Abstract
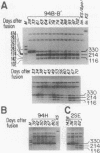
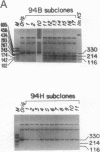
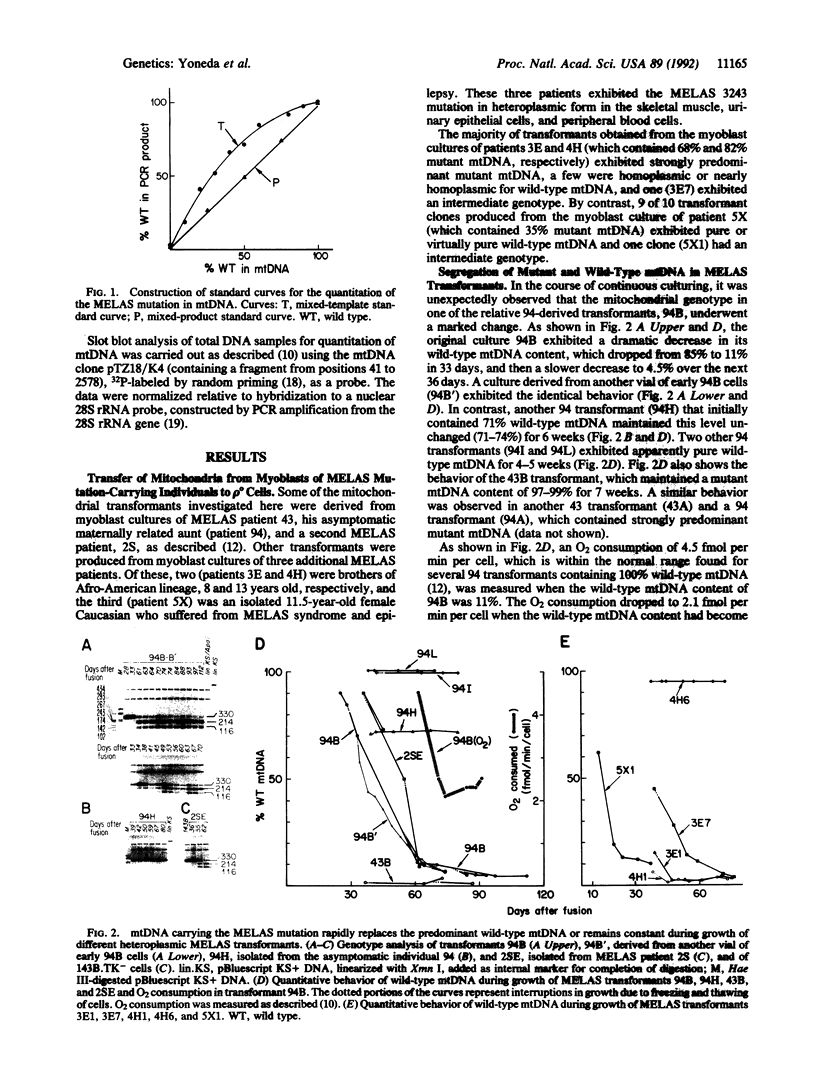
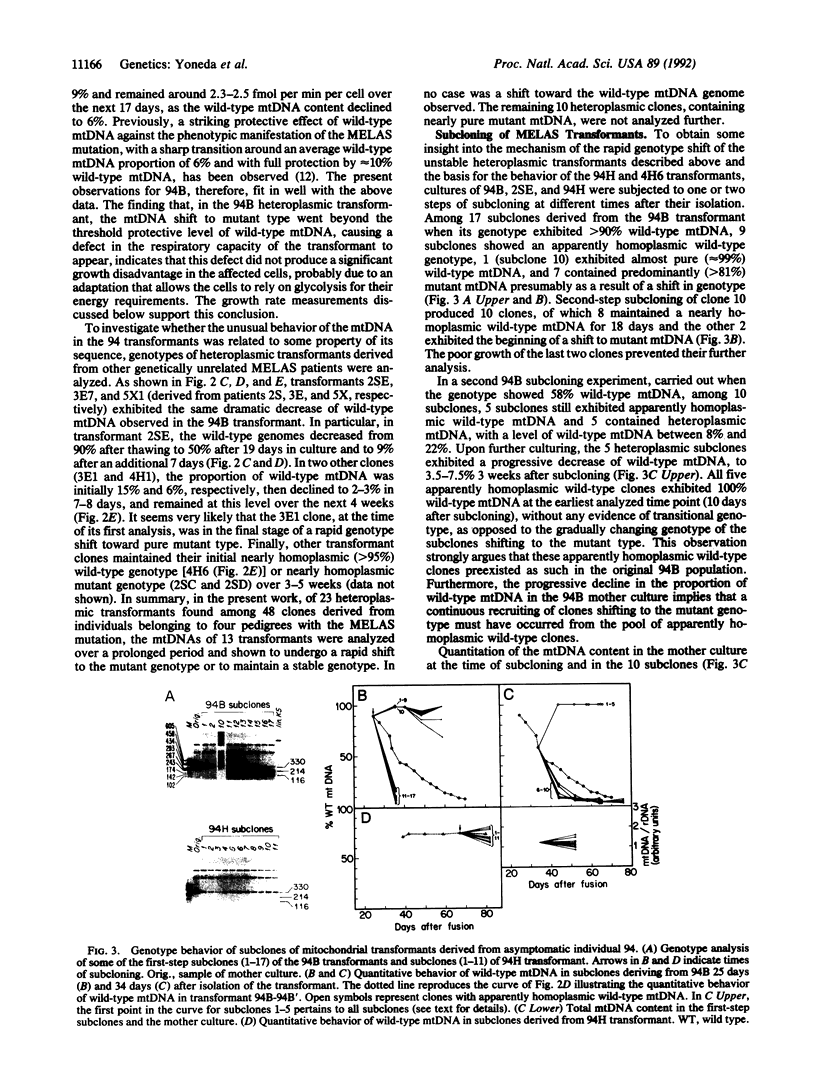
The segregation of mutant and wild-type mtDNA was investigated in transformants constructed by transferring human mitochondria from individuals belonging to four pedigrees with the MELAS encephalomyopathy-associated mtDNA mutation (MELAS is mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes) into human mtDNA-less (rho 0) cells. Five of 13 clonal cell lines containing mixtures of wild-type and mutant mtDNAs were found to undergo a rapid shift of their genotype toward the pure mutant type. The other 8 cell lines, which included 6 exhibiting nearly homoplasmic mutant mtDNA, on the contrary, maintained a stable genotype. Subcloning experiments and growth rate measurements clearly indicated that an intracellular replicative advantage of mutant mtDNA was mainly responsible for the dramatic shift toward the mutant genotype observed in the unstable cell lines.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanas V., Engel W. K. A new program for investigating adult human skeletal muscle grown aneurally in tissue culture. Neurology. 1975 Jan;25(1):58–67. doi: 10.1212/wnl.25.1.58. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Collins R. A., Stohl L. L., Goewert R. R., Lambowitz A. M. Deletion mutants of Neurospora crassa mitochondrial DNA and their relationship to the "stop-start" growth phenotype. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6032–6036. doi: 10.1073/pnas.77.10.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand H., Griffiths A. J., Court D. A., Cheng C. K. An extrachromosomal plasmid is the etiological precursor of kalDNA insertion sequences in the mitochondrial chromosome of senescent neurospora. Cell. 1986 Dec 5;47(5):829–837. doi: 10.1016/0092-8674(86)90525-8. [DOI] [PubMed] [Google Scholar]
- Bolhuis P. A., Bleeker-Wagemakers E. M., Ponne N. J., Van Schooneveld M. J., Westerveld A., Van den Bogert C., Tabak H. F. Rapid shift in genotype of human mitochondrial DNA in a family with Leber's hereditary optic neuropathy. Biochem Biophys Res Commun. 1990 Aug 16;170(3):994–997. doi: 10.1016/0006-291x(90)90490-e. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Martinuzzi A., Yoneda M., Daga A., Hurko O., Johns D., Lai S. T., Nonaka I., Angelini C., Attardi G. MELAS mutation in mtDNA binding site for transcription termination factor causes defects in protein synthesis and in respiration but no change in levels of upstream and downstream mature transcripts. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4221–4225. doi: 10.1073/pnas.89.10.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi B., de Margerie-Hottinguer H., Roman H. SUPPRESSIVENESS: A NEW FACTOR IN THE GENETIC DETERMINISM OF THE SYNTHESIS OF RESPIRATORY ENZYMES IN YEAST. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1065–1071. doi: 10.1073/pnas.41.12.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez I. L., Gorski J. L., Campen T. J., Dorney D. J., Erickson J. M., Sylvester J. E., Schmickel R. D. Variation among human 28S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7666–7670. doi: 10.1073/pnas.82.22.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kikuchi A., Takemitsu M., Goto Y., Nonaka I. Introduction of disease-related mitochondrial DNA deletions into HeLa cells lacking mitochondrial DNA results in mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10614–10618. doi: 10.1073/pnas.88.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurko O., McKee L., Zuurveld J. G. Transfection of human skeletal muscle cells with SV40 large T antigen gene coupled to a metallothionein promoter. Ann Neurol. 1986 Nov;20(5):573–582. doi: 10.1002/ana.410200504. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- King M. P., Attardi G. Injection of mitochondria into human cells leads to a rapid replacement of the endogenous mitochondrial DNA. Cell. 1988 Mar 25;52(6):811–819. doi: 10.1016/0092-8674(88)90423-0. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Momoi T., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun. 1990 Dec 31;173(3):816–822. doi: 10.1016/s0006-291x(05)80860-5. [DOI] [PubMed] [Google Scholar]
- Lazarus C. M., Earl A. J., Turner G., Küntzel H. Amplification of a mitochondrial DNA sequence in the cytoplasmically inherited 'ragged' mutant of Aspergillus amstelodami. Eur J Biochem. 1980 May;106(2):633–641. doi: 10.1111/j.1432-1033.1980.tb04611.x. [DOI] [PubMed] [Google Scholar]
- Lott M. T., Voljavec A. S., Wallace D. C. Variable genotype of Leber's hereditary optic neuropathy patients. Am J Ophthalmol. 1990 Jun 15;109(6):625–631. doi: 10.1016/s0002-9394(14)72429-8. [DOI] [PubMed] [Google Scholar]
- Meola G., Scarpini E., Velicogna M., Mottura A., Baron P. L., Beretta S., Scarlato G. Analysis of fibronectin expression during human muscle differentiation. Basic Appl Histochem. 1986;30(2):153–163. [PubMed] [Google Scholar]
- Mita S., Schmidt B., Schon E. A., DiMauro S., Bonilla E. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9509–9513. doi: 10.1073/pnas.86.23.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanske S., Moraes C. T., Lombes A., Miranda A. F., Bonilla E., Lewis P., Whelan M. A., Ellsworth C. A., DiMauro S. Widespread tissue distribution of mitochondrial DNA deletions in Kearns-Sayre syndrome. Neurology. 1990 Jan;40(1):24–28. doi: 10.1212/wnl.40.1.24. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., 4th, Wallace D. C. Oxidative phosphorylation diseases. Disorders of two genomes. Adv Hum Genet. 1990;19:267–330. [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Shoubridge E. A., Karpati G., Hastings K. E. Deletion mutants are functionally dominant over wild-type mitochondrial genomes in skeletal muscle fiber segments in mitochondrial disease. Cell. 1990 Jul 13;62(1):43–49. doi: 10.1016/0092-8674(90)90238-a. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ino H., Ohno K., Ohbayashi T., Ikebe S., Sano T., Ichiki T., Kobayashi M., Wada Y., Ozawa T. Mitochondrial DNA mutations in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Biochem Biophys Res Commun. 1991 Jan 31;174(2):861–868. doi: 10.1016/0006-291x(91)91497-z. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends Genet. 1989 Jan;5(1):9–13. doi: 10.1016/0168-9525(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wright R. M., Horrum M. A., Cummings D. J. Are mitochondrial structural genes selectively amplified during senescence in Podospora anserina? Cell. 1982 Jun;29(2):505–515. doi: 10.1016/0092-8674(82)90167-2. [DOI] [PubMed] [Google Scholar]