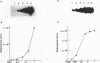Abstract
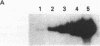
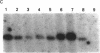
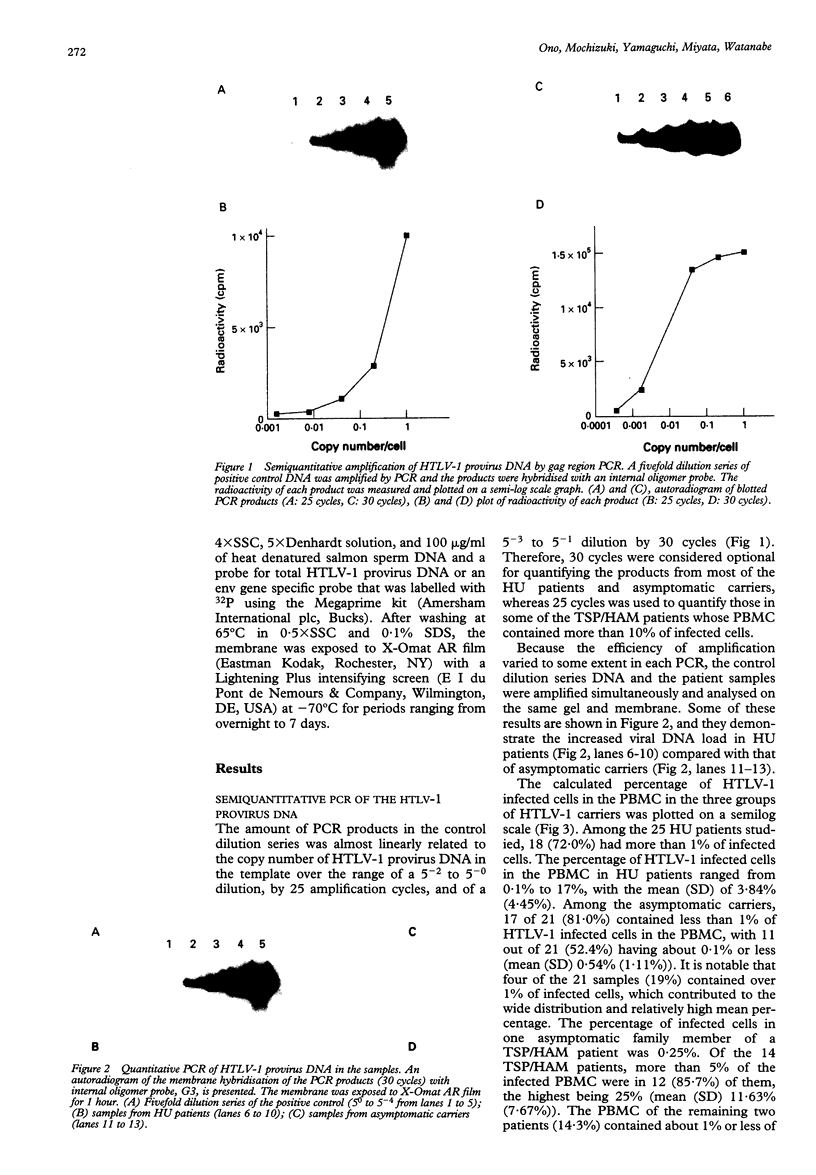
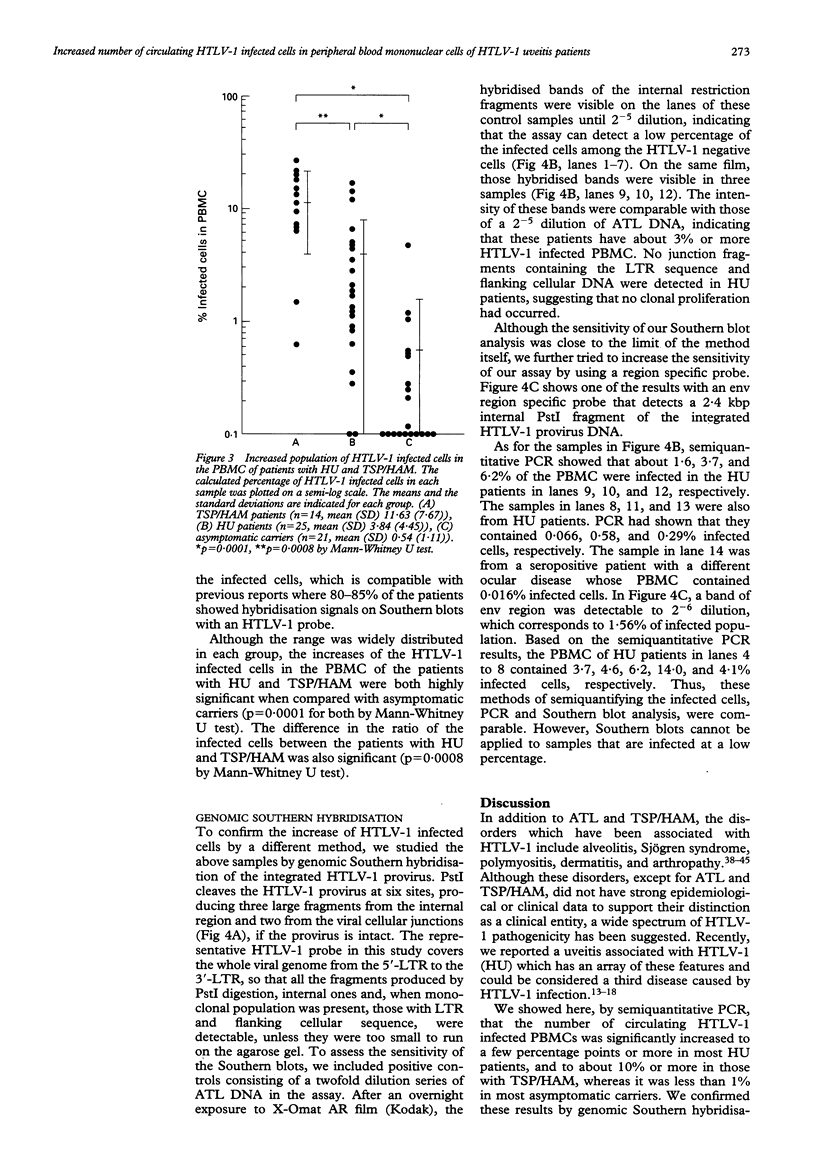
AIMS--This study aimed to characterise the status of viral infection in patients with HTLV-1 uveitis (HU) by quantifying the circulating HTLV-1 infected cells in the peripheral blood. METHODS--Genomic DNA samples of peripheral blood mononuclear cells (PBMC) were obtained from 25 patients with HU, 14 patients with tropical spastic paraparesis/HTLV-1 associated myelopathy (TSP/HAM), and 21 asymptomatic carriers of HTLV-1. Quantitative polymerase chain reaction (PCR) of the gag region of HTLV-1 provirus DNA was performed on these DNA samples. To confirm the PCR, genomic Southern blot hybridisation was performed to identify integrated HTLV-1 provirus. This procedure detected a few percent of HTLV-1 infected cells in the PBMC. RESULTS--Most of the HU patients had a significantly increased number of circulating HTLV-1 infected cells (mean (SD) 3.84% (4.45%) of the PBMC), whereas the percentage of infected cells in most asymptomatic carriers was less than 1% (0.54% (1.11%)). Most of the TSP/HAM patients also had a relatively high percentage (11.63% (7.67%)). The differences among these three groups were highly significant by the Mann-Whitney U test. CONCLUSION--The results suggested that the increase in the number of HTLV-1 infected cells is one base for the development of inflammatory HU lesions, as it is for TSP/HAM.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akizuki S., Nakazato O., Higuchi Y., Tanabe K., Setoguchi M., Yoshida S., Miyazaki Y., Yamamoto S., Sudou S., Sannomiya K. Necropsy findings in HTLV-I associated myelopathy. Lancet. 1987 Jan 17;1(8525):156–157. doi: 10.1016/s0140-6736(87)91984-2. [DOI] [PubMed] [Google Scholar]
- Arai N., Nomura D., Villaret D., DeWaal Malefijt R., Seiki M., Yoshida M., Minoshima S., Fukuyama R., Maekawa M., Kudoh J. Complete nucleotide sequence of the chromosomal gene for human IL-4 and its expression. J Immunol. 1989 Jan 1;142(1):274–282. [PubMed] [Google Scholar]
- Brew B. J., Price R. W. Another retroviral disease of the nervous system: chronic progressive myelopathy due to HTLV-1. N Engl J Med. 1988 May 5;318(18):1195–1197. doi: 10.1056/NEJM198805053181809. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Fujisawa J., Osame M., Toita M., Sonoda S., Kubota R., Ijichi S., Yoshida M. Frequent clonal proliferation of human T-cell leukemia virus type 1 (HTLV-1)-infected T cells in HTLV-1-associated myelopathy (HAM-TSP). Blood. 1992 Aug 15;80(4):1012–1016. [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Gessain A., Caudie C., Gout O., Vernant J. C., Maurs L., Giordano C., Malone G., Tournier-Lasserve E., Essex M., de-Thé G. Intrathecal synthesis of antibodies to human T lymphotropic virus type I and the presence of IgG oligoclonal bands in the cerebrospinal fluid of patients with endemic tropical spastic paraparesis. J Infect Dis. 1988 Jun;157(6):1226–1234. doi: 10.1093/infdis/157.6.1226. [DOI] [PubMed] [Google Scholar]
- Gessain A., Saal F., Gout O., Daniel M. T., Flandrin G., de The G., Peries J., Sigaux F. High human T-cell lymphotropic virus type I proviral DNA load with polyclonal integration in peripheral blood mononuclear cells of French West Indian, Guianese, and African patients with tropical spastic paraparesis. Blood. 1990 Jan 15;75(2):428–433. [PubMed] [Google Scholar]
- Greenberg S. J., Jacobson S., Waldmann T. A., McFarlin D. E. Molecular analysis of HTLV-I proviral integration and T cell receptor arrangement indicates that T cells in tropical spastic paraparesis are polyclonal. J Infect Dis. 1989 Apr;159(4):741–744. doi: 10.1093/infdis/159.4.741. [DOI] [PubMed] [Google Scholar]
- Ijichi S., Eiraku N., Osame M., Izumo S., Kubota R., Maruyama I., Matsumoto M., Niimura T., Sonoda S. Activated T lymphocytes in cerebrospinal fluid of patients with HTLV-I-associated myelopathy (HAM/TSP). J Neuroimmunol. 1989 Dec;25(2-3):251–254. doi: 10.1016/0165-5728(89)90143-4. [DOI] [PubMed] [Google Scholar]
- Itoyama Y., Minato S., Kira J., Goto I., Sato H., Okochi K., Yamamoto N. Altered subsets of peripheral blood lymphocytes in patients with HTLV-I associated myelopathy (HAM). Neurology. 1988 May;38(5):816–818. doi: 10.1212/wnl.38.5.816. [DOI] [PubMed] [Google Scholar]
- Iwakura Y., Tosu M., Yoshida E., Takiguchi M., Sato K., Kitajima I., Nishioka K., Yamamoto K., Takeda T., Hatanaka M. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science. 1991 Aug 30;253(5023):1026–1028. doi: 10.1126/science.1887217. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y., Ohara Y., Kobayashi I., Akizuki S. Infiltration of helper/inducer T lymphocytes heralds central nervous system damage in human T-cell leukemia virus infection. Am J Pathol. 1992 May;140(5):1003–1008. [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Gupta A., Mattson D., Mingioli E., McFarlin D. E. Immunological studies in tropical spastic paraparesis. Ann Neurol. 1990 Feb;27(2):149–156. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- Kim J. H., Durack D. T. Manifestations of human T-lymphotropic virus type I infection. Am J Med. 1988 May;84(5):919–928. doi: 10.1016/0002-9343(88)90072-1. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Kehrl J. H., Burton J., Tendler C. L., Jeang K. T., Danielpour D., Thevenin C., Kim K. Y., Sporn M. B., Roberts A. B. Transactivation of the transforming growth factor beta 1 (TGF-beta 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-beta 1 in adult T cell leukemia. J Exp Med. 1990 Jul 1;172(1):121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira J., Koyanagi Y., Yamada T., Itoyama Y., Goto I., Yamamoto N., Sasaki H., Sakaki Y. Increased HTLV-I proviral DNA in HTLV-I-associated myelopathy: a quantitative polymerase chain reaction study. Ann Neurol. 1991 Feb;29(2):194–201. doi: 10.1002/ana.410290214. [DOI] [PubMed] [Google Scholar]
- LaGrenade L., Hanchard B., Fletcher V., Cranston B., Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990 Dec 1;336(8727):1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Sugimoto M., Nakashima H., Imamura F., Kawano O., Uyama E., Takatsu K., Araki S. Spontaneous T cell proliferation and release of soluble interleukin-2 receptors in patients with HTLV-I-associated myelopathy. Am J Trop Med Hyg. 1990 Apr;42(4):365–373. doi: 10.4269/ajtmh.1990.42.365. [DOI] [PubMed] [Google Scholar]
- Minato S., Itoyama Y., Fujii N., Kira J., Goto I., Yamamoto N. Activated T cells in HTLV-I-associated myelopathy: autologous mixed lymphocyte reaction. Ann Neurol. 1989 Sep;26(3):398–401. doi: 10.1002/ana.410260316. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Malefijt R. D., Heike T., Fujisawa J., Takebe Y., Nishida J., Shlomai J., Yokota T., Yoshida M. Activation of T cell-derived lymphokine genes in T cells and fibroblasts: effects of human T cell leukemia virus type I p40x protein and bovine papilloma virus encoded E2 protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6547–6566. doi: 10.1093/nar/16.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Yoshida M., Arai K. T-cell activation signals and human T-cell leukemia virus type I-encoded p40x protein activate the mouse granulocyte-macrophage colony-stimulating factor gene through a common DNA element. Mol Cell Biol. 1988 Dec;8(12):5581–5587. doi: 10.1128/mcb.8.12.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Tajima K., Watanabe T., Yamaguchi K. Human T lymphotropic virus type 1 uveitis. Br J Ophthalmol. 1994 Feb;78(2):149–154. doi: 10.1136/bjo.78.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Watanabe T., Yamaguchi K., Tajima K., Yoshimura K., Nakashima S., Shirao M., Araki S., Miyata N., Mori S. Uveitis associated with human T lymphotropic virus type I: seroepidemiologic, clinical, and virologic studies. J Infect Dis. 1992 Oct;166(4):943–944. doi: 10.1093/infdis/166.4.943. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Watanabe T., Yamaguchi K., Takatsuki K., Yoshimura K., Shirao M., Nakashima S., Mori S., Araki S., Miyata N. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res. 1992 Mar;83(3):236–239. doi: 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Watanabe T., Yamaguchi K., Yoshimura K., Nakashima S., Shirao M., Araki S., Takatsuki K., Mori S., Miyata N. Uveitis associated with human T-cell lymphotropic virus type I. Am J Ophthalmol. 1992 Aug 15;114(2):123–129. doi: 10.1016/s0002-9394(14)73974-1. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Yamaguchi K., Takatsuki K., Watanabe T., Mori S., Tajima K. HTLV-I and uveitis. Lancet. 1992 May 2;339(8801):1110–1110. doi: 10.1016/0140-6736(92)90699-4. [DOI] [PubMed] [Google Scholar]
- Morgan O. S., Rodgers-Johnson P., Mora C., Char G. HTLV-1 and polymyositis in Jamaica. Lancet. 1989 Nov 18;2(8673):1184–1187. doi: 10.1016/s0140-6736(89)91793-5. [DOI] [PubMed] [Google Scholar]
- Nagasato K., Nakamura T., Ohishi K., Shibayama K., Motomura M., Ichinose K., Tsujihata M., Nagataki S. Active production of anti-human T-lymphotropic virus type I (HTLV-I) IgM antibody in HTLV-I-associated myelopathy. J Neuroimmunol. 1991 May;32(2):105–109. doi: 10.1016/0165-5728(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Osame M., Matsumoto M., Usuku K., Izumo S., Ijichi N., Amitani H., Tara M., Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987 Feb;21(2):117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Greene W. C. Molecular biology of the type I human T-cell leukemia virus (HTLV-I) and adult T-cell leukemia. J Clin Invest. 1991 Mar;87(3):761–766. doi: 10.1172/JCI115078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M., Nakashima H., Matsumoto M., Uyama E., Ando M., Araki S. Pulmonary involvement in patients with HTLV-I-associated myelopathy: increased soluble IL-2 receptors in bronchoalveolar lavage fluid. Am Rev Respir Dis. 1989 Jun;139(6):1329–1335. doi: 10.1164/ajrccm/139.6.1329. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Nakashima H., Watanabe S., Uyama E., Tanaka F., Ando M., Araki S., Kawasaki S. T-lymphocyte alveolitis in HTLV-I-associated myelopathy. Lancet. 1987 Nov 21;2(8569):1220–1220. doi: 10.1016/s0140-6736(87)91362-6. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Vernant J. C., Buisson G., Magdeleine J., De Thore J., Jouannelle A., Neisson-Vernant C., Monplaisir N. T-lymphocyte alveolitis, tropical spastic paresis, and Sjögren syndrome. Lancet. 1988 Jan 23;1(8578):177–177. doi: 10.1016/s0140-6736(88)92744-4. [DOI] [PubMed] [Google Scholar]
- Vernant J. C., Maurs L., Gessain A., Barin F., Gout O., Delaporte J. M., Sanhadji K., Buisson G., de-Thé G. Endemic tropical spastic paraparesis associated with human T-lymphotropic virus type I: a clinical and seroepidemiological study of 25 cases. Ann Neurol. 1987 Feb;21(2):123–130. doi: 10.1002/ana.410210204. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Nerenberg M., Cros D., Soto-Aguilar M. C. HTLV-I polymyositis in a patient also infected with the human immunodeficiency virus. N Engl J Med. 1989 Apr 13;320(15):992–995. doi: 10.1056/NEJM198904133201507. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K. Human T-lymphotropic virus type I in Japan. Lancet. 1994 Jan 22;343(8891):213–216. doi: 10.1016/s0140-6736(94)90994-6. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Osame M., Kawai H., Toita M., Kuwasaki N., Nishida Y., Hiraki Y., Takahashi K., Nomura K., Sonoda S. Increased replication of HTLV-I in HTLV-I-associated myelopathy. Ann Neurol. 1989 Sep;26(3):331–335. doi: 10.1002/ana.410260304. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Osame M., Usuku K., Matsumoto M., Igata A. Viruses detected in HTLV-I-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet. 1987 May 9;1(8541):1085–1086. doi: 10.1016/s0140-6736(87)90506-x. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Mochizuki M., Araki S., Miyata N., Yamaguchi K., Tajima K., Watanabe T. Clinical and immunologic features of human T-cell lymphotropic virus type I uveitis. Am J Ophthalmol. 1993 Aug 15;116(2):156–163. doi: 10.1016/s0002-9394(14)71279-6. [DOI] [PubMed] [Google Scholar]