Abstract
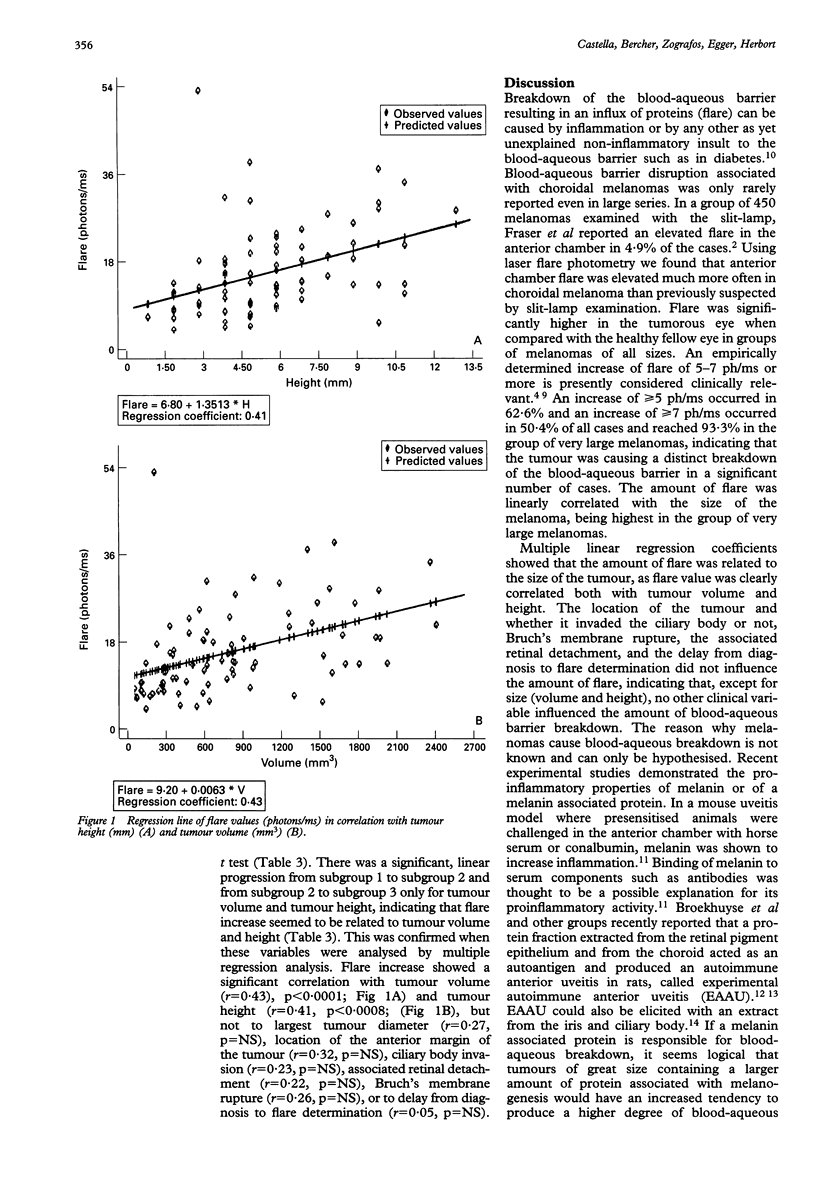
AIMS--Aqueous flare was used to determine the frequency and amount of blood-aqueous barrier breakdown and correlate it with tumour variables. METHODS--Aqueous flare was analysed prospectively by laser flare photometry in 139 consecutive patients seen in the oncology unit for choroidal melanoma. Both eyes of patients were examined with a laser flare cell meter in a standard fashion. RESULTS--Mean flare difference between healthy and tumour eyes was 3.01 (SD 2.5) photons per millisecond (ph/ms) in 32 cases of small melanomas (p < 0.0001), 10.74 (13.9) ph/ms in 92 cases of medium and large melanomas (p < 0.0001), and 19.23 (11.8) ph/ms in 15 cases of very large melanomas (p < 0.0001). This mean differential flare was significantly higher in medium and large than in small melanomas (p < 0.002) and in very large melanomas than in medium and large melanomas (p < 0.028). A difference of > or = 7 ph/ms between affected and healthy eyes was noted in 70 of 139 melanomas (50.4%). It was found in 3/32 small melanomas (9.4%), in 53/92 medium and large melanomas (57.6%), and in 14/15 very large melanomas (93.3%). CONCLUSION--Multiple linear regression analysis showed that flare was most strongly correlated with tumour volume (r = 0.43; p < 0.0001) and tumour height (r = 0.41; p < 0.0008).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altamirano D., Mermoud A., Pittet N., van Melle G., Herbort C. P. Aqueous humor analysis after Nd:YAG laser capsulotomy with the laser flare-cell meter. J Cataract Refract Surg. 1992 Nov;18(6):554–558. doi: 10.1016/s0886-3350(13)80441-5. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D., Winkens H. J., Van Vugt A. H. Experimental autoimmune anterior uveitis (EAAU), a new form of experimental uveitis. I. Induction by a detergent-insoluble, intrinsic protein fraction of the retinal pigment epithelium. Exp Eye Res. 1991 Apr;52(4):465–474. doi: 10.1016/0014-4835(91)90044-f. [DOI] [PubMed] [Google Scholar]
- Cantrill H. L., Cameron J. D., Ramsay R. C., Knobloch W. H. Retinal vascular changes in malignant melanoma of the choroid. Am J Ophthalmol. 1984 Apr;97(4):411–418. doi: 10.1016/s0002-9394(14)76123-9. [DOI] [PubMed] [Google Scholar]
- Favard C., Moukadiri H., Dorey C., Praloran V., Plouët J. Purification and biological properties of vasculotropin, a new angiogenic cytokine. Biol Cell. 1991;73(1):1–6. doi: 10.1016/0248-4900(91)90002-5. [DOI] [PubMed] [Google Scholar]
- Fraser D. J., Jr, Font R. L. Ocular inflammation and hemorrhage as initial manifestations of uveal malignant melanoma. Incidence and prognosis. Arch Ophthalmol. 1979 Jul;97(7):1311–1314. doi: 10.1001/archopht.1979.01020020053012. [DOI] [PubMed] [Google Scholar]
- Guex-Crosier Y., Pittet N., Herbort C. P. Evaluation of laser flare-cell photometry in the appraisal and management of intraocular inflammation in uveitis. Ophthalmology. 1994 Apr;101(4):728–735. doi: 10.1016/s0161-6420(13)31050-1. [DOI] [PubMed] [Google Scholar]
- Herbort C. P., Mermoud A., Schnyder C., Pittet N. Anti-inflammatory effect of diclofenac drops after argon laser trabeculoplasty. Arch Ophthalmol. 1993 Apr;111(4):481–483. doi: 10.1001/archopht.1993.01090040073033. [DOI] [PubMed] [Google Scholar]
- Kaya M., Edward D. P., Tessler H., Hendricks R. L. Augmentation of intraocular inflammation by melanin. Invest Ophthalmol Vis Sci. 1992 Mar;33(3):522–531. [PubMed] [Google Scholar]
- Kincaid M. C., Folberg R., Torczynski E., Zakov Z. N., Shore J. W., Liu S. J., Planchard T. A., Weingeist T. A. Complications after proton beam therapy for uveal malignant melanoma. A clinical and histopathologic study of five cases. Ophthalmology. 1988 Jul;95(7):982–991. doi: 10.1016/s0161-6420(88)33092-7. [DOI] [PubMed] [Google Scholar]
- Küchle M., Nguyen N. X., Naumann G. O. Aqueous flare in eyes with choroidal malignant melanoma. Am J Ophthalmol. 1992 Feb 15;113(2):207–208. doi: 10.1016/s0002-9394(14)71538-7. [DOI] [PubMed] [Google Scholar]
- MacFaul P. A., Morgan G. Histopathological changes in malignant melanomas of the choroid after cobalt plaque therapy. Br J Ophthalmol. 1977 Mar;61(3):221–228. doi: 10.1136/bjo.61.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty A. P., Spalton D. J., Moriarty B. J., Shilling J. S., Ffytche T. J., Bulsara M. Studies of the blood-aqueous barrier in diabetes mellitus. Am J Ophthalmol. 1994 Jun 15;117(6):768–771. doi: 10.1016/s0002-9394(14)70320-4. [DOI] [PubMed] [Google Scholar]
- Oshika T., Kato S., Funatsu H. Quantitative assessment of aqueous flare intensity in diabetes. Graefes Arch Clin Exp Ophthalmol. 1989;227(6):518–520. doi: 10.1007/BF02169443. [DOI] [PubMed] [Google Scholar]
- Rahi A. H., Agrawal P. K. Prognsotic parameters in choroidal melanomata. Trans Ophthalmol Soc U K. 1977 Sep;97(3):368–372. [PubMed] [Google Scholar]
- Sawa M. Clinical application of laser flare-cell meter. Jpn J Ophthalmol. 1990;34(3):346–363. [PubMed] [Google Scholar]
- Sawa M., Tsurimaki Y., Tsuru T., Shimizu H. New quantitative method to determine protein concentration and cell number in aqueous in vivo. Jpn J Ophthalmol. 1988;32(2):132–142. [PubMed] [Google Scholar]
- Shah S. M., Spalton D. J., Taylor J. C. Correlations between laser flare measurements and anterior chamber protein concentrations. Invest Ophthalmol Vis Sci. 1992 Sep;33(10):2878–2884. [PubMed] [Google Scholar]
- Zirm M. Proteins in aqueous humor. Adv Ophthalmol. 1980;40:100–172. [PubMed] [Google Scholar]


