Abstract
Context:
The epidemiology and outcome of critical illness in Mongolia remain undefined.
Aim:
The aim of this study was to evaluate the epidemiology and outcome of critical illness in Mongolia.
Settings and Design:
This is a multicenter, prospective, observational cohort study including 19 Mongolian centers.
Materials and Methods:
Demographic, clinical, and outcome data of patients >15 years admitted to the Intensive Care Units (ICUs) were collected during a 6-month period.
Statistical Analysis:
Descriptive methods, Mann–Whitney-U test, Fisher's exact or Chi-square test, and logistic regression analyses were used for statistical analysis.
Results:
Two thousand and thirty-two patients (53.6% male) with a median age of 49 years (36–62 years) were included. The most frequent ICU admission diagnoses were stroke (17.4%), liver failure (9.2%), heart failure (9%), infection (8.3%), severe trauma (7.5%), traumatic brain injury (7.1%), acute abdomen (7%), pre-eclampsia/eclampsia (5.8%), renal failure (3.9%), and postoperative care following elective and emergency surgeries (3.2%). ICU mortality was 23.5% in the study population and 26.6% when maternal cases were excluded. The five ICU admission diagnoses with the highest ICU mortality were lung tuberculosis (51.9%), traumatic brain injury (42.1%), liver failure (33.7%), stroke (31.9%), and infection (30.8%). The five ICU admission diagnoses causing most death cases were stroke (n = 113), liver failure (n = 63), traumatic brain injury (n = 61), infection (n = 52), and acute abdomen (n = 38).
Conclusion:
Critical illness in Mongolia affects younger patients compared to high-income countries. ICU admission diagnoses are similar with a particularly high incidence of stroke and liver failure. ICU mortality is approximately 25% with most deaths caused by stroke, liver failure, and traumatic brain injury.
Keywords: Asia, critical illness, epidemiology, Intensive Care Unit, Mongolia, outcome, resource-limited
INTRODUCTION
Mongolia is a Central Asian lower-middle-income country which is home to three million people.[1] Intensive care medicine in Mongolia is an under-resourced and under-developed medical specialty.[2,3] Although the national capacity of Intensive Care Unit (ICU) beds both for adult (8.1 ICU beds/100,000 inhabitants) and pediatric (3.6 ICU beds/100,000 inhabitants) patients compare favorably to other low- and middle-income countries (unpublished data), lack of equipment as well as adequately trained staff potentially makes these ICUs of only limited value for the management of critically ill patients. While only small reports from selected ICUs have been published,[4,5] the epidemiology and outcome of critical illness in Mongolia remain undefined. Knowledge of these factors would assist to understand the needs of critically ill patients and identify future directions to improve intensive care medicine in Mongolia.
In this study, we evaluated the ICU admission diagnoses and ICU mortality rates of a large collection of adult critically ill patients throughout Mongolia.
MATERIALS AND METHODS
This analysis was designed as a multicenter, prospective, observational cohort study which was performed from March 1, 2015 to August 31, 2015. The study protocol was approved by the Ethics Committee of the Mongolian National University of Medical Sciences. As only anonymous data were collected and no therapeutic changes were performed, written informed consent was waived by the committee.
Study sites
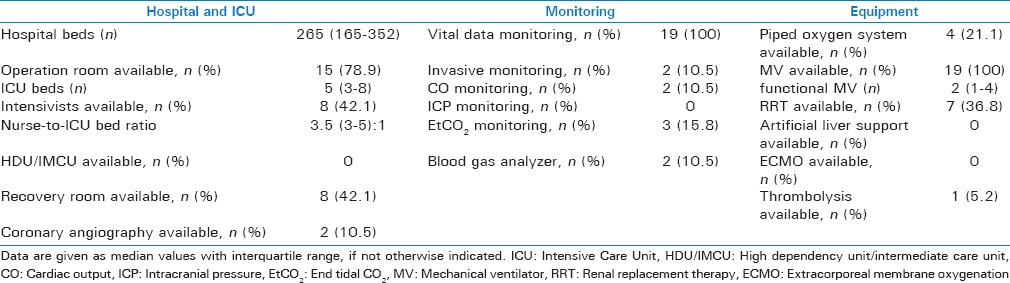
Nineteen out of the 42 Mongolian hospitals which run an adult ICU service participated in this study. In the capital city of Ulaanbaatar which is home to approximately half of the Mongolian population, five tertiary level referral teaching hospitals and five secondary level hospitals were included. In the provinces, nine secondary level provincial hospitals were selected based on their willingness to take part in the study and their geographical location to form a collective best representative of all parts of Mongolia [Figure 1]. Table 1 shows the characteristics of the study hospitals and their ICUs. No primary level hospital participated in this study as none of these hospitals in Mongolia runs an ICU. All critically ill patients admitted to primary level hospitals are transferred to the ICU service of the closest provincial secondary level hospital.
Figure 1.
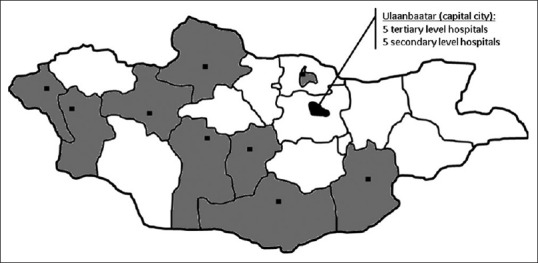
Map of Mongolia indicating the geographical location and catchment area of the 19 participating study sites
Table 1.
Characteristics of the 19 study sites
Study patients
All patients who were admitted to one of the study ICUs during the observation period were eligible for the study entry. Patients <15 years of age were excluded from the study.
Data collection
The following data were collected from all the study patients: Age, gender, origin of admission, admission diagnosis, need for emergency surgery, the Modified Early Warning Score,[6] the South African Triage Score[7] in case of trauma as the ICU admission diagnosis, access to mechanical ventilation, invasive hemodynamic monitoring (defined as the possibility to measure at least invasive arterial blood pressure) and/or renal replacement therapy, length of stay in the ICU, and ICU mortality. In case of death, autopsy results or the clinically suspected cause of death were documented. All ICU admission diagnoses were retrospectively classified into one of the following diagnostic categories: Medical, neurological, trauma (including traumatic brain injury), maternal, infection, surgical (nontrauma), and others.
Statistical analysis
No sample size calculations were performed. The SPSS software package was used for all the statistical analyses (SPSS Inc., SPSS IBM, Chicago, Illinois, United States of America). Descriptive methods were used to present data with categorical variables given as absolute numbers and percentage and continuous variables as median values with interquartile ranges. Comparisons between secondary and tertiary level hospitals were made using the Mann–Whitney-U test, Fisher's exact test, or Chi-square test, as appropriate. The risk of ICU deaths (odds ratio with 95% confidence interval) was calculated for each diagnostic category and admission diagnosis with binary logistic regression analyses using the diagnostic category or admission diagnosis with the lowest ICU mortality as a reference. All analyses were performed twice, once unadjusted and once adjusted for the Modified Early Warning Score which was included as an independent variable. P < 0.05 was considered to be statistically significant.
RESULTS
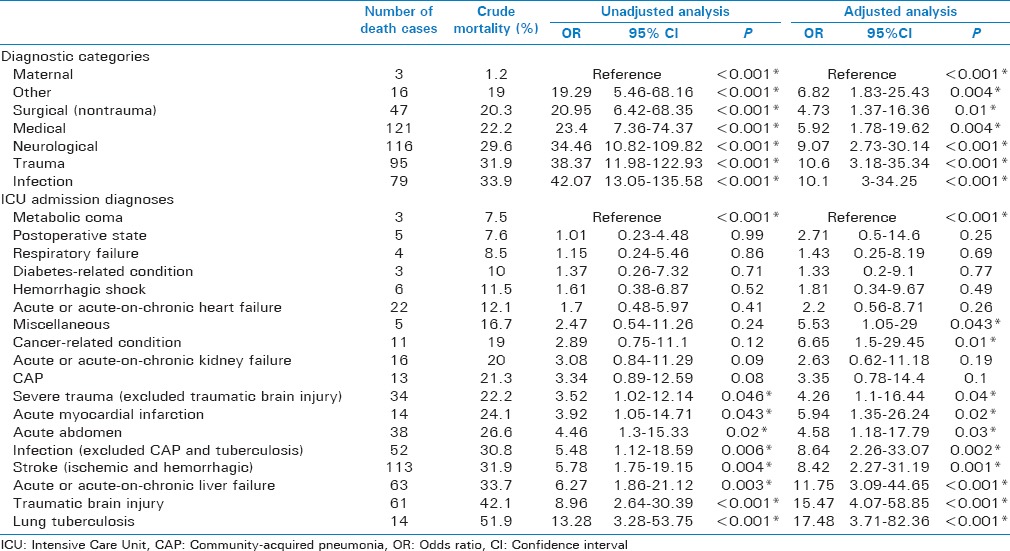
During the observation period, 2032 patients were included in the study. The median number of patients per site was 85 (interquartile range, 52–163). Demographic and clinical data of the study population are shown in Table 2. Study patients admitted to tertiary level hospitals were younger, more often females, differed in the origin of admission, the diagnostic categories, had lower Modified Early Warning Score but higher South African Triage Score counts, more frequent access to invasive hemodynamic monitoring and renal replacement therapy, as well as a higher ICU mortality (if maternal cases were excluded). The most common ICU admission diagnoses are shown in Tables 3 and 4 (total population and categorized age groups). Figure 2 displays ICU mortality rates categorized by age groups. Crude mortality rates as well as the adjusted and unadjusted risk of death for each diagnostic category and ICU admission diagnosis are shown in Table 5. Infection was the diagnostic category with the highest ICU mortality, whereas medical admissions were associated with the highest number of ICU deaths. Lung tuberculosis was the ICU admission diagnosis with the highest ICU mortality. Most ICU deaths were caused by stroke (ischemic and hemorrhagic).
Table 2.
Demographic and clinical data of the study population
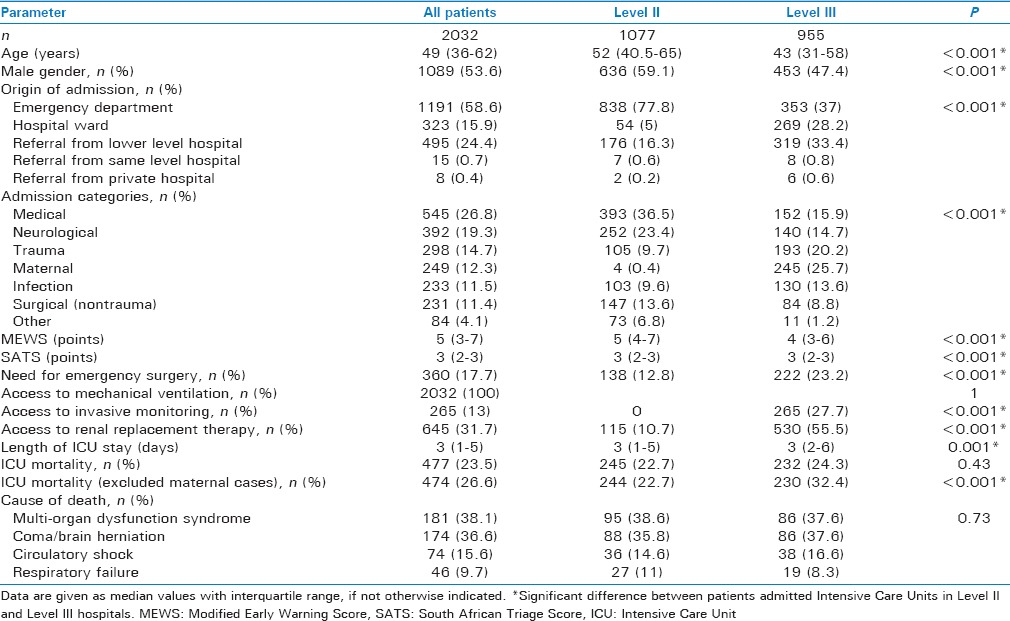
Table 3.
Description of the 20 most common Intensive Care Unit admission diagnoses in all study patients
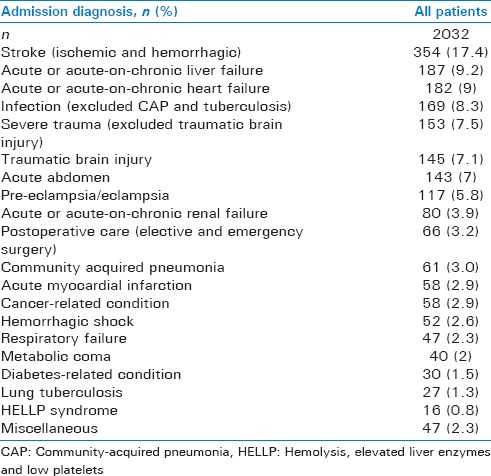
Table 4.
The five most common Intensive Care Unit admission diagnoses and related Intensive Care Unit mortalities per age group
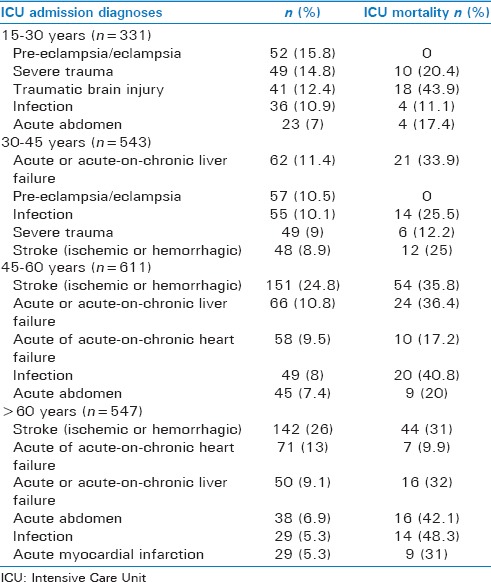
Figure 2.
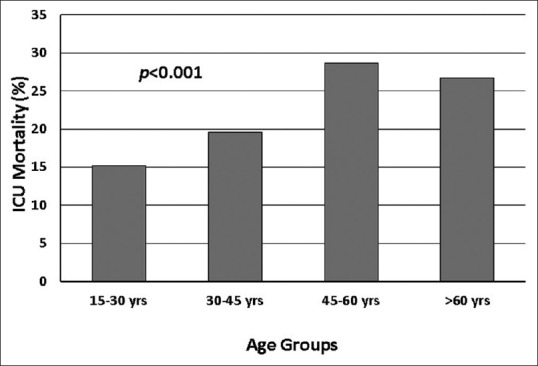
Differences between Intensive Care Unit mortality between age groups
Table 5.
Crude mortality rates and risk of death by diagnostic categories and admission diagnoses
DISCUSSION
This is the largest systematic evaluation of the epidemiology and outcome of critical illness in adults in Mongolia. Compared to the reports from high-income countries,[4,8,9,10] the demographic pattern of critically ill patients shares similarities in the gender ratio with a small over-representation of males, but differs in the median age which is 10–15 years younger in the Mongolian cohort. On the other hand, Mongolian ICU patients were relevantly older than critically ill patients in Sub-Saharan Africa.[11,12] With few exceptions, the disease spectrum causing critical illness in Mongolia resembles the one seen in high-income countries and countries located in the same geographical latitude as Mongolia. Yet, some critical illnesses such as stroke or liver failure appear to occur at a particularly high incidence.
It was outside the scope of our survey to determine the specific etiologies of the reported diseases. However, based on the published literature, certain assumptions can be made. Poor control of chronic arterial hypertension and a high prevalence of cardiovascular risk factors (e.g., fatty diet, high alcohol consumption) in the Mongolian population[13] could, for example, explain the high incidence of ischemic or hemorrhagic stroke. Similarly, an exceptionally high prevalence of liver cancer and other hepatic diseases such as chronic hepatitis viral infection in Mongolia[14] most likely explains the high rate of acute and acute-on-chronic liver failure in this ICU cohort. On the other hand, the number of patients admitted to the ICU because of acute myocardial infarction was low despite the fact that coronary heart disease is the most common cause of death in the country.[14] Considering the fact that no other high dependency or intermediate care facilities existed in the study centers during the observation period, one must assume that a relevant proportion of acute myocardial infarction patients were managed without intensive care support. Since the central referral maternal hospital, which participated in this study, cares for most critically ill obstetric cases in the capital city and for referral cases from the provinces, obstetrical diseases as a reason for ICU admission could have been overestimated in our analysis. Finally, it is interesting that only 3.2% of the study patients were admitted to the ICU for postoperative care after elective or emergency surgery (excluding surgery for severe trauma or acute abdomen). While recovery rooms frequently existed in those study centers running an operation theater, none of them admitted ventilated patients after the surgery. This observation is particularly astonishing in the light of the fact that Mongolia has one of the highest global prevalence rates of cancers typically requiring surgery (e.g., liver, gastric, or esophageal cancers).[14]
The unadjusted risk of ICU death was highest for critical illness due to infection as the diagnostic category and due to lung tuberculosis, traumatic brain injury, and liver failure as ICU admission diagnosis. Given the high rate of traumatic brain injury, liver failure, and stroke as admission diagnoses, these diseases resulted in the highest numbers of death in the ICU. While traumatic brain injury was monthly observed in young patients, both stroke and liver failure were predominantly seen in patients aged > 30 years. The incidence and mortality rates of traumatic brain injury in Mongolian ICUs is similarly high as reported from other resource-limited settings.[15,16] This finding confirms the delicate role of traumatic brain injury as the leading cause of death in young adults in middle- and low-income countries.[17] Social and economic consequences are likely to be immense, both in Mongolia and globally.
Our study indicates that efforts to develop and progress intensive care medicine in Mongolia should pay particular attention to neurocritical care. Improvement of the ICU management of patients with an infection and those with liver failure is likely to save a relevant number of lives, as well. All of these measures, however, need to go hand in hand with nationwide strategies to improve the management of the underlying disease (e.g., thrombolysis, neurointervention and neurosurgery for stroke) and its specific risk factors (e.g., control of chronic arterial hypertension, reduction of hepatitis transmission and alcohol consumption).
Certain limitations need to be taken into account when interpreting our study results. First, we did not include all ICUs in Mongolia and therefore cannot report on the entire adult ICU patient population during the observation period. Yet, given the fact that we included 19 ICUs whose catchment areas covered about half of the Mongolian territory and that the participating centers were well distributed over the country [Figure 1], it is unlikely that inclusion of more ICUs would have relevantly changed our study results. Second, our observation period spanned 6 months from March until August and did not include the Mongolian winter months with the lowest temperatures (typically January and February). It is, thus, possible that the incidence of certain diseases that are more common during winter such as respiratory tract infections have been underestimated in our report. In addition, we did not specifically address the ethical considerations such as those associated with end-of-life care in the study ICUs. Finally, our study did not include children. Therefore, our results must not be extrapolated to critically ill children (<15 years of age).
CONCLUSION
Critical illness in Mongolia affects younger patients compared to high-income countries. ICU admission diagnoses are similar with a particularly high incidence of stroke and liver failure. ICU mortality is approximately 25% with most deaths caused by stroke, liver failure, and traumatic brain injury.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thank all ICU staff of the participating study centers for their invaluable time, efforts, and support for this study.
REFERENCES
- 1.The World Factbook: Mongolia. [Last accessed on 2016 Apr 09]. Available from: https://www.cia.gov/library/publications/the.world.factbook/geos/mg.html .
- 2.Dünser MW, Bataar O, Tsenddorj G, Lundeg G, Jochberger S, Jakob S Helfen Berührt Study Team. Intensive care medicine in Mongolia's 3 largest cities: Outlining the needs. J Crit Care. 2009;24:469.e1–6. doi: 10.1016/j.jcrc.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Bataar O, Lundeg G, Tsenddorj G, Jochberger S, Grander W, Baelani I, et al. Nationwide survey on resource availability for implementing current sepsis guidelines in Mongolia. Bull World Health Organ. 2010;88:839–46. doi: 10.2471/BLT.10.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dünser MW, Bataar O, Tsenddorj G, Lundeg G, Torgersen C, Romand JA, et al. Differences in critical care practice between an industrialized and a developing country. Wien Klin Wochenschr. 2008;120:600–7. doi: 10.1007/s00508-008-1064-8. [DOI] [PubMed] [Google Scholar]
- 5.Dünser MW, Bataar O, Rusher AH, Hasibeder WR, Tsenddorj G Helfen Berührt Study Team. Report from Mongolia – How much do we know about the incidence of rare cases in less developed countries: A case series. J Med Case Rep. 2008;2:358. doi: 10.1186/1752-1947-2-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbe CP, Davies RG, Williams E, Rutherford P, Gemmell L. Effect of introducing the Modified Early Warning score on clinical outcomes, cardio-pulmonary arrests and intensive care utilisation in acute medical admissions. Anaesthesia. 2003;58:797–802. doi: 10.1046/j.1365-2044.2003.03258.x. [DOI] [PubMed] [Google Scholar]
- 7.Bell SA, Oduro G, Ampong P, Oteng R, Donkor P. The implementation of the South African Triage Score (SATS) in an urban teaching hospital, Ghana. Afr J Emerg Med. 2014;4:71–5. doi: 10.1016/j.afjem.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groeger JS, Guntupalli KK, Strosberg M, Halpern N, Raphaely RC, Cerra F, et al. Descriptive analysis of critical care units in the United States: Patient characteristics and Intensive Care Unit utilization. Crit Care Med. 1993;21:279–91. doi: 10.1097/00003246-199302000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Caironi P, Tognoni G, Masson S, Fumagalli R, Pesenti A, Romero M, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–21. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 10.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370:1583–93. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 11.Baker T, Schell CO, Lugazia E, Blixt J, Mulungu M, Castegren M, et al. Vital signs directed therapy: Improving care in an Intensive Care Unit in a low-income country. PLoS One. 2015;10:e0144801. doi: 10.1371/journal.pone.0144801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawe HR, Mfinanga JA, Lidenge SJ, Mpondo BC, Msangi S, Lugazia E, et al. Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int Health Hum Rights. 2014;14:26. doi: 10.1186/1472-698X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang L, Xu T, Li H, Zhang M, Wang A, Tong W, et al. Hypertension, alcohol drinking and stroke incidence: A population-based prospective cohort study among inner Mongolians in China. J Hypertens. 2014;32:1091–6. doi: 10.1097/HJH.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 14.World Health Rankings. Health Profile: Mongolia. [Last accessed on 2016 Apr 09]. Available from: http://www.worldlifeexpectancy.com/country.health.profile/mongolia .
- 15.Tran TM, Fuller AT, Kiryabwire J, Mukasa J, Muhumuza M, Ssenyojo H, et al. Distribution and characteristics of severe traumatic brain injury at Mulago National Referral Hospital in Uganda. World Neurosurg. 2015;83:269–77. doi: 10.1016/j.wneu.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf AS, Suleiman ZA, Olaoye I, Bolaji BO, Adeleke NA, Odebode TO. Outcomes of intensive care management of traumatic brain injury in a resource-challenged tertiary health centre. West Afr J Med. 2014;33:136–40. [PubMed] [Google Scholar]
- 17.Hyder AA, Wunderlich CA, Puvanachandra P, Gururaj G, Kobusingye OC. The impact of traumatic brain injuries: A global perspective. NeuroRehabilitation. 2007;22:341–53. [PubMed] [Google Scholar]


