Abstract
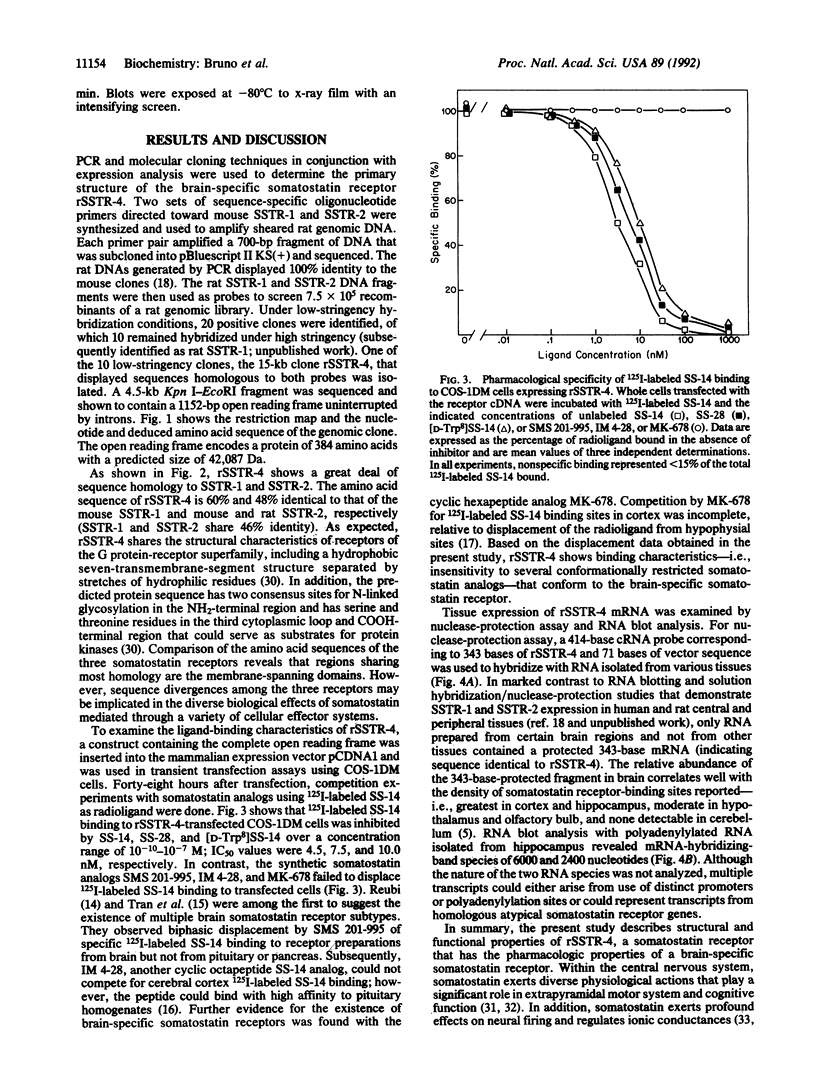
The PCR and conventional library screening were used to clone the brain-specific somatostatin receptor rSSTR-4 from a rat genomic library. The deduced amino acid sequence encodes a protein of 384 amino acids and displays structural and sequence homologies with members of the G protein-receptor superfamily. The amino acid sequence of rSSTR-4 is 60% and 48% identical to that of somatostatin receptors SSTR-1 and SSTR-2, respectively, two recently cloned subtypes. Competition curve analysis of the binding properties of the receptor transiently expressed in COS-1 cells revealed a higher apparent affinity for somatostatin 14 than for somatostatin 28. In contrast, the somatostatin analogs SMS 201-995, IM 4-28, and MK-678 failed to displace specific binding in transfected cells. These characteristics resemble the pharmacological binding properties of the previously described brain-specific somatostatin-receptor subtype. Examination of the tissue distribution of mRNA for rSSTR-4 revealed expression limited to various brain regions with highest levels in the cortex and hippocampus. Thus, based on the pharmacology and tissue localization of this receptor, we conclude that rSSTR-4 represents a brain-specific somatostatin receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger A., Labrie F., Borgeat P., Savary M., Cote J., Drouin J., Schally A. V., Coy D. H., Coy E. J., Sestanj K. Inhibition of growth hormone and thyrotropin release by growth hormone-release inhibiting hormone. Mol Cell Endocrinol. 1974 Oct;1(5):329–339. doi: 10.1016/0303-7207(74)90022-7. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P., Katzman R., Terry R. D. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature. 1980 Nov 20;288(5788):279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- DeNoble V. J., Hepler D. J., Barto R. A. Cysteamine-induced depletion of somatostatin produces differential cognitive deficits in rats. Brain Res. 1989 Mar 13;482(1):42–48. doi: 10.1016/0006-8993(89)90540-4. [DOI] [PubMed] [Google Scholar]
- Delfs J. R., Dichter M. A. Effects of somatostatin on mammalian cortical neurons in culture: physiological actions and unusual dose response characteristics. J Neurosci. 1983 Jun;3(6):1176–1188. doi: 10.1523/JNEUROSCI.03-06-01176.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., Lefkowitz R. J. A family of receptors coupled to guanine nucleotide regulatory proteins. Biochemistry. 1987 May 19;26(10):2657–2664. doi: 10.1021/bi00384a001. [DOI] [PubMed] [Google Scholar]
- Dorflinger L. J., Schonbrunn A. Somatostatin inhibits basal and vasoactive intestinal peptide-stimulated hormone release by different mechanisms in GH pituitary cells. Endocrinology. 1983 Nov;113(5):1551–1558. doi: 10.1210/endo-113-5-1551. [DOI] [PubMed] [Google Scholar]
- Epelbaum J., Tapia Arancibia L., Kordon C., Enjalbert A. Characterization, regional distribution, and subcellular distribution of 125I-Tyr1-somatostatin binding sites in rat brain. J Neurochem. 1982 Jun;38(6):1515–1523. doi: 10.1111/j.1471-4159.1982.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Grewe C., Aguilera G. Actions of growth hormone-releasing factor and somatostatin on adenylate cyclase and growth hormone release in rat anterior pituitary. Mol Cell Endocrinol. 1984 Oct;37(3):277–284. doi: 10.1016/0303-7207(84)90097-2. [DOI] [PubMed] [Google Scholar]
- Heiman M. L., Murphy W. A., Coy D. H. Differential binding of somatostatin agonists to somatostatin receptors in brain and adenohypophysis. Neuroendocrinology. 1987 Jun;45(6):429–436. doi: 10.1159/000124788. [DOI] [PubMed] [Google Scholar]
- Inoue M., Nakajima S., Nakajima Y. Somatostatin induces an inward rectification in rat locus coeruleus neurones through a pertussis toxin-sensitive mechanism. J Physiol. 1988 Dec;407:177–198. doi: 10.1113/jphysiol.1988.sp017409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluxen F. W., Bruns C., Lübbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch B. D., Dorflinger L. J., Schonbrunn A. Pertussis toxin blocks both cyclic AMP-mediated and cyclic AMP-independent actions of somatostatin. Evidence for coupling of Ni to decreases in intracellular free calcium. J Biol Chem. 1985 Oct 25;260(24):13138–13145. [PubMed] [Google Scholar]
- Koch B. D., Schonbrunn A. Characterization of the cyclic AMP-independent actions of somatostatin in GH cells. II. An increase in potassium conductance initiates somatostatin-induced inhibition of prolactin secretion. J Biol Chem. 1988 Jan 5;263(1):226–234. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Nemeroff C. B., Youngblood W. W., Manberg P. J., Prange A. J., Jr, Kizer J. S. Regional brain concentrations of neuropeptides in Huntington's chorea and schizophrenia. Science. 1983 Sep 2;221(4614):972–975. doi: 10.1126/science.6136092. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Spiess J. Evidence fore biosynthesis and differential post-translational proteolytic processing of different (pre)prosomatostatins in pancreatic islets. J Biol Chem. 1983 Jan 25;258(2):1121–1128. [PubMed] [Google Scholar]
- Raynor K., Reisine T. Analogs of somatostatin selectively label distinct subtypes of somatostatin receptors in rat brain. J Pharmacol Exp Ther. 1989 Nov;251(2):510–517. [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Reubi J. C. Evidence for two somatostatin-14 receptor types in rat brain cortex. Neurosci Lett. 1984 Aug 31;49(3):259–263. doi: 10.1016/0304-3940(84)90299-4. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Perrin M. H., Rivier J. E., Vale W. High affinity binding sites for a somatostatin-28 analog in rat brain. Life Sci. 1981 May 11;28(19):2191–2198. doi: 10.1016/0024-3205(81)90628-7. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Perrin M., Rivier J., Vale W. High affinity binding sites for somatostatin to rat pituitary. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1538–1545. doi: 10.1016/0006-291x(82)90963-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Scherer G., Schmid W., Zentgraf H., Schütz G. Isolation and characterization of the rat tyrosine aminotransferase gene. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1346–1350. doi: 10.1073/pnas.81.5.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikant C. B., Patel Y. C. Characterization of pituitary membrane receptors for somatostatin in the rat. Endocrinology. 1982 Jun;110(6):2138–2144. doi: 10.1210/endo-110-6-2138. [DOI] [PubMed] [Google Scholar]
- Tran V. T., Beal M. F., Martin J. B. Two types of somatostatin receptors differentiated by cyclic somatostatin analogs. Science. 1985 Apr 26;228(4698):492–495. doi: 10.1126/science.2858917. [DOI] [PubMed] [Google Scholar]
- Wang H. L., Reisine T., Dichter M. Somatostatin-14 and somatostatin-28 inhibit calcium currents in rat neocortical neurons. Neuroscience. 1990;38(2):335–342. doi: 10.1016/0306-4522(90)90032-y. [DOI] [PubMed] [Google Scholar]
- White J. D., Stewart K. D., McKelvy J. F. Measurement of neuroendocrine peptide mRNA in discrete brain regions. Methods Enzymol. 1986;124:548–560. doi: 10.1016/0076-6879(86)24039-2. [DOI] [PubMed] [Google Scholar]
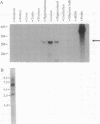
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeggari M., Viguerie N., Susini C., Esteve J. P., Vaysse N., Rivier J., Wunsch E., Ribet A. Characterization of pancreatic somatostatin binding sites with a 125I-somatostatin 28 analog. Peptides. 1986 Nov-Dec;7(6):953–959. doi: 10.1016/0196-9781(86)90120-8. [DOI] [PubMed] [Google Scholar]