (Fig. 2C), whereas before culture the tubules lacked these cells (Fig. 2D). Interstitial tissue failed to form 3βHSD-positive or CYP11A1-positive cells whether or not the tissue was encapsulated (data not shown). The abilities of encapsulated and non-encapsulated tubules and interstitial tissue (interstitial fraction, IF) to form Leydig cells also were compared by measuring their respective abilities to produce testosterone in vitro in response to LH stimulation (Fig. 2E). Testosterone, which was not detectable at the start of culture, was measurable in the culture medium of seminiferous tubules after 2 weeks whether or not the tubules were encapsulated. Thereafter, testosterone production continued to rise through 4 weeks, equivalently between encapsulated and non-encapsulated tubules. In contrast, the interstitial tissue, whether or not encapsulated, failed to produce testosterone for up to 4 weeks.
Figure 2.
Effect of encapsulation on the development of functional Leydig cells in vitro. Alginate encapsulated seminiferous tubules were stained for 3β-HSD (A, B) or CYP11A1 (C, D). Clusters of 3β-HSD (A) and CYP11A1 (C) positive cells were seen after 4 weeks in culture, but not before culture (B, D). (E) Testosterone production by cultured seminiferous tubules. Whether encapsulated or not, the cultured tubules (T) produced testosterone by 4 weeks, whereas intersitium (IF), whether or not encapsulated, produced no testosterone. Data are expressed as mean ± SEM of three separately conducted experiments. Groups with shared letters were not significantly different at P≤0.05. Groups without letters had undetectable testosterone levels.
3.2. Differentiation of testosterone-producing cells by implanted alginate-encapsulated tissues in vivo
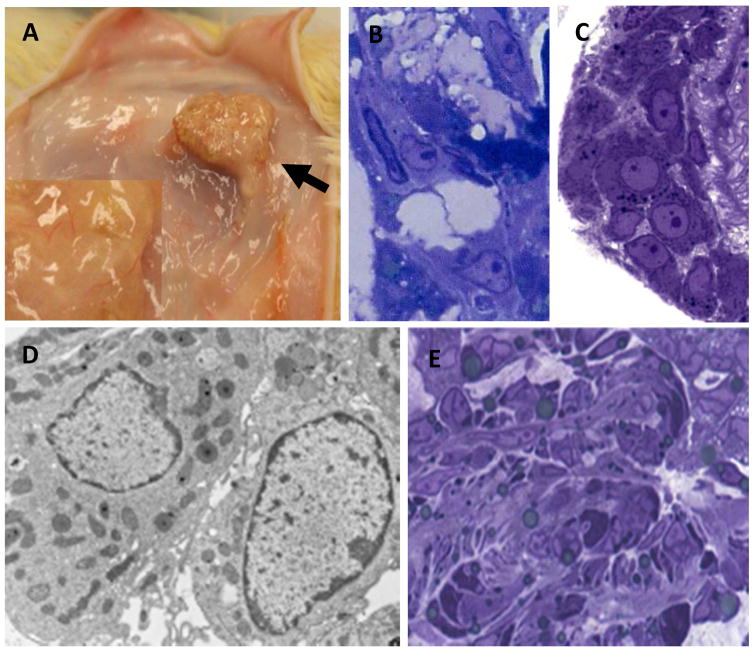
To determine whether cells associated with the seminiferous tubules and/or interstitial tissue would differentiate into testosterone-producing Leydig cells when encapsulated in alginate and implanted separately into rats outside the testis, encapsulated tubules and interstitium were implanted subcutaneously into the backs of rats that had been castrated a week earlier. Castrated rats that received alginate capsules devoid of tissue served as controls. By two months following implantation, blood vessels were seen entering the tubule-containing capsules (Fig. 3A). Leydig cells were evident in implants of seminiferous tubules removed from rats at 2 months (Fig. 3B). These cells resembled the Leydig cells restored to the testes of EDS-treated rats (Fig. 3C). As seen by electron microscopy (Fig. 3D), the restored cells in the capsules were rich in mitochondria and smooth endoplasmic reticulum, characteristic of testosterone-producing Leydig cells. In contrast, the transplanted interstitial tissue failed to develop any cells that resembled Leydig cells (Fig. 3E).
Figure 3.
In vivo development of Leydig cells by alginate-encapsulated tissue two months after implantation. (A) Seminiferous tubule-containing capsules (black arrow) showing blood vessels that entered the implants. (B) Section of implanted capsule revealing Leydig cells formed between the seminiferous tubules (white arrows). (C) Leydig cells (white arrows) restored to rat testis two months after EDS-treatment of the rats. (D) Electron micrograph of Leydig cells formed on the surface of the encapsulated tubules. (E) No Leydig cells were identified in the implanted interstitial tissue.
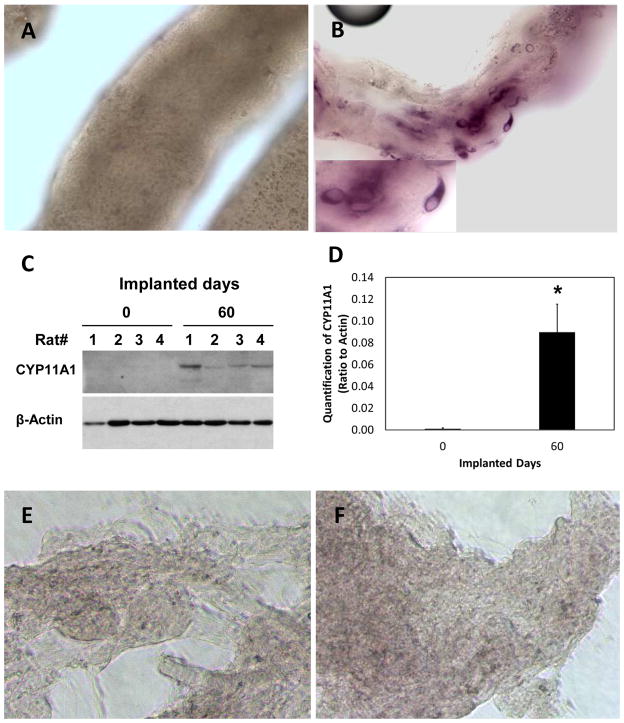
Cytochemical staining revealed that whereas there were no 3βHSD-positive cells associated with the tubules before their implantation (Fig. 4A), 3βHSD-positive cells were evident on the surfaces of the tubules two months after their implantation (Figs. 4B ). Western blot analyses of CYP11A1 expression by tubules prior to their implantation and 2 months thereafter were consistent with the 3βHSD data; before implantation, CYP11A1 was not expressed by cells associated with the isolated tubules, whereas CYP11A1 was expressed 2 months after implantation (Fig. 4C). Quantification of the Western blot results from 4 rats per group showed significant increase in CYP11A1 two months after implantation (Fig. 4D). In contrast, no 3βHSD-positive cells were seen in interstitial tissue either before (Fig. 4E) or after (Fig. 4D) implantation, and there were no cells that expressed CYP11A1 (not shown).
Figure 4.
Formation of steroidogenic cells in implanted seminiferous tubules in vivo. (A) Seminiferous tubules before their implantation, showing absence of 3βHSD-positive cells. (B) 3βHSD-positive cells were found in the seminiferous tubules two months after implantation. (C) Western blot of CYP11A1 before (0 days) and 60 days after tubule transplantation into four castrated rats. Actin served as a loading control. (D) Quantification of CYP11A1 at 0 and 60 days after tubule transplantation. *Significantly different compared to day 0 at P≤0.05 (N=4). (E, F) No 3βHSD-positive cells were seen associated with interstitium before (E) or two months after (F) implantation.
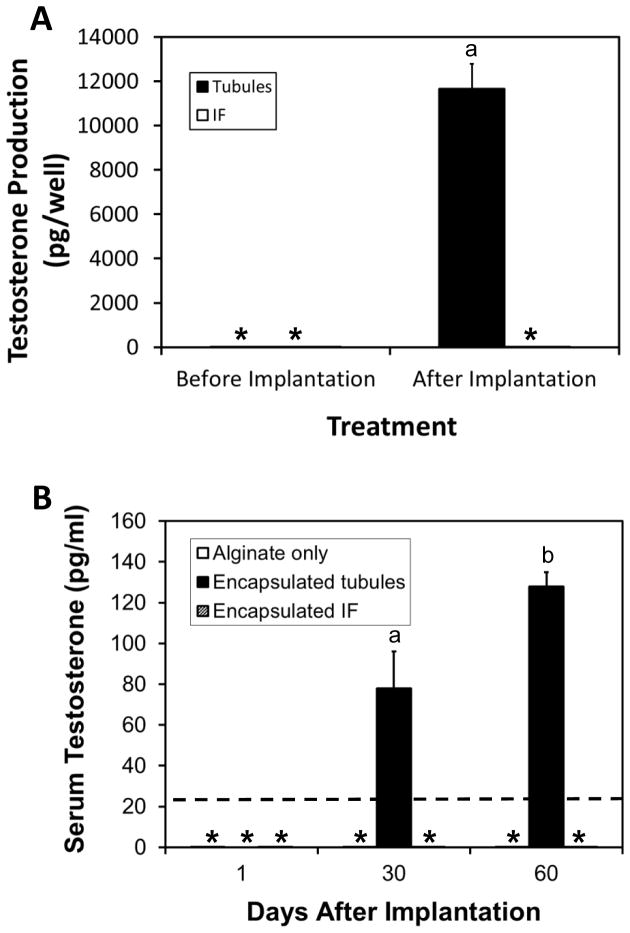
Before implantation, seminiferous tubules and interstitial tissue incubated with LH for 24 hours produced no testosterone (Fig. 5A). Two months after implantation, the implanted tissue capsules were removed from rats and incubated with LH for 24 hours. The capsules containing seminiferous tubules produced testosterone, whereas those that contained interstitial tissue did not (Fig. 5A). Measurements of serum levels of testosterone in implanted rats were consistent with these results (Fig. 5B). Serum testosterone initially was undetectable in castrated rats that received alginate only or alginate-encapsuled interstitial tissue or tubules. Serum testosterone remained below detection limits throughout the following 2 months in rats that received alginate or interstitial tissue. In rats that received seminiferous tubule-containing implants, however, serum testosterone levels rose significantly by one month, and rose further through two months. These results, taken together, indicate that cells associated with the tubules, but not with interstitial tissue, formed testosterone-producing Leydig cells during the two month in vivo implantation period.
Figure 5.
Testosterone production by seminiferous tubules and interstitium implanted in castrated rats. (A) Testosterone production by seminiferous tubules and interstitium (IF) before and two months after their implantation. The implants were removed from rats after implantation and incubated with LH in vitro for 24 hours. Testosterone was assayed in the medium. (B) Testosterone in the serum of castrated rats at 1, 30 and 60 days after rats were implanted with either seminiferous tubules or interstitium (IF). Rats that received empty implants (alginate only) served as controls. *Undetected. ----Detection limit of RIA. aSignificantly different from *groups at P≤0.05. bSignificantly different from agroup and *groups at P≥0.05.
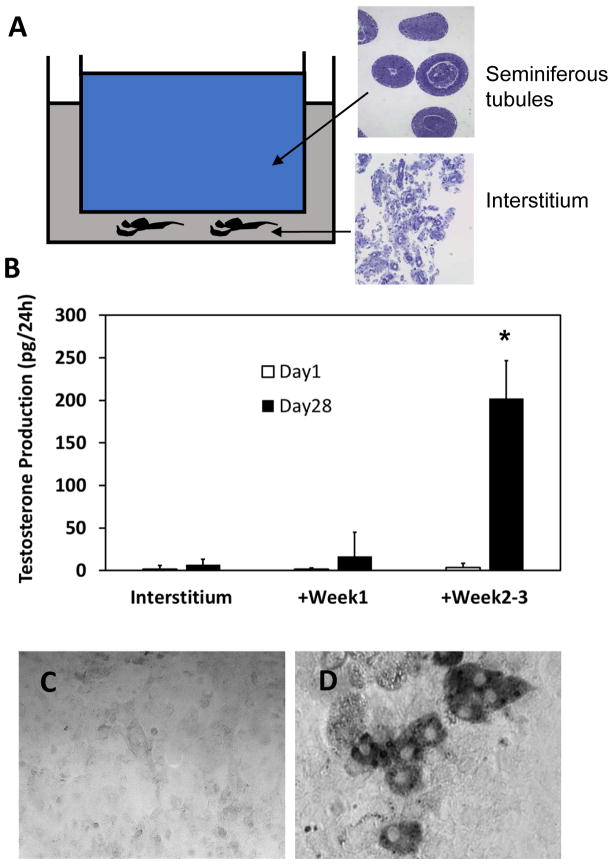
3.3. Differentiation of testosterone-producing cells by interstitial tissue when cultured together with seminiferous tubules
In both in vitro and in vivo studies described above, the interstitial tissue, by itself, was unable to form Leydig cells. In contrast, Leydig cells did form on the surfaces of seminiferous tubules. These results suggested that the interstitial tissue lacks stem cells and/or required differentiation factors, the latter presumably from seminiferous tubules. To distinguish between these possibilities, we used a dual-chamber co-culture system in which interstitum and seminiferous tubules were cultured side-by-side, separated by a filter that prevented contact between the two but allowed communication via secreted factors (Fig. 6A). Tissues were co-cultured with LH either for week 1, when SLC proliferation is known to occur, or for weeks 2–3, when SLC differentiation occurs (Odeh et al., 2014; Li et al., 2016). In the first case, after 1 week of culture together, the interstitium and the tubules were cultured separately with LH for another 3 weeks. In the second case, the interstitium was cultured alone for the first week, and then co-cultured with the tubules for weeks 2–3. This was followed by separation of the two tissues and the culture of interstitum with LH alone for another week. After the 4 week culture periods, tissue was cultured with maximally stimulating LH for 24 hours, and testosterone was measured in the medium. Co-culture of interstitium and tubules for week 1 had little effect on the ability of the interstitium to form testosterone-producing cells (Fig. 6B). However, co-culture of interstitium and tubules during weeks 2–3 resulted in significantly increased testosterone production by the interstitium (Fig 6B). Cytochemical staining of 3βHSD activity was consistent with the testosterone results; no 3βHSD-positive cells were seen in preparations of interstitium cultured alone with LH for 4 weeks (Fig. 6C), but were seen in interstitial preparations co-cultured with tubules during weeks 2–3 (Fig. 6D).
Figure 6.
Effect of seminiferous tubule-derived factors on the differentiation of Leydig cells by intersititial tissue. (A) Diagram of the system used for the co-culture of seminiferous tubules and interstitium. (B) Testosterone production by interstitium alone on Day 1 and Day 28 of culture with LH (denoted Interstitium); interstitium co-cultured with seminiferous tubules during Week 1, then separated from the tubules and cultured alone with LH for 3 additional weeks (+Week 1); interstitum alone cultured with LH for 1 week, then co-cultured with tubules for weeks 2–3, and then separated from the tubules and cultured with LH for an additional week (+Week 2–3). *Significant difference from other groups at P<0.05 based on three separately conducted experiments. (C) 3βHSD staining of cells in the intersititium after culture alone for 4 weeks with LH. (D) 3βHSD staining of cells in the interstitium after co-culture with seminiferous tubules during weeks 2–3. Arrows indicate 3βHSD+ cell cluster.
4. DISCUSSION
Although Leydig cells represent a relatively stable population in the adult testis, some turnover has been reported (Teerds et al., 1989). The observation that a new generation of Leydig cells forms after the experimental elimination of pre-existing Leydig cells from the adult testis with EDS (Jackson et al., 1986; Kerr et al., 1987) suggested that there may be stem cells in the adult testis that are capable of giving rise to the new cells (Davidoff et al., 2004; Jackson et al., 1986; Stanley et al., 2012). Indeed, there have been studies reporting the isolation, culture, and characterization of putative SLCs from neonatal and adult animals (Ge, et al., 2008; Stanley et al., 2012; Landreh et al., 2013; Jiang et al., 2014; Kilcoyne et al., 2014; Li et al., 2016). These cells have been shown to be capable of self-renewing in vitro for prolonged periods of time, and of differentiating into testosterone-producing cells (Ge et al., 2008; Stanley et al., 2012; Jiang et al., 2014). A recent study reported that SLCs from neonatal animals are multipotent (Jiang et al., 2014). The location of the SLCs and the niche factors involved in their regulation in vivo remain uncertain. There have been studies reporting that at least some Leydig cells derive from SLCs located on the outside of the seminiferous tubules (Russell et al., 1995; O’Shaughnessy et al., 2008; Stanley et al., 2012; Li et al., 2016). However, there also are studies reporting that SLCs are located in perivascular regions of the interstitial compartment of fetal, early neonatal and adult testes (Jackson et al., 1986; Davidoff et al., 2004; Defalco et al., 2013).
We showed previously that stem cells associated with the seminiferous tubules of the adult testis were able to proliferate and differentiate into functional Leydig cells in vitro, but that the culture of isolated interstitium failed to generate testosterone-producing cells (Stanley et al., 2012). The latter did not rule out the possibility that SLCs are in fact present in the interstitial tissue. Rather, we hypothesized that if indeed there were Leydig cell precursor cells in the interstitial compartment, that compartment, when physically separated from the tubules, might lack critical factors required for the differentiation of the precursor cells (Stanley et al., 2012). To begin to assess this, we first sought to determine whether functional Leydig cells would be derived from each of the seminiferous tubules and interstitial tissue in vivo under circumstances in which there was no paracrine influence of one on the other. This was done by isolating seminiferous tubules and interstitium, encapsulating each in alginate to protect against the immune system, and implanting the tissues separately in castrated rats. By two months after the implantation of tubules, testosterone was elevated in the serum of the castrated rats. Consistent with this, Leydig cells were found to have formed on the surfaces of the implanted tubules. In contrast, there was no detectable elevation of testosterone after implantation of interstitial tissue, and no evidence of Leydig cells having formed. Additionally, we found that the newly formed Leydig cells produced testosterone ex vivo in response to LH, as determined by removing the tubule-containing alginate capsules from implanted rats and then incubating the tubules with LH in vitro. In contrast, and also consistent with in vitro results and with serum testosterone measurements, there was no evidence of steroidogenic cells in the alginate-encapsulated interstitium removed from the implanted rats, and no testosterone production ex vivo in response to LH by interstitial tissue obtained from the implants. These results, taken together, suggest that if, indeed, there are SLCs associated with interstitial tissue, the cells require factors to stimulate their differentiation that are not present when the tissue is separated from the remainder of the testis.
The production of testosterone-producing Leydig cells by the SLCs associated with the seminiferous tubules both in vitro and in vivo, but the failure of the interstitial tissue to give rise to testosterone-producing cells, suggested the possibility that the seminiferous tubules may provide the required factors for SLC differentiation. Indeed, by co-culturing the interstitium with seminiferous tubules in a dual-chamber system that allowed a free exchange of paracrine factors from the two tissues, testosterone-producing Leydig cells were generated in the interstitial tissue. Interestingly, the timing of the co-culture period was important; the seminiferous tubules were effective in inducing Leydig cell development from the intersititium when the two tissues were co-cultured during weeks 2–3, a time during which SLCs have been shown to differentiate (Li et al., 2016). The formation of testosterone-producing Leydig cells by seminiferous tubules in the absence of interstitial tissue, and by the interstitial tissue only when paracrine factors were provided by the tubules in co-culture experiments, strongly suggest that the interstitium has precursor cells but lacks critical factors provided by the seminiferous tubules. This emphasizes the uniqueness of the intratesticular environment in the development of Leydig cells, a conclusion that is consistent with previous observations that stem cells from other sources can be induced to form Leydig cells in vivo if transplanted into the interstitial compartment of the testis (Yazawa et al., 2006; Lue et al., 2007). The nature of the paracrine factors, and the cells from which they are derived, are not known as yet.
The observation that stem Leydig cells are capable of giving rise to testosterone-producing Leydig cells in vivo, in locations outside testis, could have clinical implications. Hypogonadism, which is common in aging men, may be linked to a number of metabolic and quality-of-life changes, including decreased lean body mass and bone mineral density, increased visceral fat, decreased libido and sexual function, altered mood, and fatigue (Carruthers, 2009; Lang et al., 2012; Morris and Channer, 2012). These changes can be partially overcome by exogenous testosterone administration (Katznelson et al., 1996; Wang et al., 1996; Steidle et al., 2003; Page et al., 2005; Seftel et al., 2015). However, it has been reported recently that administering testosterone may increase the risks of cardiovascular disorders and prostate tumorigenesis (Klotz, 2015; Yeap et al., 2015). Thus the availability of therapies that increase serum testosterone levels more physiologically, without the need to administer testosterone, could be of benefit to the many men with primary hypogonadism. The current studies suggest that it might be possible to elevate serum testosterone levels in individuals, and thus to treat testosterone deficiency, by implanting stem cells at locations outside testis. This approach seems more realistic for therapeutic purposes than injecting stem cells directly into the testis, as has been reported recently (Jiang et al., 2014; Yang et al., 2015). However, generating Leydig cells from stem cells outside the testis apparently requires critical seminiferous tubule-derived proliferation/differentiation factors. The nature and the regulation of such potential factors are currently under study.
Highlights.
Implanted alginate-encapsulated rat seminiferous tubules produced testosterone.
Implanted alginate-encapsulated interstititium alone did not produce testosterone.
The interstitium produced testosterone when co-cultured with tubules.
Acknowledgments
This work was supported by NIH grant R37 AG21092 (B.R.Z.) and by National Natural Science Foundation of China grants NSFC30972840 (H.C.) and NSFC81471411 (H.C.). These sources did not influence the study design, the collection, analysis or interpretation of data, or the writing of the paper.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barsoum SC, Milgram W, Mackay W, Coblentz C, Delaney KH, Kwiecien JM, Kruth SA, Chang PL. Delivery of recombinant gene product to canine brain with the use of microencapsulation. J Lab Clin Med. 2003;142:399–413. doi: 10.1016/j.lab.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Benton LX, Shan LX, Hardy MP. Differentiation of adult Leydig cells. J Steroid Biochem Mol Biol. 1995;53:61–68. doi: 10.1016/0960-0760(95)00022-r. [DOI] [PubMed] [Google Scholar]
- Carruthers M. Time for international action on treating testosterone deficiency syndrome. Aging Male. 2009;12:21–28. doi: 10.1080/13685530802699067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ge RS, Zirkin BR. Leydig cells: From stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Stanley E, Jin S, Zirkin BR. Stem Leydig cells: from fetal to aged animals. Birth Defects Res C Embryo Today. 2010;90:272–283. doi: 10.1002/bdrc.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167:935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defalco T, Saraswathula A, Briot A, Iruela-Arispe ML, Capel B. Testosterone levels influence mouse fetal Leydig cell progenitors through notch signaling. Biol Reprod. 2013;88:91, 1–12. doi: 10.1095/biolreprod.112.106138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603–5617. doi: 10.1016/j.biomaterials.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP. In search of rat stem Leydig cells: identification, isolation, and lineage-specific development. Proc Natl Acad Sci USA. 2006;103:2719–2724. doi: 10.1073/pnas.0507692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol. 2001;179:47–74. doi: 10.1016/s0303-7207(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Zirkin BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in testes of the pubertal rat. Endocrinology. 1989;124:762–770. doi: 10.1210/endo-124-2-762. [DOI] [PubMed] [Google Scholar]
- Jackson AE, O’Leary PC, Ayers MM, de Kretser DM. The effects of ethylene dimethane sulphonate (EDS) on rat Leydig cells: evidence to support a connective tissue origin of Leydig cells. Biol Reprod. 1986;35:425–437. doi: 10.1095/biolreprod35.2.425. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Cai B, Tuo Y, Wang J, Zang ZJ, Tu X, Gao Y, Su Z, Li W, Li G, Zhang M, Jiao J, Wan Z, Deng C, Lahn BT, Xiang AP. Characterization of Nestin-positive stem Leydig cells as a potential source for the treatment of testicular Leydig cell dysfunction. Cell Res. 2014;24:1466–1485. doi: 10.1038/cr.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Donachie K. Regeneration of Leydig cells in unilaterally cryptorchid rats: evidence for stimulation by local testicular factors. Cell Tissue Res. 1986;245:649–655. doi: 10.1007/BF00218568. [DOI] [PubMed] [Google Scholar]
- Kerr JB, Bartlett JM, Donachie K, Sharpe RM. Origin of regenerating Leydig cells in the testis of the adult rat. An ultrastructural, morphometric and hormonal assay study. Cell Tissue Res. 1987;249:367–377. doi: 10.1007/BF00215521. [DOI] [PubMed] [Google Scholar]
- Kilcoyne KR, Smith LB, Atanassova N, Macpherson S, McKinnell C, van den Driesche S, Jobling MS, Chambers TJ, De Gendt K, Verhoeven G, O’Hara L, Platts S, Renato de Franca L, Lara NL, Anderson RA, Sharpe RM. Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci USA. 2014;111:E1924–1932. doi: 10.1073/pnas.1320735111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinefelter GR, Hall PF, Ewing LL. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod. 1987;36:769–783. doi: 10.1095/biolreprod36.3.769. [DOI] [PubMed] [Google Scholar]
- Klotz L. Testosterone therapy and prostate cancer--safety concerns are well founded. Nat Rev Urol. 2015;12:48–54. doi: 10.1038/nrurol.2014.338. [DOI] [PubMed] [Google Scholar]
- Lang PO Samaras D, Samaras N. Testosterone replacement therapy in reversing “andropause”: what is the proof-of-principle? Rejuvenation Res. 2012;15:453–65. doi: 10.1089/rej.2012.1316. [DOI] [PubMed] [Google Scholar]
- Landreh L, Stukenborg JB, Söder O, Svechnikov K. Phenotype and steroidogenic potential of PDGFRα-positive rat neonatal peritubular cells. Mol Cell Endocrinol. 2013;372:96–104. doi: 10.1016/j.mce.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Li X, Wang Z, Jiang Z, Guo J, Zhang Y, Li C, Chung J, Folmer J, Liu J, Lian Q, Ge R, Zirkin BR, Chen H. Regulation of seminiferous tubule-associated stem Leydig cells in adult rat testes. Proc Natl Acad Sci U S A. 2016;113:2666–71. doi: 10.1073/pnas.1519395113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue Y, Erkkila K, Liu PY, Ma K, Wang C, Hikim AS, Swerdloff RS. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007;170:899–908. doi: 10.2353/ajpath.2007.060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machluf M, Orsola A, Boorjian S, Kershen R, Atala A. Microencapsulation of Leydig cells: a system for testosterone supplementation. Endocrinology. 2003;144:4975–4979. doi: 10.1210/en.2003-0411. [DOI] [PubMed] [Google Scholar]
- Miyabayashi K, Katoh-Fukui Y, Ogawa H, Baba T, Shima Y, Sugiyama N, Kitamura K, Morohashi K. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS One. 2013;8:e68050. doi: 10.1371/journal.pone.0068050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- Morris PD, Channer KS. Testosterone and cardiovascular disease in men. Asian J Androl. 2012;14:428–35. doi: 10.1038/aja.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odeh HM, Kleinguetl C, Ge R, Zirkin BR, Chen H. Regulation of the proliferation and differentiation of Leydig stem cells in the adult testis. Biol Reprod. 2014;90:123–129. doi: 10.1095/biolreprod.114.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orive G, Tam SK, Pedraz JL, Hallé JP. Biocompatibility of alginate-poly-L-lysine microcapsules for cell therapy. Biomaterials. 2006;27:3691–3700. doi: 10.1016/j.biomaterials.2006.02.048. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PJ, Morris ID, Baker PJ. Leydig cell re-generation and expression of cell signaling molecules in the germ cell-free testis. Reproduction. 2008;135:851–858. doi: 10.1530/REP-07-0529. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005;90:1502–1510. doi: 10.1210/jc.2004-1933. [DOI] [PubMed] [Google Scholar]
- Qin J, Tsai MJ, Tsai SY. Essential roles of COUP-TFII in Leydig cell differentiation and male fertility. PLoS One. 2008;3:e3285. doi: 10.1371/journal.pone.0003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LD, de França LR, Hess R, Cooke P. Characteristics of mitotic cells in developing and adult testes with observations on cell lineages. Tissue Cell. 1995;27:105–128. doi: 10.1016/s0040-8166(95)80015-8. [DOI] [PubMed] [Google Scholar]
- Seftel AD, Kathrins M, Niederberger C. Critical Update of the 2010 Endocrine Society Clinical Practice Guidelines for Male Hypogonadism: A Systematic Analysis. Mayo Clin Proc. 2015;90:1104–1115. doi: 10.1016/j.mayocp.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Stanley E, Lin CY, Jin S, Liu J, Sottas CM, Ge R, Zirkin BR, Chen H. Identification, proliferation, and differentiation of adult Leydig stem cells. Endocrinology. 2012;153:5002–5010. doi: 10.1210/en.2012-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidle C, Schwartz S, Jacoby K, Sebree T, Smith T, Bachand R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- Svechnikov K, Landreh L, Weisser J, Izzo G, Colón E, Svechnikova I, Söder O. Origin, development and regulation of human Leydig cells. Horm Res Paediatr. 2010;73:93–101. doi: 10.1159/000277141. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, De Rooij DG, Rommerts FF, van der Tweel I, Wensing CJ. Turnover time of Leydig cells and other interstitial cells in testes of adult rats. Arch Androl. 1989;23:105–111. doi: 10.3109/01485018908986831. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Huhtaniemi IT. Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update. 2015;21:310–328. doi: 10.1093/humupd/dmv008. [DOI] [PubMed] [Google Scholar]
- Toppari J, Kangasniemi M, Kaipia A, Mali P, Huhtaniemi I, Parvinen M. Stage- and cell-specific gene expression and hormone regulation of the seminiferous epithelium. J Electron Microsc Tech. 1991;19:203–214. doi: 10.1002/jemt.1060190207. [DOI] [PubMed] [Google Scholar]
- Tuck SP, Francis RM. Testosterone, bone and osteoporosis. Front Horm Res. 2009;37:123–132. doi: 10.1159/000176049. [DOI] [PubMed] [Google Scholar]
- Wang C, Eyre DR, Clark R, Kleinberg D, Newman C, Iranmanesh A, Veldhuis J, Dudley RE, Berman N, Davidson T, Barstow TJ, Sinow R, Alexander G, Swerdloff RS. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men--a clinical research center study. Clin Endocrinol Metab. 1996;81:3654–3662. doi: 10.1210/jcem.81.10.8855818. [DOI] [PubMed] [Google Scholar]
- Yang Y, Su Z, Xu W, Luo J, Liang R, Xiang Q, Zhang Q, Ge RS, Huang Y. Directed mouse embryonic stem cells into leydig-like cells rescue testosterone-deficient male rats in vivo. Stem Cells Dev. 2015;24:459–470. doi: 10.1089/scd.2014.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, Kajitani T, Shou Z, Umezawa A, Miyamoto K. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006;147:4104–4111. doi: 10.1210/en.2006-0162. [DOI] [PubMed] [Google Scholar]
- Yeap BB. Testosterone and cardiovascular disease risk. Curr Opin Endocrinol Diabetes Obes. 2015;22:193–202. doi: 10.1097/MED.0000000000000161. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang H, Yang Y, Liu H, Zhang Q, Xiang Q, Ge R, Su Z, Huang Y. NGF induces adult stem Leydig cells to proliferate and differentiate during Leydig cell regeneration. Biochem Biophys Res Commun. 2013;436:300–305. doi: 10.1016/j.bbrc.2013.05.098. [DOI] [PubMed] [Google Scholar]