Abstract
The identification of nearly 3,500 cases of chikungunya virus (CHIKV) infection in U.S. residents returning in 2014 and 2015 from areas in which it is endemic has raised concerns within the transplant community that, should recently infected individuals become organ and/or tissue donors, CHIKV would be transmitted to transplant recipients. Thus, tests designed to detect recent CHIKV infection among U.S. organ and tissue donors may become necessary in the future. Accordingly, we evaluated 2 enzyme-linked immunosorbent assays (ELISAs) for CHIKV IgM readily available in the United States using 1,000 deidentified serum or plasma specimens collected from donors between November 2014 and March 2015. The Euroimmun indirect ELISA identified 38 reactive specimens; however, all 38 were negative for CHIKV IgG and IgM in immunofluorescence assays (IFAs) conducted at a reference laboratory and, thus, were falsely reactive in the Euroimmun CHIKV IgM assay. The InBios IgM-capture ELISA identified 26 reactive samples, and one was still reactive (index ≥ 1.00) when retested using the InBios kit with a background subtraction modification to identify false reactivity. This reactive specimen was CHIKV IgM negative but IgG positive by IFAs at two reference laboratories; plaque reduction neutralization testing (PRNT) demonstrated CHIKV-specific reactivity. The IgG and PRNT findings strongly suggest that the InBios CHIKV IgM-reactive result represents true reactivity, even though the IgM IFA result was negative. If testing organ/tissue donors for CHIKV IgM becomes necessary, the limitations of the currently available CHIKV IgM ELISAs and options for their optimization must be understood to avoid organ/tissue wastage due to falsely reactive results.
INTRODUCTION
Chikungunya virus (CHIKV) is an alphavirus transmitted from person to person via mosquito bites (1). Symptoms include fever, rash, and debilitating arthralgia; 15% to 60% of patients develop chronic arthralgia leading to arthritic joint damage (2). After a large CHIKV outbreak in India and southeast Asia in 2004 through 2006, in which nearly 2 million people became infected (3, 4), epidemiologists predicted that CHIKV might move to other geographic areas where the mosquito vectors are found (5). This prediction was realized in December 2013, when local transmission of CHIKV was reported on the Caribbean island of St. Martin (6). CHIKV infection has since spread throughout the Caribbean basin (7) and is now also endemic in Mexico, Central America, and South America and in the Caribbean island nations. In conjunction with this outbreak, 3,490 cases in U.S. residents (from 49 of 50 states) were reported to the CDC during 2014 and 2015; 3,478 cases represented infections acquired during international travel to areas where CHIKV is endemic, whereas 12 cases represented local transmission (8).
There is concern within the transplant community that CHIKV could be transmitted from organ and/or tissue donors to recipients. Donor-derived transmission of other mosquito-borne viruses with similar epidemiologic and biologic features, notably dengue virus and West Nile virus, has been documented (9, 10). Although no cases of CHIKV transmission by transplantation have yet been reported, studies have shown that CHIKV can be isolated from corneas of acutely infected individuals (11), and atypical manifestations of CHIKV infection were reported in a recipient who became infected 7 years after receiving a liver transplant (12). However, the likelihood of CHIKV transmission by transplantation, and what organs/tissues might harbor the virus, remains unknown.
As additional information regarding CHIKV transmission by transplantation becomes available, there might be a future need for tests to identify recent CHIKV infection, particularly among U.S. donors living in geographic areas where many residents travel internationally and have close ethnic ties to areas where CHIKV is endemic (13). There are two accepted methods for identifying recent CHIKV infection, detection of CHIKV IgM and CHIKV RNA. CHIKV RNA is detectable in serum within the first week after symptom onset but then subsides to undetectable levels. CHIKV IgM, in contrast, becomes detectable by day 5 after the onset of symptoms and remains detectable for approximately 4 months. Thus, CHIKV RNA testing would identify organ/tissue donors who were infected within 1 week prior to donation, whereas CHIKV IgM detection would identify donors who were infected 5 days to 4 months prior to donation. From this standpoint, CHIKV IgM appears to be the more robust indicator of recent infection in organ/tissue donors (14, 15, 16). Thus, we evaluated the performance of two commercially available CHIKV IgM enzyme-linked immunosorbent assay (ELISA) kits using 1,000 archived serum or plasma samples from organ or tissue donors.
MATERIALS AND METHODS
Donor specimen selection and deidentification.
The 1,000 specimens evaluated were collected from organ or tissue donors in the United Network for Organ Sharing (UNOS) region 5, composed of several southwestern and western U.S. states, including California. All six UNOS region 5 organ procurement organizations (OPOs) served by our laboratory provided permission to utilize their specimens for the study. For each month of the 5-month period (November 2014 through March 2015), the first 60 serum or plasma samples from deceased prospective organ (heart-beating) donors and the first 140 serum or plasma samples from deceased prospective tissue (cadaveric) donors submitted to our facility were retrieved from −80° storage. The study panel thus contained 300 organ donor samples and 700 tissue donor samples; this distribution reflects the relative proportions of deceased organ donor versus deceased tissue donor specimens submitted to our facility for infectious disease screening. Samples were deidentified as requested by the participating OPOs; a sample within a given group (defined by donor type [organ or tissue] and month of collection) was randomly selected, and 1 ml of the sample was transferred to a randomly selected, numbered plastic vial within a designated numeric range. No key was created to enable tracking of which donor specimen was transferred to which numbered vial. Thus, the only demographic data available for a given specimen were donor type (organ or tissue) and month of collection; no result could be linked to a donor's protected health information.
Known positive specimens.
Five serum samples known to be positive for CHIKV IgM by an immunofluorescence assay (IFA) were obtained from Focus Diagnostics (San Juan Capistrano, CA); titers ranged from 1:40 to 1:640 (reference value, <1:10) (16).
Euroimmun assay.
Samples were tested for CHIKV IgM using the anti-chikungunya virus ELISA (IgM) kit (designated for research use only) purchased from Euroimmun US (Mountain Lakes, NJ), according to the manufacturer's instructions (17). Briefly, serum or plasma samples diluted 1:101 in sample diluent containing anti-human IgG were added to microtiter wells coated with a proprietary mixture of recombinant CHIKV antigens. After the wells sat for 1 h at 37°C and were washed, peroxidase-labeled anti-human IgM was added to them. After 30 min at room temperature (RT), the wells were washed again and then reacted with a chromogen-substrate solution (tetramethylbenzidine [TMB] plus hydrogen peroxide) for 15 min at RT; the color reaction was stopped by the addition of 0.5 M sulfuric acid. Optical densities (ODs) were measured at 450 nm and 630 nm (reference wavelength) for each well using a BioTek ELx800 photometric reader (Winooski, VT). Per the manufacturer's instructions, the results were expressed as index values, calculated by dividing a specimen's OD by the OD of a kit-supplied calibrator serum included in the same run. Index values of <0.8 were considered negative, values of 0.8 to <1.1 were considered equivocal, and values of ≥1.1 were deemed positive.
InBios screening assay.
Samples were also tested for CHIKV IgM using the CHIKjj Detect IgM-capture ELISA kit (designated for research use only) purchased from InBios International (Seattle, WA) (18). Briefly, samples were diluted 1:100 in dilution buffer and added to microtiter wells coated with polyclonal anti-human IgM (the animal source is considered proprietary). After wells sat for 30 min at 37°C and were washed, they then were incubated for 30 min at 37°C with an antigen solution containing a recombinant CHIKV envelope (E2/E1) glycoprotein produced in C7/10 mosquito cells (19). Wells were again washed and then incubated with peroxidase-labeled monoclonal anti-CHIKV antibody (CHK-175) for 30 min at 37°C. After the wells underwent a final wash, a TMB substrate solution was added, and after 10 min at RT, the color reaction was stopped by the addition of 1 N sulfuric acid. OD at 450 nm (no reference wavelength) was measured for each well using a BioTek ELx800 photometric reader. Per the manufacturer's instructions, results were expressed as index values, calculated by dividing a specimen's OD by the OD of a kit-supplied cutoff serum included in the same run. Index values of <1.00 were considered nonreactive, and values of ≥1.00 were considered reactive.
InBios background subtraction modification.
All samples reactive in the InBios screening assay were retested using the InBios kit modified to identify samples falsely reactive due to IgM antibodies that recognize immunoglobulins of other animal species, known as heterophilic IgM antibodies. These antibodies, like all IgM antibodies, are captured by the immobilized anti-IgM antibody attached to the microtiter well. Then, due to their heterophilic activity, they bind the peroxidase-labeled monoclonal anti-CHIKV antibody reagent when added, both in the presence and in the absence of antigen (20). For this modified assay, the diluted kit-supplied cutoff control and each diluted donor sample were added to 2 wells, and after the initial incubation and washing, one well received CHIKV antigen and the other well received sample dilution buffer. The assay was then finished per the manufacturer's instructions, and the OD of each well was measured as described for the screening assay. For each sample, the net OD was calculated by subtracting the OD of the well receiving dilution buffer from the OD of the well receiving antigen (hence the designation of this modification as background subtraction [BS]). These net OD values were then used to calculate the BS index, defined as the net sample OD divided by the net cutoff OD (21); the same interpretive guidelines employed for the screening assay were used for the modified assay. In addition, the value observed from the antigen well OD (Ag OD) was divided by the value observed from the diluent well OD (dil OD) to calculate the Ag OD/dil OD ratio; this value has proven useful for identifying false-reactive samples in the InBios dengue IgM-capture and West Nile virus IgM-capture ELISAs (22, 23).
Reference laboratory testing.
All samples considered reactive (equivocal or positive) in the Euroimmun assay and 2 samples with InBios BS index values of >0.80 were sent to Focus Diagnostics, where they were tested for CHIKV IgG and IgM using validated IFAs (16). For both IFAs, samples were screened at a 1:10 dilution. The 5 samples with the highest positive Euroimmun ELISA index values and the 2 samples with InBios BS index values of >0.80 were also sent to the California Department of Public Health (CDPH) Viral and Rickettsial Disease Laboratory (Richmond, CA), where they were tested for CHIKV IgG and IgM by IFA; for both IFAs, samples were screened at a 1:8 dilution, and the titers of the positive samples were determined by endpoint dilution. Per CDPH protocol, one sample positive for CHIKV IgG was reflexed to alphavirus plaque reduction neutralization testing (PRNT), which assessed the ability of various dilutions of the sample to inhibit the infectivity of CHIKV and western equine encephalitis virus (WEEV).
Statistics.
Differences between proportions were assessed by calculating the chi-square statistic (23), with significance defined as a P value of <0.01.
RESULTS
Reactivity in screening assays.
Table 1 presents a summary of the findings observed for organ and tissue donor serum or plasma specimens when tested for CHIKV IgM using the Euroimmun and InBios screening assays. Of 1,000 donor samples, 38 (3.8%) were reactive (equivocal or positive) in the Euroimmun ELISA, with 6.0% of 300 organ donor samples and 2.86% of 700 tissue donor samples reactive. Similarly, 26 of 1,000 (2.6%) samples were reactive in the InBios screening assay, with 2.3% of samples from organ donors and 2.7% of samples from tissue donors reactive. Only one specimen was reactive in both the Euroimmun (equivocal) and InBios screening assays. When assessing differences between proportions for various groups, the only difference that reached statistical significance was the proportion of organ donor versus tissue donor samples with an equivocal Euroimmun result (4.3% versus 1.1%, P = 0.001). The index range of positive donor samples in the Euroimmun ELISA was 1.13 to 3.21, with 13/17 (76%) between 1.13 and 2.00; the index range of reactive donor samples in the InBios ELISA was 1.06 to 5.98, with 22/26 (85%) between 1.06 and 2.00. All 5 known CHIKV IgM-positive samples obtained from Focus Diagnostics were positive in the Euroimmun ELISA (index range, 2.07 to 6.91) and reactive in the InBios screening assay (index range, 6.48 to 12.75).
TABLE 1.
Results for deceased-donor specimens tested for CHIKV IgM using Euroimmun and InBios screening ELISAs
| Donor group | n | Euorimmun assay results (n [%]) |
InBios assay results (n [%]) |
|||
|---|---|---|---|---|---|---|
| Negative | Equivocal | Positive | Nonreactive | Reactive | ||
| Organ | 300 | 282 | 13 (4.3) | 5 (1.7) | 293 | 7 (2.3) |
| Tissue | 700 | 680 | 8 (1.1) | 12 (1.7) | 681 | 19 (2.7) |
| All | 1,000 | 962 | 21 (2.1) | 17 (1.7) | 974 | 26 (2.6) |
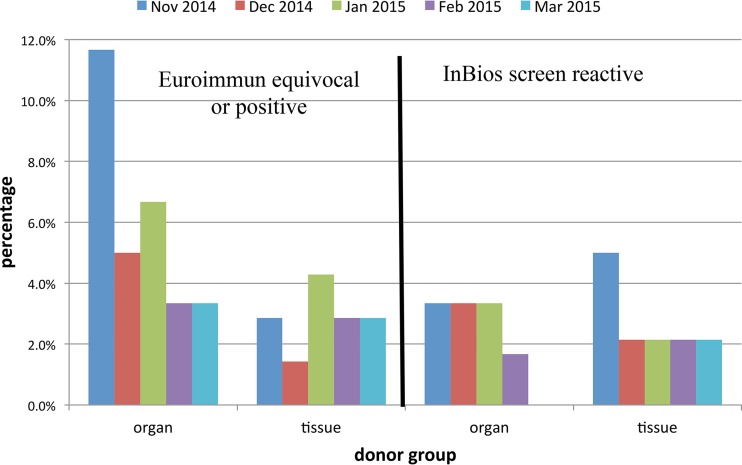
Figure 1 presents the proportions of donor samples reactive in the 2 assays by month of sample submission and donor group. With one exception, reactive results were observed in both assays from both donor groups during each month; the exception was that no InBios-reactive samples were found among the March 2015 organ donor samples. Although the proportion of organ donor samples reactive in the Euroimmun assay was higher in those obtained in November 2014 than in those obtained in other months, this difference was not statistically significant. Similarly, the proportion of tissue donor samples reactive in the InBios assay was higher in samples obtained in November 2014 than in those obtained in other months, but this difference was not statistically significant.
FIG 1.
Proportions of samples reactive in screening assays in relation to donor group and month of sample collection.
Reactivity in the InBios assay with background subtraction.
Samples reactive in the InBios CHIKV IgM screening assay were retested using the BS modification of the InBios assay to identify samples falsely reactive due to heterophilic IgM antibodies (20). Table 2 provides examples of various reactivity data patterns observed in the BS assay. Note in particular that the false-reactive sample exhibited a reactive screening index (1.23) but a nonreactive BS index (0.14) and an Ag OD/dil OD ratio that was identical to that of the nonreactive sample (1.08). In contrast, the true-reactive sample (from Focus Diagnostics) exhibited both screening index and BS index values of >1.00 and an Ag OD/dil OD ratio that was markedly higher than the ratios of the nonreactive and false-reactive samples.
TABLE 2.
Examples of BS index and Ag OD/dil OD ratios in the InBios assay with BS modification
| Sample | Antigen well OD | Diluent well OD | Net OD (Ag OD − dil OD) | Screen index | BS index | Ag OD/dil OD |
|---|---|---|---|---|---|---|
| Calibrator | 0.145 | 0.049 | 0.096 | 2.96 | ||
| Nonreactive | 0.060 | 0.051 | 0.009 | 0.41 | 0.09 | 1.08 |
| False reactive | 0.178 | 0.165 | 0.013 | 1.23 | 0.14 | 1.08 |
| True reactive | 0.818 | 0.052 | 0.766 | 5.64 | 7.98 | 15.73 |
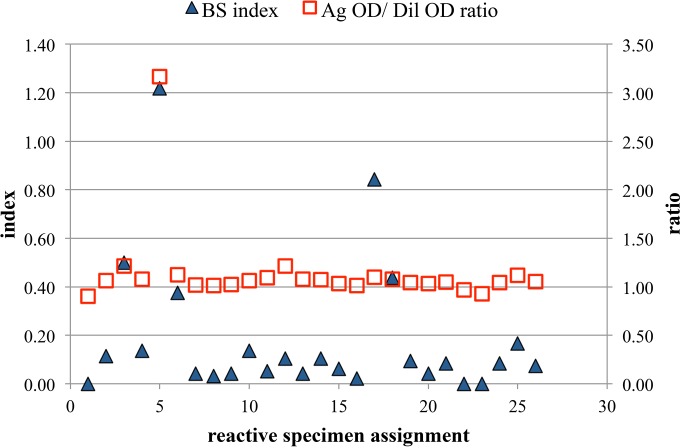
Figure 2 presents the In Bios BS index and Ag OD/dil OD ratio results for the 26 donor specimens that were reactive in the InBios screening assay. Screen-reactive specimen 5 was clearly distinctive, exhibiting a BS index of >1.00 and an Ag OD/dil OD ratio that was more than 2.5-fold higher than the ratios observed for the other 25 samples. Screen-reactive specimen 17 also exhibited a BS index that was markedly higher than the indices of all other samples (except specimen 5), but the Ag OD/dil OD ratio of specimen 17 was not increased compared to the ratios of other samples (except specimen 5). Screen-reactive specimen 1 was the sample that exhibited reactive results in both the Euroimmun and InBios screening ELISAs; as shown in Fig. 2, this sample yielded a negative result in the InBios BS assay. As expected, all 5 known positive samples from Focus Diagnostics exhibited increased BS index values (≥7.98) and increased Ag OD/dil OD ratios (≥15.73) (data not shown).
FIG 2.
InBios background subtraction (BS) assay results for 26 specimens reactive in the InBios screening assay.
Reference laboratory test results.
All 38 samples reactive in the Euroimmun ELISA were negative for CHIKV IgG and CHIKV IgM when tested by IFA at Focus Diagnostics; similarly, the 5 samples with the highest positive index values in the Euroimmun ELISA were also negative for CHIKV IgG and IgM when tested by IFA at CDPH. The 2 samples with a BS index value of >0.80 in the InBios BS assay (screen-reactive specimens 5 and 17) were also sent to both Focus Diagnostics and CDPH for CHIKV antibody testing; the results are summarized in Table 3. Reactive specimen 17 was negative for CHIKV IgG and IgM at both reference laboratories. In contrast, reactive specimen 5 was negative for CHIKV IgM but positive for CHIKV IgG at both reference laboratories, with similar CHIKV IgG titers. PRNT results from the CDPH for reactive specimen 5 demonstrated that it had specific neutralizing activity toward CHIKV (titer, 1:40) but not WEEV (titer, <1:20). These results are consistent with the interpretation that the InBios CHIKV IgM-reactive result for reactive specimen 5 (collected from an organ donor in January 2015) represents a true-reactive result.
TABLE 3.
Reference laboratory testing results for 2 samples with InBios BS index values of >0.80
| Parameter | Specimen 17 | Specimen 5 |
|---|---|---|
| Euroimmun index | 0.38 | 0.28 |
| InBios screen index | 5.98 | 1.12 |
| InBios BS index | 0.84 | 1.22 |
| InBios Ag OD/Dil OD | 1.10 | 3.17 |
| Focus IgG IFA | Negative | Positive (titer 1:80) |
| Focus IgM IFA | Negative | Negative |
| CDPH IgG IFA | Negative | Positive (titer 1:128) |
| CDPH IgM IFA | Negative | Negative |
| CDPH PRNT | Not tested | Positive (titer 1:40), WEEV negative |
DISCUSSION
A forecasting model recently identified several U.S. urban centers as high-risk areas for travel-associated CHIKV infections (13). Should a CHIKV-infected traveler become an organ/tissue donor within a few months of his or her return to the United States, it is possible that the transplanted organ/tissue will still harbor enough of the virus to transmit the infection to the recipient. Thus, if the number of travel-related CHIKV cases continues to increase, or local mosquito-borne transmission escalates, an assay for identifying donors recently infected with CHIKV might be required by UNOS and other transplant organizations. Thus, the goal of our study was to evaluate the suitability of commercially available CHIKV IgM ELISA kits for testing samples from deceased organ and tissue donors, most of whom resided in southern California, an area at high risk for CHIKV transmission. The investigation produced several interesting findings regarding the performance characteristics of the CHIKV IgM ELISA kits evaluated.
On the basis of the small number (n = 5) of known CHIKV IgM-positive samples evaluated, both the Euroimmun and InBios assays exhibited excellent sensitivity. Specificity, however, was another matter. We found that 3.8% of donor samples were reactive (equivocal or positive) in the Euroimmun indirect ELISA, a value nearly 5-fold higher than the reactive rate of 0.8% presented in the package insert for a panel of 498 blood donors (17). Interestingly, the package insert value was based on a single cutpoint index of 1.0 to define reactivity as negative or positive rather than following the package insert guidelines for defining negative, equivocal, and positive reactivity. If we used this single cutpoint approach, our reactivity value would be 2.4%, which is still 3-fold higher than the value presented by the manufacturer. This difference most likely reflects differences in the populations studied (deceased organ/tissue donors versus living blood donors). Further, we found that all 38 samples reactive in the Euroimmun ELISA were falsely reactive on the basis of IFA results. The factors responsible for this false reactivity remain unknown; however, the observation that the proportion of samples exhibiting an equivocal Euroimmun result was significantly higher in organ donors than the proportion observed in tissue donors suggests that some types of false reactivity may be related to organ donor-specific predonation treatment factors. Further studies are needed to characterize these factors and to identify ways to optimize the assay to reduce or eliminate false reactivity.
Similarly, 2.6% of donor samples were reactive in the InBios IgM-capture ELISA when performed in accordance with the package insert. Although the data supplied in the package insert did not include expected reactivity levels among blood donors or a similar population (18), this finding was not unexpected on the basis of published findings for similar IgM-capture ELISAs (20, 24). As discussed by Levinson and Miller (20), the majority of samples reactive in IgM-capture assays that target pathogens in areas of low endemicity are falsely reactive due to heterophilic antibodies, which recognize immunoglobulins of other animal species. Inherent to the IgM-capture format, however, is the ability to distinguish false reactivity from true reactivity using the BS modification. When this modified assay is performed, samples falsely reactive in the screening assay exhibit essentially the same signal intensity in the presence and absence of antigen, whereas truly reactive samples exhibit much higher signal intensity in the presence of antigen than in the absence of antigen (21, 25). This signal difference is the basis for calculating the BS index and the Ag OD/dil OD ratio; both have been used to distinguish false from true reactivity (21–23, 25). Thus, IgM-capture ELISAs offer a major advantage over indirect IgM ELISAs, since identification of falsely reactive samples by a simple assay modification is not possible for the indirect format (22, 23, 25).
The most interesting finding in our study was the identification of a specimen apparently truly reactive for CHIKV IgM using the InBios IgM-capture ELISA with BS modification, even though it was CHIKV IgM negative by IFA. This sample exhibited a BS index higher than 1.00 and an Ag OD/dil OD ratio more than 2.5-fold higher than the ratios of 25 falsely reactive samples. As further support for this true-reactive interpretation, the sample was CHIKV IgG positive by IFA at 2 different reference laboratories, and this reactivity was specific for CHIKV by PRNT; these observations indicate that the donor was truly exposed to CHIKV, and thus, the InBios IgM reactivity represents true reactivity. The explanation for why the sample yielded a positive signal using the InBios ELISA but not IFA remains unknown; one possibility is that the antigenic epitopes on the recombinant protein used in the ELISA are of sufficient abundance to yield a reactive signal but are too sparsely distributed or insufficiently exposed within infected cells to yield a positive signal in the IFA. Additional studies are needed to determine the source of these discrepant reactivities.
Our study has some limitations. The InBios CHIKV IgM ELISA package insert does not include instructions for performing the BS modification; thus, interpretation of a BS index of >1.0 as reactive has not been validated by the kit manufacturer. However, data from the reference laboratories for the two samples with the highest InBios BS index values suggest that a BS index of 1.0 is an appropriate cutpoint for distinguishing positive from negative reactivity. Similarly, there has been no systematic determination of the appropriate Ag OD/dil OD ratio cutpoint for discriminating true reactivity from false reactivity in the InBios CHIKV IgM assay. The InBios dengue IgM and West Nile virus IgM ELISAs both employ BS modification, with the results expressed as Ag OD/dil OD ratios (21, 22); however, the cutpoints are different in the 2 assays, indicating that the cutpoint for CHIKV IgM is assay specific. It remains unclear why InBios elected to market the CHIKV IgM assay without BS modification, since other InBios IgM-capture ELISAs employ this format. A limitation from the specimen standpoint is that some of the donor samples evaluated may have been collected after the donors were transfused with blood products, crystalloids, and/or colloids, which might have altered the results via blood volume dilution. Lastly, the sample deidentification process precluded us from accessing demographic information for the donor who supplied the specimen reactive in the InBios BS assay; such information would have enabled a clearer assessment of the likelihood that this donor was truly infected with CHIKV and when and where the exposure occurred.
If CHIKV IgM ELISAs are ever utilized to screen organ/tissue donors for recent CHIKV infection, a complete understanding of the limitations of the currently available assays will be required to prevent organ/tissue wastage due to falsely reactive results. Thus, further studies are needed to optimize currently available assays (or develop new assays) for identifying individuals with recent CHIKV infection.
ACKNOWLEDGMENTS
We thank the following UNOS Region 5 OPOs for permission to include their samples in this study: Donor Network West, Intermountain Donor Services, LifeSharing, Nevada Donor Network, OneLegacy, and Sierra Donor Services. We also thank Dr. Sharon Messenger and the staff of the CDPH for performing IFA and PRNT.
REFERENCES
- 1.Powers AM, Logue CH. 2007. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol 88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 2.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F. 2008. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on Reunion Island. Clin Infect Dis 47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 3.Staikowsky F, Talarmin F, Grivard P, Souab A, Schuffenecker I, Le Roux K, Lecuit M, Michault A. 2009. Prospective study of chikungunya virus acute infection in the Island of La Reunion during the 2005–2006 outbreak. PLoS One 4:e7603. doi: 10.1371/journal.pone.0007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakshmi V, Neeraja M, Subbalaxmi MVS, Parida MM, Dash PK, Santhosh SR, Rao PV. 2008. Clinical features and molecular diagnosis of chikungunya fever from south India. Clin Infect Dis 46:1436–1442. doi: 10.1086/529444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. 2012. Chikungunya: a re-emerging virus. Lancet 379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Chikungunya fact sheet no. 327. http://www.who.int/mediacentre/factsheets/fs327/en/ Accessed 23 October 2014.
- 7.Pan American Health Organization. Number of reported cases of chikungunya fever in the Americas, by country or territory 2015. http://www.paho.org/hq/index.php?option=com_topics&view=article&id=343&Itemid=40931 Accessed 14 December 2015.
- 8.Centers for Disease Control and Prevention. Chikungunya virus in the United States. http://www.cdc.gov/chikungunya/geo/united-states.html Accessed 23 June 2016.
- 9.Saigal S, Choudhary NS, Saraf N, Kataria S, Mohanka R, Soin AS. 2013. Transmission of dengue virus from a donor to a recipient after living donor liver transplantation. Liver Transpl 19:1413–1414. doi: 10.1002/lt.23755. [DOI] [PubMed] [Google Scholar]
- 10.Winston DJ, Vikram HR, Rabe IB, Dhillon G, Mulligan D, Hong JC, Busuttil RW, Nowicki MJ, Mone T, Civen R, Tecle SA, Trivedi KK, Hocevar SN; West Nile Virus Transplant-Associated Transmission Investigation Team. 2014. Donor-derived West Nile virus infection in solid organ transplant recipients: report of four additional cases and review of clinical, diagnostics, and therapeutic features. Transplantation 97:881–889. doi: 10.1097/TP.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couderc T, Gangneux N, Chretien F, Caro V, Luong TL, Ducloux B, Tolou H, Lecuit M, Grandadam M. 2012. Chikungunya virus infection of corneal grafts. J Infect Dis 206:851–859. doi: 10.1093/infdis/jis296. [DOI] [PubMed] [Google Scholar]
- 12.Kee ACL, Yan S, Tambyah P. 2010. Atypical chikungunya virus infections in immune-compromised patients. Emerg Infect Dis 16:1038–1040. doi: 10.3201/eid1606.091115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredericks AC, Fernandez-Sesma A. 2014. The burden of dengue and chikungunya worldwide: implications for the southern United States and California. Ann Glob Health 80:466–475. doi: 10.1016/j.aogh.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staples JE, Beiman RF, Powers AM. 2009. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis 49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 15.Renault P, Solet J-L, Sissoko D, Balleydier E, Larrieu S, Filleul L, Lassalle C, Thiria J, Rachou E, de Valk H, Ilef D, Ledrans M, Quatresous I, Quenel P, Pierre V. 2007. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg 77:727–731. [PubMed] [Google Scholar]
- 16.Prince HE, Seaton BL, Matud JL, Batterman HJ. 2015. Chikungunya virus RNA and antibody testing at a national reference laboratory since the emergence of chikungunya virus in the Americas. Clin Vaccine Immunol 22:291–297. doi: 10.1128/CVI.00720-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Euroimmun Medizinische Labordiagnostika AG. Anti-chikungunya virus ELISA (IgM) test instruction. EI_293aM-_A_UK_C02.doc, version 15 September 2014. Euroimmun Medizinische Labordiagnostika AG, Mountain Lakes, NJ. [Google Scholar]
- 18.InBios International. CHIKjj Detect IgM ELISA. Insert part no. 900179-03. Effective date: 15 September 2015. InBios International, Seattle, WA. [Google Scholar]
- 19.Erasmus JH, Needham J, Raychaudhuri S, Diamond MS, Beasley DWC, Morkowski S, Salje H, Fernandez Salas I, Kim DY, Frolov I, Nasar F, Weaver SC. 2015. Utilization of an Eilat virus-based chimera for serological detection of chikungunya infection. PLoS Negl Trop Dis 9:e0004119. doi: 10.1371/journal.pntd.0004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levinson SS, Miller JJ. 2002. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta 325:1–15. doi: 10.1016/S0009-8981(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 21.Prince HE, Yeh C, Lapé-Nixon M. 2008. Development of a more efficient algorithm for identifying false-positive reactivity results in a dengue virus immunoglobulin M screening assay. Clin Vaccine Immunol 15:1304–1306. doi: 10.1128/CVI.00157-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch RJ, Anderson BL, Litwin CM. 2008. Evaluation of a new commercial enzyme immunoassay for the detection of IgM antibodies to West Nile virus using a ratio method to eliminate nonspecific reactivity. J Clin Lab Anal 22:362–366. doi: 10.1002/jcla.20271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welch RJ, Chang GJ, Litwin CM. 2014. Comparison of a commercial dengue IgM capture ELISA with dengue antigen focus reduction microneutralization test and the Centers for Disease Control dengue IgM capture-ELISA. J Virol Methods 195:247–249. doi: 10.1016/j.jviromet.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Stangroom J. Chi-square calculator. www.socscistatistics.com/tests/chisquare Accessed 12 September 15.
- 25.Hogrefe WR, Moore R, Lapé-Nixon M, Wagner M, Prince HE. 2004. Performance of immunoglobulin G (IgG) and IgM enzyme-linked immunosorbent assays using a West Nile virus recombinant antigen (preM/E) for detection of West Nile virus- and other flavivirus-specific antibodies. J Clin Microbiol 42:4641–4648. doi: 10.1128/JCM.42.10.4641-4648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]