Abstract
Multifunctional T cells have been shown to be protective in chronic viral infections. In mycobacterial infections, however, evidence for a protective role of multifunctional T cells remains inconclusive. Short-term cultures of peripheral blood mononuclear cells stimulated with the Mycobacterium tuberculosis RD1 antigens 6-kDa early secretory antigenic target (ESAT6) and 10-kDa culture filtrate antigen (CFP10), which are induced in the early infection phase, have been mainly used to assess T cell multifunctionality, although long-term culture assays have been proposed to be more sensitive than short-term assays for assessment of memory T cells, which are essential for long-term immunity. Here we used a long-term culture assay system to study the T cell immune responses to the M. tuberculosis latency-associated DosR antigens and reactivation-associated Rpf antigens, compared to ESAT6 and CFP10, in patients with pulmonary tuberculosis (PTB) and household contacts of PTB patients with long-term latent tuberculosis infection (ltLTBI), in a community in which M. tuberculosis is endemic. Our results showed that the DosR antigens Rv1737c (narK2) and Rv2029c (pfkB) and the Rv2389c (rpfD) antigen of M. tuberculosis induced higher frequencies of CD4+ or CD8+ mono- or bifunctional (but not multifunctional) T cells producing interferon gamma (IFN-γ) and/or tumor necrosis alpha (TNF-α) in ltLTBI, compared to PTB. Moreover, the frequencies of CD4+ and/or CD8+ T cells with a CD45RO+ CD27+ phenotype were higher in ltLTBI than in PTB. Thus, the immune responses to selected DosR and Rpf antigens may be associated with long-term latency, correlating with protection from M. tuberculosis reactivation in ltLTBI. Further study of the functional and memory phenotypes may contribute to further discrimination between the different states of M. tuberculosis infections.
INTRODUCTION
Upon Mycobacterium tuberculosis infection, T cell populations (including Th1, Th2, Th17, and T regulatory cells) are induced that display both proinflammatory and anti-inflammatory responses, which are finely coordinated by secreted cytokines. Among the cytokines, interferon gamma (IFN-γ), tumor necrosis alpha (TNF-α), and interleukin 2 (IL-2) are considered major players in the Th1 response. Targeting of the ifng gene in mice, leading to deficient IFN-γ production, resulted in increased susceptibility to M. tuberculosis infection, identifying this cytokine as critical for host defense (1, 2). Regulated levels of IFN-γ are also important for the control of mycobacterial infections in humans. Mutations in the IFNGR genes or in genes that control IFN-γ production or signal transduction, such as IL12B, IL12RB1, IFNGR1, IFNGR2, STAT1, ISG15, IRF8, NEMO, and CYBB, have been all associated with susceptibility to mycobacterial infections (3, 4). In addition to IFN-γ, TNF-α plays a significant role in the promotion of monocyte and macrophage effector mechanisms and the maintenance of granuloma integrity (5, 6), contributing to infection control. The increased incidence of tuberculosis (TB) in autoimmune disease patients treated with anti-TNF-α antibodies (7) underscores the importance of this cytokine. Finally, IL-2 is essential for T cell differentiation and survival, the maintenance of effector functions, cell renewal, and T cell memory (8). Low levels of IL-2 have been observed in patients with active TB, compared to healthy controls (9, 10), and restoration of normal levels upon anti-TB treatment has been reported (11).
T cells capable of simultaneously producing two (bifunctional) or three (multifunctional) cytokines have been described in recent years. In chronic viral infections such as HIV and hepatitis C virus (HCV) infections, such multifunctional T cells have been associated with protective immune responses (12, 13). These data were extended to murine models of leishmaniasis (14), suggesting that similar immune responses might be important in protection against mycobacterial infections. To date, however, results have been inconclusive. A higher frequency of multifunctional CD4+ T cells, producing IFN-γ, TNF-α, and IL-2, in peripheral blood mononuclear cells (PBMCs) from patients with active pulmonary tuberculosis (PTB), compared to individuals with latent TB infection (LTBI), was reported (15–17) and decreased following anti-TB treatment (16, 17). However, others observed a lower frequency of multifunctional T cells in PTB, compared to LTBI (18–20), which increased after anti-TB treatment (18, 19). Furthermore, an increase in the frequency of bifunctional CD4+ T cells producing IFN-γ and IL-2 was reported for PTB patients after anti-TB therapy (11). Thus, a consensus on this important issue of the immune response to M. tuberculosis infection in humans has not been reached to date.
Immunological memory is the hallmark of the specific recall response (21, 22). Central memory T (TCM) cells are characterized by a long life and high proliferative potential upon antigen reencounter (22) and may originate from effector T (TEFF) cells (22). On the other hand, effector memory T (TEM) cells can rapidly proliferate upon antigen reencounter and produce effector cytokines (22). Different studies have shown that individuals with LTBI display greater frequencies of TCM cells upon in vitro stimulation with RD1 antigens and purified protein derivative (PPD), compared to PTB patients, in long-term culture assays (23–25). In contrast, PTB patients display greater frequencies of TEM or TEFF cells (26–28). Thus, phenotypic and functional studies of T cell responses to particular mycobacterial antigens may help to define correlates of protection (29, 30). Most of the studies concerning ex vivo analysis of multifunctional T cell responses and T cell memory phenotypes have used the RD1 antigens 6-kDa early secretory antigenic target (ESAT6) and 10-kDa culture filtrate antigen (CFP10), while little research has been performed with antigens that are expressed at high levels during M. tuberculosis latency and reactivation.
Our laboratory and others have provided evidence showing that proteins encoded by the DosR regulon are preferentially recognized in LTBI (31–36). This regulon encodes 48 proteins and is expressed in vitro and in vivo under conditions that may exist in the lung granulomas of infected individuals, such as acidic pH, nutrient starvation, and hypoxia, among other conditions (37–39). Also, individuals with LTBI, compared to PTB patients, preferentially recognize resuscitation-promoting factors (Rpfs) (34, 36, 40–42), proteins known to participate in bacterial reactivation from a quiescent state and to be present in M. tuberculosis (43, 44).
In this study, we characterized the functions and phenotypes of CD4+ and CD8+ multifunctional T cells in response to PPD, the RD1 antigen ESAT6-CFP10 fusion protein, the DosR regulon-encoded antigens Rv1737c (narK2), Rv2029c (pfkB), and Rv2628, and the Rpf antigens Rv0867c (rpfA) and Rv2389c (rpfD) in patients with PTB and individuals with LTBI who had remained healthy for a long time (5 to 7 years) after initial exposure to the index case (i.e., long-term LTBI [ltLTBI]), in a community in the city of Medellín, Colombia, in which M. tuberculosis is endemic. Our results showed that NarK2, PfkB, and RpfD antigens induced higher frequencies of CD4+ or CD8+ mono- or bifunctional T cells producing IFN-γ and/or TNF-α in ltLTBI, compared to PTB, confirming and significantly extending previous results obtained with other populations. The frequencies of CD4+ and/or CD8+ T cells with a TCM phenotype were also higher in ltLTBI, compared to PTB. A possible interpretation of these results is that the immune responses to the selected DosR and Rpf M. tuberculosis antigens are associated with latency maintenance and protection against M. tuberculosis reactivation in LTBI. Thus, the study of functional profiles and memory phenotypes may contribute to discriminating between different states of TB disease and identifying potential correlates of natural protection.
MATERIALS AND METHODS
Study population.
This study focused on a previously characterized community in the city of Medellín, Colombia, in which the disease is endemic, with a high incidence of M. tuberculosis infections (exceeding 70 cases per 100,000 population) (45, 46). In a previous study, 2,060 household contacts (HHCs) of 433 TB index cases were monitored for 3 years (2005 to 2008). According to IFN-γ levels in supernatants of long-term whole-blood cultures, HHC positive rates were 90.1%, 79.4%, 37.8%, and 31.6% for a culture filtrate protein preparation (CFP), CFP10, HspX, and antigen 85A, respectively (45). In the present study, 22 HHCs of recently diagnosed patients with PTB and 20 PTB patients from this community were included. An HHC (>18 years of age) was considered to be someone who had spent time regularly (weekly) in the same household as the index case for at least 1 month before the time at which the diagnosis for the index case was confirmed (45). The HHC group of individuals was initially selected from a previous cohort (45), based on having a positive IFN-γ response (≥22 pg/ml) to the specific M. tuberculosis antigen CFP10 at the initiation of the cohort study, remaining healthy for a long time (5 to 7 years) after initial exposure to the index case (i.e., ltLTBI), and continuing to live in the same area with endemic disease as at the time of original contact. All of the HHCs were healthy at the time of blood sample collection, and all were negative for HIV. In this study, the HHCs did not receive anti-TB treatment (according to the regulations of the Colombian Ministry of Health). PTB patients had received a recent diagnosis of PTB, which was confirmed microbiologically or by culture, with no more than 2 weeks of antibiotic treatment. Mycobacterium bovis BCG vaccination status was determined according to the presence or absence of the typical scar.
Ethical clearance.
Blood samples were collected only after written informed consent was obtained. Study protocols were approved by the Ethics Committee of the Instituto de Investigaciones Médicas, Facultad de Medicina, Universidad de Antioquia (Medellín, Colombia).
Reagents.
RPMI 1640 medium and Dulbecco's phosphate-buffered saline (DPBS) were obtained from Gibco (Grand Island, NY); Ficoll-Hypaque and penicillin-streptomycin solution from Bio-Whittaker (Walkersville, MD); dimethyl sulfoxide (DMSO), brefeldin A (BFA), bovine serum albumin (BSA), and sodium azide from Sigma-Aldrich (St. Louis, MO); pooled human serum (PHS) from Invitrogen (Eugene, OR); paraformaldehyde (PFA) from Mallinckrodt Baker (Phillipsburg, NJ); and Tween 20 from Promega (Madison, WI). The antibodies anti-CD4-phycoerythrin (PE)-Cy7 (clone OKT4), anti-CD27-peridinin chlorophyll (PerCp)-Cy5.5 (clone O323), anti-CD45RO-Pacific blue (clone UCHL1), anti-IFN-γ-PE (clone B27), anti-TNF-α-allophycocyanin (APC) (clone Mab11), and anti-IL-2 (clone MQ1-17H12) were obtained from BioLegend (San Diego, CA), and the antibody anti-CD8-APC-H7 (clone SK1) was obtained from Becton Dickinson (San Diego, CA).
Mycobacterial antigens.
The RD1 ESAT6-CFP10 (E6-C10) fusion protein, DosR (Rv1737c, Rv2029c, and Rv2628) and Rpf (Rv0867c and Rv2389c) antigens used throughout this study were described previously (34, 47). Additionally, PPD (RT50) from Staten Serum Institute (Copenhagen, Denmark) was included in this study. The concentrations of the DosR, Rpf, and RD1 antigens tested were the same as those reported previously (34).
Isolation of PBMCs and culture conditions.
PBMCs were collected from sodium heparin-anticoagulated venous blood (10 ml) and were separated by Ficoll-Hypaque density gradient centrifugation. PBMCs were washed twice in DPBS and counted in a hemocytometer, and cell viability was determined by trypan blue exclusion (>94% for all experiments). The cell culture protocol was described previously (34, 36). In summary, 1.5 × 105 cells/well were seeded in triplicate in 96-well U-bottom plates (Corning Costar Inc., Corning, NY), in a final volume of 200 μl/well of RPMI 1640 medium supplemented with 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 10% PHS. Cells were cultured in the presence or absence of 5 μg/ml (final concentration) of PPD, the fusion protein ESAT6-CFP10, or the selected DosR and Rpf antigens. Cell cultures were incubated for 168 h (7 days) at 37°C in 5% CO2 and 90% relative humidity.
Flow cytometric data analysis.
To determine T cell phenotypes and cytokine production, cells were treated with 10 μg/ml of BFA 4 h before the end of culture, collected in polystyrene tubes, washed with DPBS, incubated with blocking buffer (2% PHS, 0.05% NaN3) for 20 min at 4°C, and then stained with anti-CD4-PE-Cy7, anti-CD8-APC-H7, anti-CD27-PerCp-Cy5.5, and anti-CD45RO-Pacific blue. The cells were then fixed, permeabilized, and stained with anti-IFN-γ-PE, anti-TNF-α-APC, and anti-IL-2-fluorescein isothiocyanate (FITC), using a commercial kit from eBioscience, following the manufacturer's recommendations. Data for 200,000 cells were acquired with a FACSCanto II flow cytometer (Becton Dickinson). The voltages were fixed using unstained cells within the lymphocyte gate, and then a compensation matrix was calculated using CompBeads (BD Biosciences). The compensation was verified using single staining of cells and checking that fluorescence from the singly stained samples did not overlap on any other channel.
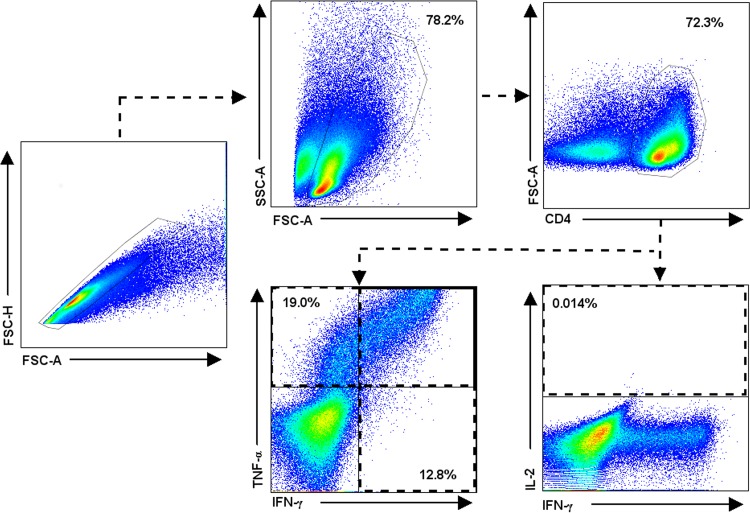
Data analysis was carried out using the FlowJo v7.6.1 (Tree Star Inc., Ashland, OR) software package. The numbers of negative and positive cells producing cytokines were determined by the fluorescence minus one (FMO) method (see Fig. S1 and S2 in the supplemental material). Dead cells were determined by staining with 7-aminoactinomycin D (7-AAD) (Thermo Fisher Scientific, Carlsbad, CA) and were excluded from the analysis. The percentage of viable cells at the end of the culture period was >80% for all experiments. Data analysis was performed as described previously (48). Briefly, doublets were excluded by using a forward scatter A (FSC-A)/forward scatter H (FSC-H) dot plot, followed by the selection of lymphocytes in an FSC-A/side scatter A (SSC-A) dot plot, and then CD4+ or CD8+ T cells were plotted versus FSC-A. Gated CD4+ and CD8+ T cells were evaluated for the frequency of IFN-γ-, TNF-α-, or IL-2-producing cells (Fig. 1). The frequencies of monofunctional, bifunctional, and multifunctional CD4+ and CD8+ T cells were evaluated using the combination gate tool in FlowJo v7.6.1. To identify the phenotypes of cytokine-producing cells, IFN-γ- and/or TNF-α-producing gated CD4+ and CD8+ T cells were evaluated for the expression of CD27 and CD45RO. Based on the presence of T cell surface markers, including CD45RO and the TNF receptor family member CD27 costimulatory molecule, T memory cells have been classified as central memory (CD45RO+ CD27+) and effector memory (CD45RO+ CD27−) cells (25, 34, 36, 49–51). In this context, early/naive T (TE/N) cells display a CD45RO− CD27+ phenotype, while effector T (TEFF) cells display none of these markers (CD45RO− CD27−).
FIG 1.
Representative flow cytometric analysis showing the gating strategy for identification of monofunctional and multifunctional CD4+ and CD8+ T cells. A total of 1.5 × 105 PBMCs were cultured in triplicate for 7 days in the presence or absence of PPD, E6-C10, and DosR and Rpf antigens. At the end of the cultures, the cells were stained with anti-CD4-PE-Cy7, anti-CD8-APC-H7, anti-CD45RO-Pacific blue, and anti-CD27-PerCp-Cy5.5, fixed, permeabilized, and stained with anti-IFN-γ-PE, anti-TNF-α-APC, and anti-IL-2-FITC. A representative experiment with a sample from a latently infected individual and PPD stimulation is shown. Analyses were similar for CD4+ and CD8+ T cells. Briefly, after exclusion of doublets, CD4+ and CD8+ T cells were gated versus FSC-A and analyzed for intracellular IFN-γ, TNF-α, and IL-2. Analysis was performed with FlowJo v7.6.1, using the combination gate tool in order to obtain the frequencies of single- and multiple-cytokine-producing cells.
Statistical analysis.
A chi-square test was used to test for differences in gender and the presence or absence of a BCG scar. The frequencies of the different combinations of IFN-γ-, IL-2-, and TNF-α-positive cells following antigenic stimulation were calculated within the total population of CD4+ and CD8+ T cells. Net values were obtained by subtracting the background values (nonstimulated cells). Data normality was tested with the Shapiro-Wilk normality test. The significance of median differences in the frequencies of single- or multiple-producer cells and the memory phenotype between individuals with ltLTBI and PTB patients was determined with the nonparametric Mann-Whitney U test. All statistical analyses were performed using GraphPad Prism v6.0 (GraphPad Software, San Diego, CA). Statistical differences were considered significant for P values of ≤0.05.
RESULTS
Study population.
This study focused on a previously characterized community in the city of Medellín, Colombia, in which TB is endemic, with a high prevalence of M. tuberculosis infections (45). HHCs for whom peripheral blood cultures stimulated with the RD1 antigen CFP10 produced ≥22 pg/ml IFN-γ in a 7-day culture assay were considered infected (79.4%) (45). In the present study, we included 22 previously identified HHCs who had remained healthy for at least 5 years, with no clinical signs of active TB, and were negative for HIV (individuals with ltLTBI) and 20 patients with confirmed (sputum and/or culture) PTB from the same community. The median age was 37 years (range, 18 to 65 years) in the ltLTBI group; 54% of the subjects were male, and 82% had been vaccinated with M. bovis BCG. The PTB group showed a median age of 29 years (range, 19 to 58 years), 60% of the subjects were male, and 95% had been vaccinated with BCG. No significant differences in age and BCG vaccination rates were found between the ltLTBI and PTB groups (Table 1).
TABLE 1.
Characteristics of the study population
| Characteristic | ltLTBI (n = 22) | PTB (n = 20) |
|---|---|---|
| Age (median [range]) (yr) | 37 (18–65) | 29 (19–58) |
| Male/female (%) | 54/46 | 60/40 |
| BCG scar positive (%) | 82 | 95 |
Multifunctional CD4+ and CD8+ T cell responses to DosR and Rpf antigens in ltLTBI and PTB.
Most studies evaluating T cell responses to M. tuberculosis DosR and Rpf antigens in LTBI and PTB have been based on IFN-γ detection (31, 32, 34, 35, 40, 52–54). Here, we used a 7-day stimulation assay to optimize sensitivity to detect latent infection, since short-term cultures (25, 55–57) are less sensitive in settings of high levels of endemicity, where mixtures of recent and old infections are commonly found (55).
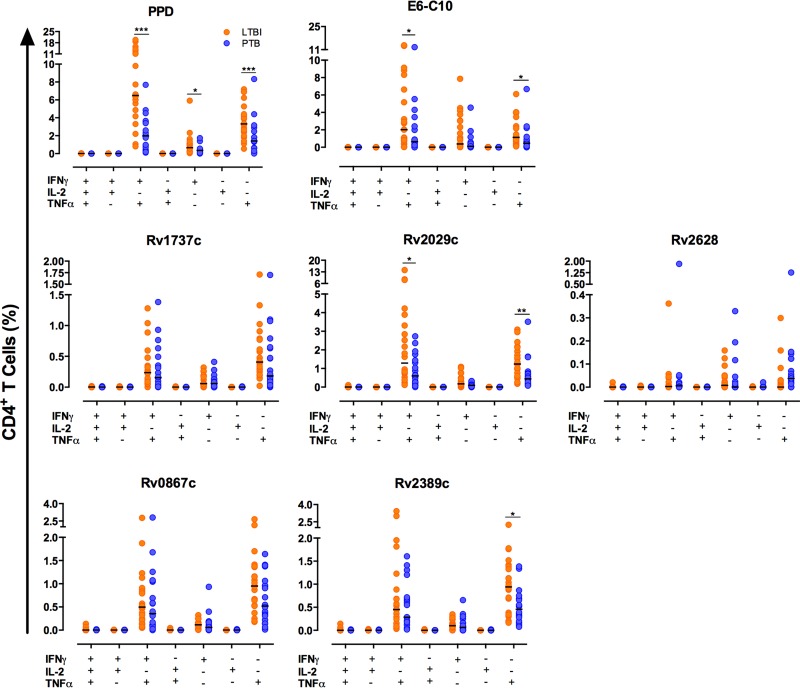
Figure 2 and Table 2 show the responses of CD4+ T cells to E6-C10 and DosR and Rpf antigens. Individuals with ltLTBI displayed higher frequencies of monofunctional TNF-α+ CD4+ T cells (P < 0.05) and bifunctional IFN-γ+ TNF-α+ CD4+ T cells (P < 0.05), compared to PTB patients; similar differences were observed for Rv2029c (PfkB) (P < 0.01 and P < 0.05, respectively). No significant differences in the frequencies of mono- and bifunctional CD4+ T cells in the ltLTBI and PTB groups in response to Rv1737c (Nark2) and Rv2628 were observed, although there was a trend for increasing frequencies in the ltLTBI group, compared to the PTB group. The frequency of monofunctional TNF-α+ CD4+ T cells in response to the Rpf antigen Rv2389c (P < 0.05), but not Rv0867c, was higher for individuals with ltLTBI (Fig. 2). In response to PPD, individuals with ltLTBI displayed higher frequencies of monofunctional CD4+ TNF-α+ (P < 0.05) and CD4+ IFN-γ+ (P < 0.05) T cells and bifunctional IFN-γ+ TNF-α+ CD4+ T cells (P < 0.001), compared to PTB patients (Fig. 2).
FIG 2.
Frequencies of single- and multiple-cytokine-producing CD4+ T cells in ltLTBI and PTB. A total of 1.5 × 105 PBMCs from individuals with ltLTBI or PTB were cultured in triplicate for 7 days in the presence or absence of PPD, E6-C10, and DosR and Rpf antigens. CD4+ T cells were stained as described in Materials and Methods and then analyzed for intracellular production of IFN-γ, TNF-α, and IL-2. The frequencies of single- and multiple-cytokine-producing CD4+ T cells were evaluated using the combination gate tool from FlowJo v7.6.1. Statistical differences between the groups were calculated with the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 2.
Frequencies of monofunctional (IFN-γ+ or TNF-α+), bifunctional (IFN-γ+ TNF-α+), and multifunctional (IFN-γ+ TNF-α+ IL-2+) CD4+ T cells in ltLTBI and PTB
| Antigen | Frequency (median [interquartile range]) (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD4+ IFN-γ+ T cells |
CD4+ TNF-α+ T cells |
CD4+ IFN-γ+ TNF-α+ T cells |
CD4+ IFN-γ+ TNF-α+ IL-2+ T cells |
|||||
| PTB | LTBI | PTB | LTBI | PTB | LTBI | PTB | LTBI | |
| PPD | 0.36 (0.09–0.40) | 0.66 (0.25–1.13)a | 1.41 (0.61–2.32) | 3.32 (2.29–4.63)b | 1.98 (0.52–3.61) | 6.49 (4.69–11.59)b | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) |
| RD1 antigens | ||||||||
| E6-C10 | 0.11 (0.03–0.46) | 0.38 (0.08–3.03) | 0.46 (0.13–0.85) | 1.14 (0.60–2.69)a | 0.63 (0.24–2.30) | 2.02 (0.77–7.05)a | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| DosR antigens | ||||||||
| Rv1737c | 0.06 (0.0–0.12) | 0.06 (0.02–0.18) | 0.18 (0.06–0.64) | 0.41 (0.24–0.77) | 0.15 (0.03–0.46) | 0.24 (0.09–0.48) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Rv2029c | 0.09 (0.05–0.18) | 0.17 (0.07–0.46) | 0.44 (0.18–0.73) | 1.24 (0.59–1.97)c | 0.60 (0.22–1.41) | 1.29 (0.68–3.46)a | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Rv2628 | 0.0 (0.0–0.02) | 0.01 (0.0–0.04) | 0.04 (0.01–0.11) | 0.0 (0.0–0.03) | 0.01 (0.00–0.02) | 0.00 (0.0–0.01) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Rpf antigens | ||||||||
| Rv0867c | 0.06 (0.01–0.16) | 0.11 (0.02–0.16) | 0.52 (0.24–1.04) | 0.95 (0.53–1.36) | 0.35 (0.10–1.06) | 0.50 (0.20–0.88) | 0.00 (0.00–0.00) | 0.00 (0.00–0.01) |
| Rv2389c | 0.06 (0.00–0.23) | 0.10 (0.06–0.18) | 0.46 (0.22–0.68) | 0.94 (0.34–1.39)a | 0.28 (0.12–0.71) | 0.45 (0.13–0.97) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
P < 0.05.
P < 0.001.
P < 0.01.
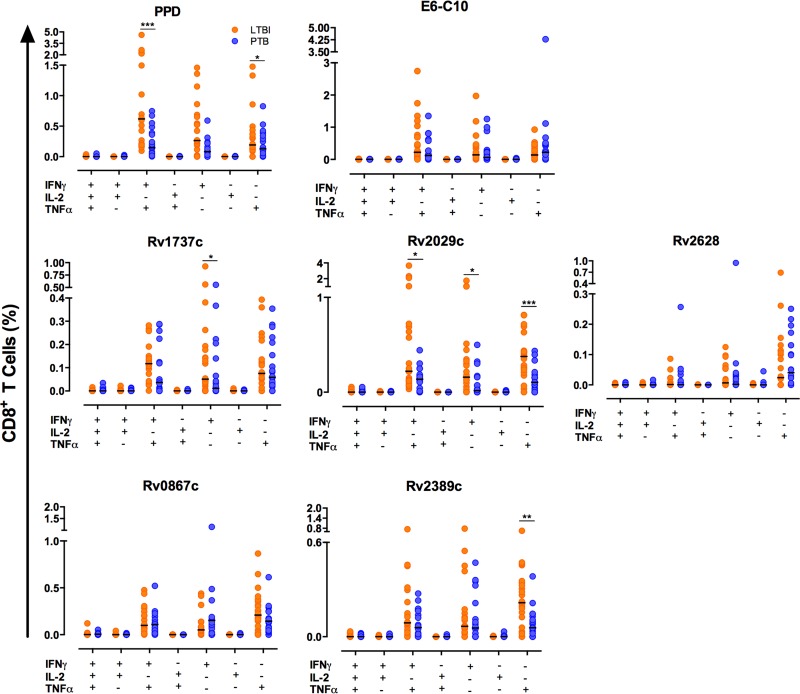
Figure 3 and Table 3 show the responses of CD8+ T cells. In this case, individuals with ltLTBI displayed higher frequencies of monofunctional IFN-γ+ T cells in response to Rv1737c and Rv2029c (P < 0.05), compared to patients with PTB. In addition, individuals with ltLTBI displayed higher frequencies of monofunctional TNF-α+ T cells (P < 0.05) and bifunctional IFN-γ+ TNF-α+ T cells (P < 0.001) in response to Rv2029c, compared to PTB patients. The frequency of monofunctional TNF-α+ CD8+ T cells in response to the Rpf antigen Rv2389c was also higher in the ltLTBI group (P < 0.01). In response to PPD, individuals with ltLTBI displayed higher frequencies of monofunctional TNF-α+ T cells (P < 0.05) and bifunctional IFN-γ+ TNF-α+ T cells (P < 0.001), compared to PTB patients. No significant differences in the frequencies of mono- or bifunctional T cells in response to E6-C10, the DosR antigen Rv2628, or the Rpf antigen RpfA (Rv0867c) were observed, although similar trends could be observed.
FIG 3.
Frequencies of single- and multiple-cytokine-producing CD8+ T cells in ltLTBI and PTB. A total of 1.5 × 105 PBMCs from individuals with ltLTBI or PTB were cultured in triplicate for 7 days in the presence or absence of PPD, E6-C10, and DosR and Rpf antigens. CD8+ T cells were stained as described in Materials and Methods and then analyzed for intracellular production of IFN-γ, TNF-α, and IL-2. The frequencies of single- and multiple-cytokine-producing CD8+ T cells were evaluated using the combination gate tool from FlowJo v7.6.1. Statistical differences between the groups were calculated with the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
TABLE 3.
Frequencies of monofunctional (IFN-γ+ or TNF-α+), bifunctional (IFN-γ+ TNF-α+), and multifunctional (IFN-γ+ TNF-α+ IL-2+) CD8+ T cells in ltLTBI and PTB
| Antigen | Frequency (median [interquartile range]) (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| CD8+ IFN-γ+ T cells |
CD8+ TNF-α+ T cells |
CD8+ IFN-γ+ TNF-α+ T cells |
CD8+ IFN-γ+ TNF-α+ IL-2+ T cells |
|||||
| PTB | LTBI | PTB | LTBI | PTB | LTBI | PTB | LTBI | |
| PPD | 0.08 (0.00–0.22) | 0.26 (0.09–0.66) | 0.13 (0.03–0.33) | 0.19 (0.11–0.39)a | 0.15 (0.06–0.38) | 0.62 (0.20–2.19)b | 0.00 (0.00–0.00) | 0.00 (0.00–0.01) |
| RD1 antigens | ||||||||
| E6-C10 | 0.06 (0.0–0.24) | 0.14 (0.03–0.43) | 0.22 (0.06–0.48) | 0.14 (0.03–0.43) | 0.12 (0.05–0.61) | 0.22 (0.06–0.83) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| DosR antigens | ||||||||
| Rv1737c | 0.01 (0.00–0.19) | 0.05 (0.02–0.18)a | 0.06 (0.02–0.19) | 0.08 (0.01–0.22) | 0.04 (0.00–0.12) | 0.12 (0.04–0.14) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Rv2029c | 0.02 (0.00–0.16) | 0.16 (0.02–0.37)a | 0.10 (0.03–0.18) | 0.38 (0.20–0.49)a | 0.14 (0.01–0.18) | 0.22 (0.11–0.70)a | 0.00 (0.00–0.02) | 0.00 (0.00–0.01) |
| Rv2628 | 0.00 (0.00–0.03) | 0.01 (0.00–0.06) | 0.04 (0.00–0.13) | 0.02 (0.00–0.11) | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Rpf antigens | ||||||||
| Rv0867c | 0.08 (0.00–0.16) | 0.05 (0.01–0.13) | 0.11 (0.04–0.23) | 0.21 (0.08–0.36) | 0.08 (0.02–0.15) | 0.10 (0.03–0.22) | 0.00 (0.00–0.01) | 0.00 (0.00–0.01) |
| Rv2389c | 0.06 (0.0–0.19) | 0.07 (0.03–0.16) | 0.06 (0.0–0.12) | 0.21 (0.05–0.35) | 0.06 (0.00–0.17) | 0.09 (0.03–0.24) | 0.00 (0.00–0.00) | 0.00 (0.00–0.01) |
P < 0.05.
P < 0.001.
Memory phenotypes of mono- and bifunctional CD4+ and CD8+ T cells.
Joint analysis of T cell functions and phenotypes may help establish whether a particular immune response is associated with protective immunity (29). Therefore, we evaluated the memory phenotypes of monofunctional (IFN-γ+ or TNF-α+) (Fig. 4 and 5) and bifunctional (IFN-γ+ TNF-α+) (Fig. 6) CD4+ and CD8+ T cells upon stimulation with PPD, E6-C10, and the selected DosR and Rpf antigens in the same 7-day-stimulated PBMC cultures.
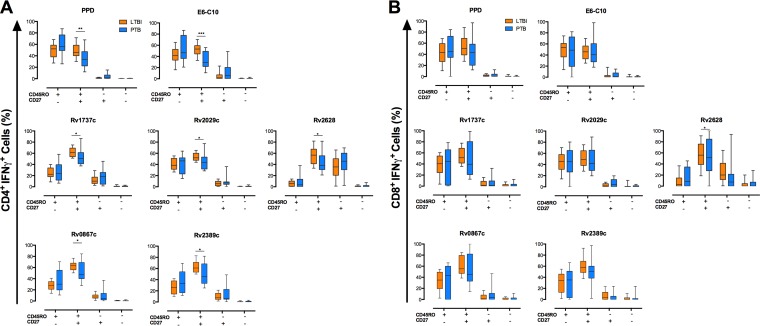
FIG 4.
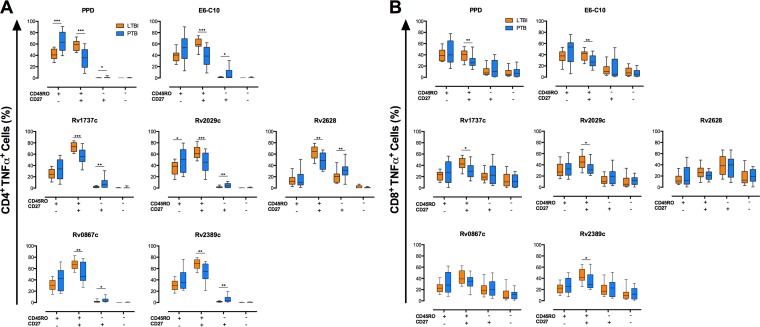
Memory phenotypes of monofunctional IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells in ltLTBI and PTB. A total of 1.5 × 105 PBMCs from individuals with ltLTBI or PTB were cultured in triplicate for 7 days in the presence or absence of PPD, E6-C10, and DosR and Rpf antigens. CD4+ and CD8+ T cells were stained as described in Materials and Methods and then analyzed for intracellular production of IFN-γ, TNF-α, and IL-2. The frequencies of single- and multiple-cytokine-producing CD4+ and CD8+ T cells were evaluated using the combination gate tool from FlowJo v7.6.1. The memory phenotypes of monofunctional IFN-γ+ CD4+ (A) and IFN-γ+ CD8+ (B) T cells were evaluated by flow cytometry according the surface expression of CD45RO and CD27. Statistical differences between the groups were calculated with the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 5.
Memory phenotypes of monofunctional TNF-α+ CD4+ and TNF-α+ CD8+ T cells in ltLTBI and PTB. A total of 1.5 × 105 PBMCs from individuals with ltLTBI or PTB were cultured in triplicate for 7 days in the presence or absence of PPD, E6-C10, and DosR and Rpf antigens. CD4+ and CD8+ T cells were stained as described in Materials and Methods and then analyzed for intracellular production of IFN-γ, TNF-α, and IL-2. The frequencies of single- and multiple-cytokine-producing CD4+ and CD8+ T cells were evaluated using the combination gate tool from FlowJo v7.6.1. The memory phenotypes of monofunctional TNF-α+ CD4+ (A) and TNF-α+ CD8+ (B) T cells were evaluated by flow cytometry according the surface expression of CD45RO and CD27. Statistical differences between the groups were calculated with the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
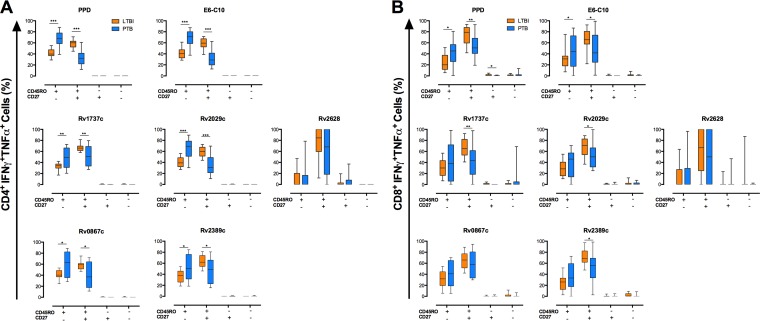
FIG 6.
Memory phenotypes of bifunctional IFN-γ+ TNF-α+ CD4+ and IFN-γ+ TNF-α+ CD8+ T cells in ltLTBI and PTB. A total of 1.5 × 105 PBMCs from individuals with ltLTBI or PTB were cultured in triplicate for 7 days in the presence or absence of PPD, E6-C10, and DosR and Rpf antigens. CD4+ and CD8+ T cells were stained as described in Materials and Methods and then analyzed for intracellular production of IFN-γ, TNF-α, and IL-2. The frequencies of single- and multiple-cytokine-producing CD4+ and CD8+ T cells were evaluated using the combination gate tool from FlowJo v7.6.1. The memory phenotypes of bifunctional IFN-γ+ TNF-α+ CD4+ (A) and IFN-γ+ TNF-α+ CD8+ (B) T cells were evaluated by flow cytometry according the surface expression of CD45RO and CD27. Statistical differences between the groups were calculated with the Mann-Whitney U test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Increased frequencies of monofunctional (IFN-γ+ or TNF-α+) CD4+ T cells with a CD45RO+ CD27+ phenotype (TCM) were observed for individuals with ltLTBI, compared to PTB patients, in response to E6-C10 (P < 0.001 and P < 0.001, respectively), the DosR antigens Rv1737c (P < 0.05 and P < 0.001, respectively), Rv2029c (P < 0.05 and P < 0.001, respectively), and Rv2628 (P < 0.05 and P < 0.01, respectively), and the Rpf antigens Rv0867c (P < 0.05 and P < 0.01, respectively) and Rv2389c (P < 0.05 and P < 0.01, respectively) (Fig. 4 and 5); similar findings were observed upon stimulation with PPD (P < 0.01 and P < 0.001, respectively) (Fig. 4 and 5). In addition, individuals with ltLTBI displayed higher frequencies of bifunctional (IFN-γ+ TNF-α+) CD4+ T cells with a TCM phenotype in response to PPD (P < 0.001), E6-C10 (P < 0.001), Rv1737c (P < 0.01), Rv2029c (P < 0.001), Rv0867c (P < 0.05), and Rv2389c (P < 0.05) (Fig. 6).
In contrast, PTB patients displayed higher frequencies of monofunctional TNF-α+ CD4+ T cells with a CD45RO+ CD27− (TEM) phenotype in response to PPD (P < 0.001) and the DosR antigen Rv2029c (P < 0.05) (Fig. 5). Also, PTB patients displayed higher frequencies of bifunctional IFN-γ+ TNF-α+ CD4+ T cells with a TEM phenotype upon stimulation with PPD (P < 0.001), E6-C10 (P < 0.001), Rv1737c (P < 0.01), Rv2029c (P < 0.001), Rv0867c (P < 0.05), and Rv2389 (P < 0.05), compared to individuals with ltLTBI (Fig. 6). Of note, high frequencies of monofunctional CD4+ TNF-α+ T cells with a CD45RO− CD27+ (TE/N) phenotype were observed for PTB patients in response to PPD (P < 0.05), E6-C10 (P < 0.05), Rv1737c (P < 0.01), Rv2029c (P < 0.01), Rv2628 (P < 0.01), Rv0867c (P < 0.05), and Rv2389 (P < 0.01) (Fig. 5).
Considering CD8+ T cells, individuals with ltLTBI displayed a higher frequency of monofunctional IFN-γ+ cells with a TCM phenotype in response to the DosR antigen Rv2628 (P < 0.05), compared to PTB patients (Fig. 4). In addition, the ltLTBI group displayed higher frequencies of monofunctional (TNF-α+) and bifunctional (IFN-γ+ TNF-α+) CD8+ T cells with a TCM phenotype in response to PPD (P < 0.01 and P < 0.01, respectively), E6-C10 (P < 0.01 and P < 0.05, respectively), Rv1737c (P < 0.05 and P < 0.01, respectively), Rv2029c (P < 0.05 and P < 0.05, respectively), and Rv2389c (P < 0.05 and P < 0.05, respectively) (Fig. 5 and 6). In contrast, PTB patients displayed higher frequencies of bifunctional (IFN-γ+ TNF-α+) CD8+ T cells with a TEM phenotype upon stimulation with PPD (P < 0.05) and E6-C10 (P < 0.05), compared to individuals with ltLTBI (Fig. 6).
DISCUSSION
In this study, which was performed in a community in Colombia in which TB is endemic, we characterized the functions and phenotypes of CD4+ and CD8+ T cells (by flow cytometry) in response to DosR regulon-encoded antigens and resuscitation Rpf antigens in individuals with ltLTBI and patients with PTB. We found that Rv1737c (NarK2), Rv2029c (PfkB), and Rv2389c (RpfD) antigens induced higher frequencies of CD4+ or CD8+ mono- or bifunctional T cells (producing IFN-γ and/or TNF-α) in ltLTBI, compared to PTB. In addition, higher frequencies of CD4+ and/or CD8+ mono- or bifunctional T cells with a TCM phenotype (CD45RO+ CD27+) in response to RD1, DosR, and Rpf antigens were observed in ltLTBI, compared to PTB. Conversely, higher frequencies of bifunctional CD4+ or CD8+ T cells with a TEM phenotype (CD45RO+ CD27−) in response to RD1, DosR, and Rpf antigens were observed in PTB, compared with ltLTBI. All of these data suggest that the response to M. tuberculosis DosR and Rpf antigens may contribute to mycobacterial control in latent infection and may help to discriminate further between the different states of M. tuberculosis infections.
In previous studies in the same community in which TB is endemic, we showed that HHCs with LTBI displayed higher frequencies of CD4+ IFN-γ+ T cells with a TCM phenotype (CD45RO+ CD27+) in response to M. tuberculosis DosR and Rpf antigens, compared to PTB patients (34). More recently, we monitored the T cell immune responses to RD1, DosR, and Rpf antigens in HHCs with LTBI over a 12-month period after TB index case diagnosis. At 12 months, E6-C10+ HHCs displayed decreases in IFN-γ levels in response to E6-C10, DosR, and Rpf antigens and a generalized decrease in cytokine production. Conversely, E6-C10− HHCs at the end of the follow-up period (12 months) showed increases in the IFN-γ responses and cytokine levels in response to E6-C10 (36). The maintenance of CD45RO+ CD27+ CD4+ T cells in E6-C10+ HHCs and their increase in E6-C10− HHCs suggested that CD45RO+ CD27+ T cells may play a protective role in the immune response controlling M. tuberculosis infection and may be leading to a state of controlled latent infection (36). In viral infections, however, protective immune responses have been associated with the presence of multifunctional T cells, producing IFN-γ, TNF-α, and IL-2 (12, 13). Therefore, the presence and association of multifunctional T cell responses in TB have been examined by several groups, mostly in response to in vitro stimulation with ESAT6 and CFP10; as discussed above, however, results have been inconclusive (15–20).
It is now well established that, as a consequence of adaptation to the infected host cell intracellular milieu, M. tuberculosis changes its gene expression profile (37, 39). This implies that the immune response may develop a different specificity profile depending on the immunodominant antigens newly expressed by M. tuberculosis during its adaptation to the host. The DosR antigens become strongly expressed under stress conditions, including hypoxia, nutrient starvation, low pH, and high concentrations of reactive oxygen and nitrogen intermediates, all of which may mimic conditions inside granulomas (37–39). Also, expression patterns of rpfA-E genes have been observed during acute infection with M. tuberculosis (43, 44). Of note, the multifunctional T cell responses to DosR and Rpf antigens in communities in which M. tuberculosis is endemic have been poorly characterized.
With respect to the DosR antigens, our results showed that individuals with ltLTBI displayed higher frequencies of mono- and bifunctional CD4+ and CD8+ T cells producing IFN-γ and/or TNF-α in response to Rv2029c (pfkB), compared to PTB patients. Moreover, individuals with ltLTBI displayed a higher frequency of monofunctional CD8+ IFN-γ+ T cells in response to Rv1737c (narK2), compared to PTB patients, suggesting that a protective immune response may develop in ltLTBI in response to DosR antigens. Indeed, greater immune responses to Rv1737c and Rv2029c in ltLTBI, compared to PTB, were reported previously for different human populations (31, 32, 34, 35). Furthermore, it has been reported that DosR antigens, including Rv2029c, induce predominant mono- and bifunctional (IFN-γ and/or TNF-α) CD4+ and/or CD8+ T cell responses in LTBI (33). Rv2029c (pfkB), a probable phosphofructokinase, is a key enzyme in glycolysis (58). A recent study suggests that, in M. tuberculosis, glycolysis leads to the accumulation of toxic metabolites, limiting M. tuberculosis survival under hypoxic conditions (58). Rv1737c (narK2) is a probable nitrite/nitrate transporter that participates in the regulation of the nitrate reductase activity of M. tuberculosis under hypoxic conditions (59, 60). It has been suggested that nitrate reduction may play an important role in M. tuberculosis survival during dormancy (61). Thus, the evidence presented in this paper and a previous paper (34), as well as others (62), reinforces the notion that M. tuberculosis DosR regulon-encoded antigens, including Rv1737c and Rv2029c, may be interesting biomarkers associated with a protective immune response and might be potential candidates for postexposure vaccines.
An immune response to Rpf antigens of M. tuberculosis has been preferentially associated with individuals with LTBI (34, 36, 40, 42). In a previous study, we reported a higher frequency of CD4+ IFN-γ+ T cells in HHCs with LTBI, compared to PTB, in response to RpfD (Rv2389c) (34). The higher frequencies of monofunctional CD4+ TNF-α+ and CD8+ TNF-α+ T cells in ltLTBI that we observed in the present study are concordant with the observation of the presence of monofunctional CD4+ TNF-α+ and CD8+ TNF-α+ T cells in M. tuberculosis-infected nonprogressors of Norwegian origin (41). Thus, monofunctional CD4+ TNF-α+ T cells may play an important role in controlling M. tuberculosis reactivation in latently infected individuals. We previously observed a lower frequency of IFN-γ+ T cells and lower levels of IFN-γ production in response to Rv0867c (RpfA), compared to RpfD (34, 36). It has been reported that the rpfA and rpfD genes are differentially expressed during M. tuberculosis growth and under stress conditions (63). It is tempting to speculate that differences in the expression and/or function of RpfA and RpfD in M. tuberculosis may lead to different immune responses during latency.
Our results showed that HHCs with ltLTBI displayed significant increases in the frequencies of monofunctional (IFN-γ+ or TNF-α+) or bifunctional (IFN-γ+ TNF-α+) CD4+ and CD8+ T cells in response to stimulation with the RD1 and PPD antigens. Both IFN-γ and TNF-α play important roles in the protective immune response against M. tuberculosis infection, participating in the activation of effector mechanisms of monocytes and macrophages and in granuloma integrity (5, 6). Thus, our observations may suggest that mono- and bifunctional CD4+ and CD8+ T cells producing IFN-γ and/or TNF-α can contribute to effective mycobacterial growth control in individuals with LTBI (34, 48, 64). Although in other studies higher frequencies of mono- and bifunctional CD4+ T cells producing IFN-γ and/or TNF-α in response to RD1 were found in PTB, compared to LTBI (18, 19, 27, 28), the difference in the lengths of the in vitro cultures might explain this difference. Short-term cultures (24 h) have been mainly associated with the detection of a T cell effector memory phenotype, while long-term cultures (5 to 7 days), as used in this study, have been mainly associated with the detection of a T central memory phenotype (25, 36, 56, 57, 64). An alternative explanation for the observed reductions in the frequencies of mono- and bifunctional CD4+ T cells in peripheral blood samples from PTB patients may involve the previously reported sequestration of CD4+ T cells at the site of infection (65, 66).
T cell memory generation is critical for specific immune responses. Studies in viral models of chronic infection showed that effector T cells expanded during viral replication, while memory cells were detected upon virus control (30). Memory generation is also critical for protective immunity to M. tuberculosis (67). PBMCs from tuberculin skin test-positive and cured TB patients that were stimulated with RD1 antigens displayed a higher frequency of CD4+ T cells with a TCM phenotype, compared to patients with moderate or severe TB, who displayed a preferential TEM phenotype (23). In a previous study, we found a higher frequency of CD4+ T cells with a TCM phenotype (CD45RO+ CD27+) in LTBI, compared to PTB, in response to the fusion protein ESAT6-CFP10 (34). Furthermore, BCG vaccination induces the expansion of a CD4+ T cell population with a TCM phenotype (68), although it is not clear whether this expansion may result in long-term protection. In the present study, we found that individuals with ltLTBI displayed higher frequencies of mono- and bifunctional CD4+ and/or CD8+ TCM cells that produced IFN-γ and/or TNF-α, compared to PTB patients. Conversely, higher frequencies of bifunctional CD4+ and CD8+ T cells with a TEM phenotype (CD45RO+ CD27−) that produced IFN-γ and TNF-α were found in PTB patients, compared to individuals with ltLTBI. Overall, these results suggest that mono- and bifunctional T cells with a TCM phenotype may play an important role in M. tuberculosis infection control in latently infected individuals, while TEM cells may be associated with the presence of replicating mycobacteria in PTB patients and may represent biomarkers of the mycobacterial load. Commandeur and colleagues reported higher frequencies of mono- and bifunctional T cells with a TEM phenotype in response to stimulation with DosR and Rpf antigens in LTBI (33, 41). This difference from our results may be explained by the length of the in vitro cultures. While we used long-term cultures (7 days), Commandeur and colleagues used short-term cultures (24 h) (33, 41). It has been argued that long-term cultures (5 to 7 days), in contrast to short-term cultures (24 h), select potentially long-lived T cells, particularly central memory T cells (55–57). This may also explain the absence (or lower levels) of effector T cells (CD45RO− CD27−) for single-cytokine-producing CD4+ and CD8+ T cells. Under our experimental conditions using long-term cultures, we have observed enrichment of the TCM phenotype (CD45RO+ CD27+ T cells) (25, 34, 36, 64).
Recent studies demonstrated that RD1 antigen stimulation induces greater proportions of multifunctional CD4+ T cells in individuals with LTBI, compared to patients with active TB (18–20). In this study, we found that mono- and bifunctional CD4+ and CD8+ T cells producing IFN-γ and/or TNF-α were observed more frequently than monofunctional IL-2+ or multifunctional (IFN-γ+ TNF-α+ IL-2+) T cells, independent of the antigen and disease status, as observed by others (48). A similar observation in the macaque model was recently published (69). The low frequency of antigen-specific T cells uniquely producing IL-2 may be a consequence of the long-term culture used in our study, as reported previously (40, 48). In a recent study, Han and colleagues, using a short-term stimulation assay, reported that the T cell multifunctional response is the result of sequential production of cytokines for short times, during which T cells simultaneously secrete multiple cytokines (70). More recently, it has been shown that some methodological factors, including the source of T cells (fresh whole blood, fresh PBMCs, or frozen PBMCs), the length of the culture (short term versus long term), and the use of costimulatory antibodies, can affect the sensitivity of intracellular cytokine assays (71). These results may explain the contrasting results of the T cell cytokine profiles observed in our study.
Another potential limitation of the present study is that we analyzed the immune responses of a relatively small number of subjects in each group, which is why this constitutes a pilot study. Although larger populations and longitudinal studies are needed to confirm these observations, the results generated in this study are consistent with results observed in other human populations.
In this study, PTB patients are defined as having received a recent diagnosis of PTB, which was confirmed microbiologically or by culture, with no more than 2 weeks of antibiotic treatment. There is some evidence in the literature of changes to the transcriptome and cell populations as early as 1 week posttreatment, which may affect the composition of the T cell population responding to the infection (72). However, it has been reported that significant changes in the immune response and cell populations in the host are principally observed 1 to 3 or 6 months after the initiation of TB treatment (73–75), indicating that changes at a transcriptomic level are not translated immediately into protein and cellular changes in the host. We think that our results may not be affected by the anti-TB treatment. Also, some reports have presented evidence suggesting that the immune responses of patients with multidrug-resistant (MDR) TB may be different from those of patients with drug-sensitive TB (76, 77). Since all of our PTB samples were collected within the first 2 weeks of treatment, no testing for antibiotic resistance was performed. However, the frequency of MDR TB in our population is low (estimated rate, 2.4%) (78), and thus our results may not be affected by this factor.
In conclusion, we have shown that individuals with ltLTBI display prominent mono- and bifunctional (IFN-γ+ and/or TNF-α+) T cell responses, with a CD45RO+ CD27+ phenotype, to M. tuberculosis DosR and Rpf antigens, which we hypothesize are associated with maintenance of immune control of latent M. tuberculosis infection and protection from disease reactivation. To our knowledge, the present data represent the first description of the multifunctional T cell responses to DosR and Rpf antigens in individuals with ltLTBI and PTB in a community in which TB is endemic. Our results may contribute to a better understanding of latency and the definition of predictive biomarkers of latency and reactivation.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the TB patients and latently infected individuals who consented to participate in this study.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00217-16.
REFERENCES
- 1.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcais A, Fieschi C, Abel L, Casanova JL. 2005. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med 202:1617–1621. doi: 10.1084/jem.20052302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottenhoff TH, Verreck FA, Lichtenauer-Kaligis EG, Hoeve MA, Sanal O, van Dissel JT. 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat Genet 32:97–105. doi: 10.1038/ng0902-97. [DOI] [PubMed] [Google Scholar]
- 5.Algood HM, Lin PL, Flynn JL. 2005. Tumor necrosis factor and chemokine interactions in the formation and maintenance of granulomas in tuberculosis. Clin Infect Dis 41(Suppl 3):S189–S193. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs M, Samarina A, Grivennikov S, Botha T, Allie N, Fremond C, Togbe D, Vasseur V, Rose S, Erard F, Monteiro A, Quesniaux V, Ryffel B. 2007. Reactivation of tuberculosis by tumor necrosis factor neutralization. Eur Cytokine Netw 18:5–13. [DOI] [PubMed] [Google Scholar]
- 7.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345:1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 8.Hoyer KK, Dooms H, Barron L, Abbas AK. 2008. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev 226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Lin Y, Iyer DV, Gong J, Abrams JS, Barnes PF. 1995. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun 63:3231–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sargentini V, Mariotti S, Carrara S, Gagliardi MC, Teloni R, Goletti D, Nisini R. 2009. Cytometric detection of antigen-specific IFN-γ/IL-2 secreting cells in the diagnosis of tuberculosis. BMC Infect Dis 9:99. doi: 10.1186/1471-2334-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, Guyot-Revol V, Gunatheesan R, Klenerman P, Lalvani A. 2007. Dynamic relationship between IFN-γ and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J Immunol 178:5217–5226. doi: 10.4049/jimmunol.178.8.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciuffreda D, Comte D, Cavassini M, Giostra E, Buhler L, Perruchoud M, Heim MH, Battegay M, Genne D, Mulhaupt B, Malinverni R, Oneta C, Bernasconi E, Monnat M, Cerny A, Chuard C, Borovicka J, Mentha G, Pascual M, Gonvers JJ, Pantaleo G, Dutoit V. 2008. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol 38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 14.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med 13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MO. 2009. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur J Immunol 39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 16.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. 2010. Multifunctional CD4+ T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol 40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 17.Young JM, Adetifa IM, Ota MO, Sutherland JS. 2010. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One 5:e11237. doi: 10.1371/journal.pone.0011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. 2011. Dominant TNF-α+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med 17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtner M, Mascia C, Sauzullo I, Mengoni F, Vita S, Marocco R, Belvisi V, Russo G, Vullo V, Mastroianni CM. 2015. Multifunctional analysis of CD4+ T-cell response as immune-based model for tuberculosis detection. J Immunol Res 2015:217287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed R, Gray D. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 22.Sallusto F, Geginat J, Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 23.Goletti D, Butera O, Bizzoni F, Casetti R, Girardi E, Poccia F. 2006. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J Infect Dis 194:984–992. doi: 10.1086/507427. [DOI] [PubMed] [Google Scholar]
- 24.Geldmacher C, Ngwenyama N, Schuetz A, Petrovas C, Reither K, Heeregrave EJ, Casazza JP, Ambrozak DR, Louder M, Ampofo W, Pollakis G, Hill B, Sanga E, Saathoff E, Maboko L, Roederer M, Paxton WA, Hoelscher M, Koup RA. 2010. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med 207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marin ND, Paris SC, Rojas M, Garcia LF. 2012. Reduced frequency of memory T cells and increased Th17 responses in patients with active tuberculosis. Clin Vaccine Immunol 19:1667–1676. doi: 10.1128/CVI.00390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K, Kaufmann SH, Jacobsen M. 2008. Mycobacterium tuberculosis-specific CD4+, IFNγ+, and TNFα+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43:143–148. doi: 10.1016/j.cyto.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano G, Girardi E, Palmieri F, Goletti D. 2013. IFNγ/TNFα specific-cells and effector memory phenotype associate with active tuberculosis. J Infect 66:475–486. doi: 10.1016/j.jinf.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Pollock KM, Whitworth HS, Montamat-Sicotte DJ, Grass L, Cooke GS, Kapembwa MS, Kon OM, Sampson RD, Taylor GP, Lalvani A. 2013. T-cell immunophenotyping distinguishes active from latent tuberculosis. J Infect Dis 208:952–968. doi: 10.1093/infdis/jit265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 30.Zielinski CE, Corti D, Mele F, Pinto D, Lanzavecchia A, Sallusto F. 2011. Dissecting the human immunologic memory for pathogens. Immunol Rev 240:40–51. doi: 10.1111/j.1600-065X.2010.01000.x. [DOI] [PubMed] [Google Scholar]
- 31.Leyten EM, Lin MY, Franken KL, Friggen AH, Prins C, van Meijgaarden KE, Voskuil MI, Weldingh K, Andersen P, Schoolnik GK, Arend SM, Ottenhoff TH, Klein MR. 2006. Human T-cell responses to 25 novel antigens encoded by genes of the dormancy regulon of Mycobacterium tuberculosis. Microbes Infect 8:2052–2060. doi: 10.1016/j.micinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Black GF, Thiel BA, Ota MO, Parida SK, Adegbola R, Boom WH, Dockrell HM, Franken KL, Friggen AH, Hill PC, Klein MR, Lalor MK, Mayanja H, Schoolnik G, Stanley K, Weldingh K, Kaufmann SH, Walzl G, Ottenhoff TH. 2009. Immunogenicity of novel DosR regulon-encoded candidate antigens of Mycobacterium tuberculosis in three high-burden populations in Africa. Clin Vaccine Immunol 16:1203–1212. doi: 10.1128/CVI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Commandeur S, Lin MY, van Meijgaarden KE, Friggen AH, Franken KL, Drijfhout JW, Korsvold GE, Oftung F, Geluk A, Ottenhoff TH. 2011. Double- and monofunctional CD4+ and CD8+ T-cell responses to Mycobacterium tuberculosis DosR antigens and peptides in long-term latently infected individuals. Eur J Immunol 41:2925–2936. doi: 10.1002/eji.201141602. [DOI] [PubMed] [Google Scholar]
- 34.Riano F, Arroyo L, Paris S, Rojas M, Friggen AH, van Meijgaarden KE, Franken KL, Ottenhoff TH, Garcia LF, Barrera LF. 2012. T cell responses to DosR and Rpf proteins in actively and latently infected individuals from Colombia. Tuberculosis (Edinb) 92:148–159. doi: 10.1016/j.tube.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Hozumi H, Tsujimura K, Yamamura Y, Seto S, Uchijima M, Nagata T, Miwa S, Hayakawa H, Fujisawa T, Hashimoto D, Inui N, Suda T, Chida K, Koide Y. 2013. Immunogenicity of dormancy-related antigens in individuals infected with Mycobacterium tuberculosis in Japan. Int J Tuberc Lung Dis 17:818–824. doi: 10.5588/ijtld.12.0695. [DOI] [PubMed] [Google Scholar]
- 36.Arroyo L, Rojas M, Ortiz BL, Franken KL, Garcia LF, Ottenhoff TH, Barrera LF. 2016. Dynamics of the T cell response to Mycobacterium tuberculosis DosR and Rpf antigens in a Colombian population of household contacts of recently diagnosed pulmonary tuberculosis patients. Tuberculosis (Edinb) 97:97–107. doi: 10.1016/j.tube.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. 2003. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehra S, Foreman TW, Didier PJ, Ahsan MH, Hudock TA, Kissee R, Golden NA, Gautam US, Johnson AM, Alvarez X, Russell-Lodrigue KE, Doyle LA, Roy CJ, Niu T, Blanchard JL, Khader SA, Lackner AA, Sherman DR, Kaushal D. 2015. The DosR regulon modulates adaptive immunity and is essential for Mycobacterium tuberculosis persistence. Am J Respir Crit Care Med 191:1185–1196. doi: 10.1164/rccm.201408-1502OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boon C, Dick T. 2012. How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later. Future Microbiol 7:513–518. doi: 10.2217/fmb.12.14. [DOI] [PubMed] [Google Scholar]
- 40.Schuck SD, Mueller H, Kunitz F, Neher A, Hoffmann H, Franken KL, Repsilber D, Ottenhoff TH, Kaufmann SH, Jacobsen M. 2009. Identification of T-cell antigens specific for latent Mycobacterium tuberculosis infection. PLoS One 4:e5590. doi: 10.1371/journal.pone.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Commandeur S, van Meijgaarden KE, Lin MY, Franken KL, Friggen AH, Drijfhout JW, Oftung F, Korsvold GE, Geluk A, Ottenhoff TH. 2011. Identification of human T-cell responses to Mycobacterium tuberculosis resuscitation-promoting factors in long-term latently infected individuals. Clin Vaccine Immunol 18:676–683. doi: 10.1128/CVI.00492-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang W, Qi Y, Ren C, Wen H, Franken KL, Ottenhoff TH, Shen J. 2013. Interferon-γ responses to Mycobacterium tuberculosis Rpf proteins in contact investigation. Tuberculosis (Edinb) 93:612–617. doi: 10.1016/j.tube.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Mukamolova GV, Turapov OA, Young DI, Kaprelyants AS, Kell DB, Young M. 2002. A family of autocrine growth factors in Mycobacterium tuberculosis. Mol Microbiol 46:623–635. doi: 10.1046/j.1365-2958.2002.03184.x. [DOI] [PubMed] [Google Scholar]
- 44.Gupta RK, Srivastava R. 2012. Resuscitation promoting factors: a family of microbial proteins in survival and resuscitation of dormant mycobacteria. Indian J Microbiol 52:114–121. doi: 10.1007/s12088-011-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.del Corral H, Paris SC, Marin ND, Marin DM, Lopez L, Henao HM, Martinez T, Villa L, Barrera LF, Ortiz BL, Ramirez ME, Montes CJ, Oquendo MC, Arango LM, Riano F, Aguirre C, Bustamante A, Belisle JT, Dobos K, Mejia GI, Giraldo MR, Brennan PJ, Robledo J, Arbelaez MP, Rojas CA, Garcia LF. 2009. IFNγ response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One 4:e8257. doi: 10.1371/journal.pone.0008257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Secretaría de Salud de Medellín. 2015. Boletín epidemiológico Medellín, ciudad saludable, número 4. Secretaría de Salud de Medellín, Medellín, Colombia. [Google Scholar]
- 47.Franken KL, Hiemstra HS, van Meijgaarden KE, Subronto Y, den Hartigh J, Ottenhoff TH, Drijfhout JW. 2000. Purification of His-tagged proteins by immobilized chelate affinity chromatography: the benefits from the use of organic solvent. Protein Expr Purif 18:95–99. doi: 10.1006/prep.1999.1162. [DOI] [PubMed] [Google Scholar]
- 48.Marin ND, Paris SC, Rojas M, Garcia LF. 2013. Functional profile of CD4+ and CD8+ T cells in latently infected individuals and patients with active TB. Tuberculosis (Edinb) 93:155–166. doi: 10.1016/j.tube.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Appay V, van Lier RA, Sallusto F, Roederer M. 2008. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 73:975–983. [DOI] [PubMed] [Google Scholar]
- 50.Tapaninen P, Korhonen A, Pusa L, Seppala I, Tuuminen T. 2010. Effector memory T-cells dominate immune responses in tuberculosis treatment: antigen or bacteria persistence? Int J Tuberc Lung Dis 14:347–355. [PubMed] [Google Scholar]
- 51.Chatterjee S, Clark CE, Lugli E, Roederer M, Nutman TB. 2015. Filarial infection modulates the immune response to Mycobacterium tuberculosis through expansion of CD4+ IL-4 memory T cells. J Immunol 194:2706–2714. doi: 10.4049/jimmunol.1402718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goletti D, Butera O, Vanini V, Lauria FN, Lange C, Franken KL, Angeletti C, Ottenhoff TH, Girardi E. 2010. Response to Rv2628 latency antigen associates with cured tuberculosis and remote infection. Eur Respir J 36:135–142. doi: 10.1183/09031936.00140009. [DOI] [PubMed] [Google Scholar]
- 53.Govender L, Abel B, Hughes EJ, Scriba TJ, Kagina BM, de Kock M, Walzl G, Black G, Rosenkrands I, Hussey GD, Mahomed H, Andersen P, Hanekom WA. 2010. Higher human CD4 T cell response to novel Mycobacterium tuberculosis latency associated antigens Rv2660 and Rv2659 in latent infection compared with tuberculosis disease. Vaccine 29:51–57. doi: 10.1016/j.vaccine.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutherland JS, Lalor MK, Black GF, Ambrose LR, Loxton AG, Chegou NN, Kassa D, Mihret A, Howe R, Mayanja-Kizza H, Gomez MP, Donkor S, Franken K, Hanekom W, Klein MR, Parida SK, Boom WH, Thiel BA, Crampin AC, Ota M, Walzl G, Ottenhoff TH, Dockrell HM, Kaufmann SH. 2013. Analysis of host responses to Mycobacterium tuberculosis antigens in a multi-site study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS One 8:e74080. doi: 10.1371/journal.pone.0074080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cehovin A, Cliff JM, Hill PC, Brookes RH, Dockrell HM. 2007. Extended culture enhances sensitivity of a gamma interferon assay for latent Mycobacterium tuberculosis infection. Clin Vaccine Immunol 14:796–798. doi: 10.1128/CVI.00093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leyten EM, Arend SM, Prins C, Cobelens FG, Ottenhoff TH, van Dissel JT. 2007. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short and prolonged in vitro incubation. Clin Vaccine Immunol 14:880–885. doi: 10.1128/CVI.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanekom WA, Dockrell HM, Ottenhoff TH, Doherty TM, Fletcher H, McShane H, Weichold FF, Hoft DF, Parida SK, Fruth UJ. 2008. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med 5:e145. doi: 10.1371/journal.pmed.0050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phong WY, Lin W, Rao SP, Dick T, Alonso S, Pethe K. 2013. Characterization of phosphofructokinase activity in Mycobacterium tuberculosis reveals that a functional glycolytic carbon flow is necessary to limit the accumulation of toxic metabolic intermediates under hypoxia. PLoS One 8:e56037. doi: 10.1371/journal.pone.0056037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hutter B, Dick T. 2000. Analysis of the dormancy-inducible narK2 promoter in Mycobacterium bovis BCG. FEMS Microbiol Lett 188:141–146. doi: 10.1111/j.1574-6968.2000.tb09185.x. [DOI] [PubMed] [Google Scholar]
- 60.Sohaskey CD, Wayne LG. 2003. Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol 185:7247–7256. doi: 10.1128/JB.185.24.7247-7256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khan A, Sarkar D. 2012. Nitrate reduction pathways in mycobacteria and their implications during latency. Microbiology 158:301–307. doi: 10.1099/mic.0.054759-0. [DOI] [PubMed] [Google Scholar]
- 62.Singh S, Saraav I, Sharma S. 2014. Immunogenic potential of latency associated antigens against Mycobacterium tuberculosis. Vaccine 32:712–716. doi: 10.1016/j.vaccine.2013.11.065. [DOI] [PubMed] [Google Scholar]
- 63.Gupta RK, Srivastava BS, Srivastava R. 2010. Comparative expression analysis of rpf-like genes of Mycobacterium tuberculosis H37Rv under different physiological stress and growth conditions. Microbiology 156:2714–2722. doi: 10.1099/mic.0.037622-0. [DOI] [PubMed] [Google Scholar]
- 64.Rueda CM, Marin ND, Garcia LF, Rojas M. 2010. Characterization of CD4 and CD8 T cells producing IFN-γ in human latent and active tuberculosis. Tuberculosis (Edinb) 90:346–353. doi: 10.1016/j.tube.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Jafari C, Thijsen S, Sotgiu G, Goletti D, Dominguez Benitez JA, Losi M, Eberhardt R, Kirsten D, Kalsdorf B, Bossink A, Latorre I, Migliori GB, Strassburg A, Winteroll S, Greinert U, Richeldi L, Ernst M, Lange C. 2009. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trialsgroup study. Am J Respir Crit Care Med 180:666–673. doi: 10.1164/rccm.200904-0557OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jafari C, Kessler P, Sotgiu G, Ernst M, Lange C. 2011. Impact of a Mycobacterium tuberculosis-specific interferon-γ release assay in bronchoalveolar lavage fluid for a rapid diagnosis of tuberculosis. J Intern Med 270:254–262. doi: 10.1111/j.1365-2796.2011.02378.x. [DOI] [PubMed] [Google Scholar]
- 67.Prezzemolo T, Guggino G, La Manna MP, Di Liberto D, Dieli F, Caccamo N. 2014. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front Immunol 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soares AP, Kwong Chung CK, Choice T, Hughes EJ, Jacobs G, van Rensburg EJ, Khomba G, de Kock M, Lerumo L, Makhethe L, Maneli MH, Pienaar B, Smit E, Tena-Coki NG, van Wyk L, Boom WH, Kaplan G, Scriba TJ, Hanekom WA. 2013. Longitudinal changes in CD4+ T-cell memory responses induced by BCG vaccination of newborns. J Infect Dis 207:1084–1094. doi: 10.1093/infdis/jis941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gideon HP, Phuah J, Myers AJ, Bryson BD, Rodgers MA, Coleman MT, Maiello P, Rutledge T, Marino S, Fortune SM, Kirschner DE, Lin PL, Flynn JL. 2015. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog 11:e1004603. doi: 10.1371/journal.ppat.1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han Q, Bagheri N, Bradshaw EM, Hafler DA, Lauffenburger DA, Love JC. 2012. Polyfunctional responses by human T cells result from sequential release of cytokines. Proc Natl Acad Sci U S A 109:1607–1612. doi: 10.1073/pnas.1117194109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith SG, Smits K, Joosten SA, van Meijgaarden KE, Satti I, Fletcher HA, Caccamo N, Dieli F, Mascart F, McShane H, Dockrell HM, Ottenhoff TH. 2015. Intracellular cytokine staining and flow cytometry: considerations for application in clinical trials of novel tuberculosis vaccines. PLoS One 10:e0138042. doi: 10.1371/journal.pone.0138042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cliff JM, Kaufmann SH, McShane H, van Helden P, O'Garra A. 2015. The human immune response to tuberculosis and its treatment: a view from the blood. Immunol Rev 264:88–102. doi: 10.1111/imr.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mattos AMM, Almeida CDS, Franken KLMC, Alves CCDS, Abramo C, de Souza MA, L'Hotellier M, Alves MJM, Ferreira AP, Oliveira SC, Ottenhoff THM, Teixeira HC. 2010. Increased IgG1, IFN-γ, TNF-α and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol 22:775–782. doi: 10.1093/intimm/dxq429. [DOI] [PubMed] [Google Scholar]
- 74.Adetifa IM, Ota MO, Walther B, Hammond AS, Lugos MD, Jeffries DJ, Donkor SA, Adegbola RA, Hill PC. 2010. Decay kinetics of an interferon gamma release assay with anti-tuberculosis therapy in newly diagnosed tuberculosis cases. PLoS One 5:e12502. doi: 10.1371/journal.pone.0012502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X, Cao Z, Jiang J, Niu H, Dong M, Tong A, Cheng X. 2010. Association of mycobacterial antigen-specific CD4+ memory T cell subsets with outcome of pulmonary tuberculosis. J Infect 60:133–139. doi: 10.1016/j.jinf.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 76.Tan Q, Xie WP, Min R, Dai GQ, Xu CC, Pan HQ, Miao CD, Yang Z, Xu WG, Wang H. 2012. Characterization of Th1- and Th2-type immune response in human multidrug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis 31:1233–1242. doi: 10.1007/s10096-011-1434-4. [DOI] [PubMed] [Google Scholar]
- 77.Li N, Xie WP, Kong H, Min R, Hu CM, Zhou X, Lu ZM, Ji XH, Wang H. 2015. Enrichment of regulatory T-cells in blood of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 19:1230–1238. doi: 10.5588/ijtld.15.0148. [DOI] [PubMed] [Google Scholar]
- 78.World Health Organization. 2015. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.