Abstract
OBJECTIVE:
This study examined 12-month neurobehavioral outcomes in children who survived out-of-hospital cardiac arrest (OH-CA), were comatose after resuscitation, and were enrolled in a clinical trial to evaluate targeted temperature management to hypothermia (33.0°C) or normothermia (36.8°C) (Therapeutic Hypothermia after Pediatric Cardiac Arrest, Out-of-Hopsital [THAPCA-OH]; NCT00878644).
METHODS:
Baseline functioning was assessed by caregiver responses on the Vineland Adaptive Behavior Scales–Second Edition (VABS-II) soon after OH-CA (based on functioning before OH-CA); children with broadly normal baseline functioning (VABS-II ≥70) were included in the THAPCA-OH primary outcome. VABS-II was completed again 12 months later. Then, face-to-face cognitive evaluations were completed. Analyses evaluated changes in VABS-II composite, domain, and subdomain scores and cognitive functioning at follow-up.
RESULTS:
Ninety-six of 295 enrolled children were alive at 12 months; 87 of 96 had broadly normal baseline functioning (VABS-II ≥70). Follow-up was obtained on 85/87. Forty-two of 85 had VABS-II ≥70 at 12 months. VABS-II composite, domain, and subdomain scores declined significantly between baseline and 12-month follow-up (P < .001). Declines were greatest in older children. Most children displayed well below average cognitive functioning. Older age at cardiac arrest and higher baseline VABS-II scores were predictive of greater decline in neurobehavioral function. Treatment with hypothermia did not influence neurobehavioral outcomes.
CONCLUSIONS:
This is the largest study exploring long-term neurobehavioral outcomes in children surviving OH-CA who were comatose after resuscitation. Results revealed significant neurobehavioral morbidity across multiple functional domains, based both on caregiver reports and performance on objective cognitive measures, in survivors 1 year later.
What’s Known on This Subject:
Children who survive out-of-hospital cardiac arrest (OH-CA) are at risk for poor neurologic outcome. No prospective study has examined long-term neurobehavioral outcome in detail in survivors of OH-CA or examined variables associated with these outcome measures.
What This Study Adds:
Among children who survived OH-CA, were comatose after resuscitation, and were enrolled in a targeted temperature-management trial, many had significant neurobehavioral morbidity 1 year later. Older age was associated with worse outcomes, whereas cardiac arrest and family variables were not.
Neurobehavioral outcome in pediatric survivors of out-of-hospital cardiac arrest (OH-CA) is uncertain. Studies exploring long-term neurobehavioral outcomes in children surviving OH-CA are limited by small samples, single sites, specific etiologies, and restricted ages.1–7 Some studies include children who sustained in-hospital or unspecified cardiac arrest (CA) location.1–4,7 Children who sustain in-hospital CA undergo more rapid resuscitation and less incremental brain injury.8 Only 2 studies examined long-term neurobehavioral outcomes in children who were unresponsive in the early postresuscitation recovery period.1,9
Multicenter or population-based studies conducted in pediatric OH-CA have focused on short-term, global outcome, described as “favorable” versus “poor” at time of hospital discharge.8,10 Recently, a prospective, multicenter trial, entitled Therapeutic Hypothermia after Pediatric Cardiac Arrest, Out-of-Hospital (THAPCA-OH) evaluated 2 targeted temperature management strategies, hypothermia or normothermia, in children who were comatose after OH-CA. One year later, only 16% of enrolled children displayed favorable outcome, defined as survival and broadly normal functioning (score ≥70 on Vineland Adaptive Behavior Scales, Second Edition [VABS-II], a caregiver report measure of neurobehavioral outcome). Outcomes did not differ between treatment groups.9 This secondary analysis of data, collected for THAPCA-OH, reports details of neurobehavioral and cognitive outcomes 1 year after OH-CA in children with broadly normal baseline function.
Methods
Study Population
A total of 295 children, ages 2 days to 18 years, were enrolled in THAPCA-OH; children with CA associated with trauma were excluded. Full inclusion and exclusion criteria, randomization, and enrollment details are described elsewhere.9 At 12-month follow-up, there were 96 confirmed survivors and 8 for whom vital status could not be determined. Of the 96 survivors, 87 with pre-CA VABS-II scores ≥70 were eligible for the THAPCA-OH primary outcome. This report analyzes 12-month neurobehavioral outcomes in 85 of these 87 survivors; 2 cases were lost to follow-up.
Assessment Measures
Family Functioning
Pre–OH-CA family functioning was measured using the General Functioning Scale of the Family Assessment Device (FAD), a 12-item self-reported measure, scored 0 to 4; scores ≥2 indicate abnormal functioning.11
Global Functioning Measures
Pediatric Cerebral Performance Category (PCPC) and Pediatric Overall Performance Category (POPC)12,13: PCPC measures neurologic functioning, whereas POPC measures overall health (including neurologic functioning). These clinician-rated scales have been recommended for reporting outcome after pediatric CA14; they provide no detailed measurements or age-specific normative data.
Neurobehavioral Outcome Measures
Vineland Adaptive Behavior Scales-Second Edition (VABS-II)15: VABS-II measures functional skills and provides age-corrected standard scores (mean = 100, SD = 15) in 4 domains (communication, daily living, socialization, motor skills) and an overall adaptive behavior composite. Each domain includes subdomains with developmentally sequenced items, starting with skills typically observed in infancy. Subdomain raw scores are age-corrected and standardized as v-scores. With means of 15 (SD = 3), v-scores range from 4.67 SDs below to 3 SDs above means, allowing for more precise measurement of low-functioning individuals. VABS-II includes a parent/caregiver rating form and a survey interview (using caregiver as informant) that yield comparable scores.15 Telephone administration of VABS-II is validated16 and a Spanish translation of the interview version is available.15
Wechsler Abbreviated Scale of Intelligence (WASI)17: WASI measures intellectual or general cognitive functioning. Normative data are based on a standardization sample highly representative of the English-speaking US population aged from 6 to 89. The Vocabulary subtest requires individuals to orally define words. The Matrix Reasoning subtest, a measure of nonverbal fluid reasoning, requires individuals to view incomplete gridded patterns and select correct responses. Age-corrected standardized t-scores are available for both. When combined, these subtests yield age-corrected standard scores (mean = 100, SD = 15) for general intellectual functioning (Full Scale IQ).
Mullen Scales of Early Learning (Mullen)18: The Mullen, a measure of cognitive functioning designed for infants and young children, has 4 scales (visual reception, fine motor, receptive language, and expressive language). Normative data are available through age 5 years 8 months. Age-corrected standardized scores are available for each scale as t-scores and for overall early learning composite as a standard score.
For this report, all t-scores (Mullen and WASI) and v-scores (VABS-II) were transformed to standard scores. Scores >115 are above average, 85 to 115 are average, 70 to 84 are below average, and 50 to 69 are well below average. The lowest possible Mullen composite score is 49. For Mullen scales, raw scores below the lowest score on the normative table for age were referred to as lowest possible scores.
Procedures
Within 24 hours of enrollment, a primary caregiver completed the VABS-II rating form to determine baseline functioning. Site research coordinators reviewed instructions for form completion and responses for accuracy. In some cases, coordinators read items to caregivers and recorded responses. Demographic variables (age, gender, race, ethnicity, caregiver education level, and family functioning) were collected. Baseline neurologic and overall functioning was rated by research staff by using medical records or caregiver report. CA-related variables (etiology, epinephrine doses, randomization treatment) were collected.
Twelve months after OH-CA, a trained research assistant at 1 site (Kennedy Krieger Institute, Baltimore, MD), unaware of treatment group assignment, conducted a semistructured telephone interview to assess neurobehavioral function (including VABS-II). Subsequently, children participated in on-site cognitive testing. Children ≥6 years who were reported to have no consistent means of functional communication on the 12-month VABS-II did not undergo additional testing and were assigned lowest possible scores for outcome analyses. For Spanish-speaking caregivers, telephone VABS-II interviews were completed in Spanish. Spanish-speaking children were tested by Spanish-speaking examiners and for those ≥6 years, only WASI matrix reasoning was administered.
Data Analysis
Change in VABS-II scores were calculated for each child (12-month baseline score). Distributions of continuous variables were compared between groups by using t-tests or analysis of variance. Paired t-tests were used to test differences between 2 continuous variables (eg, between baseline and 12-month scores). Categorical variables were examined by using Fisher’s exact test. Standard linear regression models were fit with change in VABS-II score as the outcome variable and baseline continuous and categorical factors as predictors. A multivariable regression model was fit by using baseline predictors that showed a trend of association (P < .10) in univariate models. Spearman’s rank correlation coefficients were used to measure relationships between VABS-II overall and domain scores and Mullen overall and scale scores. All analyses were performed by using SAS software, version 9.4 (SAS Institute, Inc, Cary, NC).
Results
Demographics and Baseline Functioning
There were no differences in demographic variables at age of follow-up among infants/toddlers (<3 years), preschool-aged children (3 to <6 years), or older children (≥6 years) (Table 1). Most were <6 years at 12-month follow-up (range 1.1–18.9 years), white, and not Hispanic. Average family functioning fell within the “normal” range. Mean baseline VABS-II scores were average for age. Almost all children obtained normal PCPC ratings; 5 scored in mild and 3 in moderate disability categories. In all groups, OH-CA etiology was primarily respiratory.
TABLE 1.
Characteristics of Study Population
| Age at Time of 12-mo Follow-up, y | |||
|---|---|---|---|
| 0 to <3, n = 28 | 3 to <6, n = 24 | ≥6, n = 33 | |
| Age at Randomization, y, mean (SD)a | 0.8 (0.6) | 3.3 (1.0) | 12.8 (3.8) |
| Gender: boys, n (%) | 19 (68) | 17 (71) | 26 (79) |
| Race, n (%) | |||
| White | 19 (68) | 17 (71) | 15 (45) |
| Black or African American | 4 (14) | 5 (21) | 13 (39) |
| Other/Unknown | 5 (18) | 2 (8) | 5 (15) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 5 (18) | 5 (21) | 8 (24) |
| Not Hispanic or Latino | 21 (75) | 19 (79) | 24 (73) |
| Stated as Unknown | 2 (7) | 0 (0) | 1 (3) |
| Caregiver’s highest level of education, n (%) | |||
| Some high school or less | 2 (7) | 4 (17) | 12 (36) |
| High school graduate or GED | 11 (39) | 6 (25) | 4 (12) |
| Vocational school or some college | 5 (18) | 8 (33) | 5 (15) |
| College degree | 6 (21) | 3 (13) | 6 (18) |
| Graduate or doctoral degree | 4 (14) | 3 (13) | 6 (18) |
| Average FAD score, mean (SD)b | 1.4 (0.4) | 1.3 (0.4) | 1.6 (0.5) |
| Pre-CA VABS-II Adaptive Behavior Composite Score, mean (SD) | 96 (14.7) | 102 (15.0) | 105 (15.8) |
| Pre-CA PCPC, n (%) | |||
| Normal = 1 | 24 (86) | 23 (96) | 30 (91) |
| Mild disability = 2 | 1 (4) | 1 (4) | 3 (9) |
| Moderate disability = 3 | 3 (11) | 0 (0) | 0 (0) |
| Pre-CA POPC, n (%) | |||
| Good = 1 | 20 (71) | 23 (96) | 26 (79) |
| Mild disability = 2 | 4 (14) | 0 (0) | 6 (18) |
| Moderate disability = 3 | 4 (14) | 1 (4) | 1 (3) |
| Total no. of doses of epinephrine administered by EMS and at hospital, median (interquartile range)c | 3.0 (2.0–4.0) | 2.0 (0.5–3.5) | 2.0 (1.0–3.0) |
| Primary etiology of CA (fewer categories), n (%) | |||
| Cardiovascular event | 3 (11) | 4 (17) | 7 (21) |
| Respiratory event | 21 (75) | 19 (79) | 19 (58) |
| Other/Unknown | 4 (14) | 1 (4) | 7 (21) |
| Randomized treatment, n (%) | |||
| Hypothermia | 17 (61) | 14 (58) | 20 (61) |
| Normothermia | 11 (39) | 10 (42) | 13 (39) |
EMS, emergency medical services.
P < .05 for comparison between age groups. P > .05 for all other comparisons between age groups.
Missing for 1 subject in the 0 to <3 age group. A FAD score < 2 is considered normal family functioning.
Missing for 1 subject in the 0 to <3 age group and 2 subjects in the ≥6 age group.
Neurobehavioral Functioning
Table 2 displays mean baseline and 12-month follow-up scores for VABS-II adaptive behavior composite, domain, and subdomain scores in both treatment groups. Composite, domain, and subdomain scores declined significantly. Mean baseline scores ranged from 95 to 106, mean follow-up scores from 68 to 81 and mean change from −23 to −35, and did not differ between hypothermia and normothermia groups. At 12 months, one-third had average functioning and one-third had severely deficient functioning (Supplemental Table 7).
TABLE 2.
Mean VABS-II Scores at Baseline and 12-mo Follow-Up and Mean Change
| VABS-II | Overall, n = 85 | Hypothermia Group, n = 51 | Normothermia Group, n = 34 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| na | Baseline Scores | Follow-up Scores | Changeb | Baseline Scores | Follow-up Scores | Changeb | Baseline Scores | Follow-up Scores | Changeb | |
| Adaptive behavior composite | 85 | 101 | 69 | −33 | 102 | 70 | −32 | 101 | 67 | −34 |
| Communication | 85 | 101 | 71 | −30 | 102 | 72 | −31 | 100 | 70 | −30 |
| Receptive | 85 | 103 | 76 | −27 | 104 | 76 | −27 | 102 | 76 | −26 |
| Expressive | 85 | 102 | 71 | −30 | 104 | 72 | −32 | 99 | 71 | −28 |
| Written | 47 | 95 | 70 | −25 | 96 | 71 | −26 | 93 | 69 | −24 |
| Daily living | 85 | 104 | 69 | −35 | 104 | 70 | −35 | 104 | 67 | −36 |
| Personal | 84 | 103 | 69 | −34 | 103 | 69 | −34 | 102 | 69 | −33 |
| Domestic | 67 | 105 | 78 | −27 | 106 | 81 | −25 | 102 | 73 | −29 |
| Community | 67 | 105 | 76 | −29 | 103 | 78 | −25 | 108 | 72 | −36 |
| Socialization | 85 | 101 | 76 | −26 | 100 | 76 | −23 | 104 | 75 | −29 |
| Interpersonal relationships | 85 | 101 | 76 | −25 | 100 | 75 | −25 | 103 | 76 | −26 |
| Play and leisure | 85 | 100 | 76 | −23 | 98 | 77 | −20 | 103 | 75 | −28 |
| Coping skills | 66 | 106 | 81 | −25 | 105 | 83 | −23 | 107 | 79 | −28 |
| Motor functioning | 80 | 100 | 68 | −32 | 104 | 69 | −34 | 95 | 65 | −29 |
| Gross | 81 | 99 | 71 | −28 | 101 | 72 | −29 | 94 | 68 | −26 |
| Fine | 81 | 102 | 74 | −28 | 105 | 75 | −30 | 98 | 73 | −25c |
P values were >.05 for comparisons of change between treatment groups.
VABS-II Subdomain scores were transformed to correspond to a scale with mean 100 and SD 15.
The n’s vary because of age differences and missing data. Domestic, community, and coping skills subdomains are not administered to children <1 y of age. Written subdomain is not administered to children <3 y of age. Score for baseline coping and for baseline personal functioning were each missing for 1 subject. Scores for baseline gross motor functioning scores were missing for 4 subjects. Scores for baseline fine motors skills were also missing for 4 subjects.
P < .001 for all comparisons of baseline and follow-up scores except where noted.
P = .003 for comparison of baseline and follow-up scores.
At 12-month follow-up, 49% (42/85) had composite VABS-II scores ≥70 and 38% (32/85) had composite scores within 1 SD (15 points) of their baselines. Similar fractions had follow-up domain scores within 1 SD of baselines (Communication, 32/85 [38%]; Daily Living, 28/85 [33%]; Socialization, 37/85 [44%]; Motor, 37/80 [46%]).
Table 3 displays mean change from baseline to follow-up by age group. For overall adaptive behavior composite and daily living domain, older children had greater declines in functioning than infants/toddlers and preschool children. For communication, socialization, and motor domains, change was significantly greater for older children compared with infants/toddlers. For socialization, preschool children also had greater declines than infants/toddlers.
TABLE 3.
Age Group Comparison of Mean Change in VABS-II Composite and Domain Scores from Baseline to 12-mo Follow-up
| VABS-II | Age, y at Time of 12-mo Follow-up | |||
|---|---|---|---|---|
| 0 to <3, n = 28 | 3 to <6, n = 24 | ≥6, n = 33 | Pa | |
| Adaptive behavior composite | −21 | −28 | −46 | .004b,c |
| Communication | −19 | −28 | −41 | .02b |
| Receptive | −16 | −24 | −38 | .01b |
| Expressive | −20 | −29 | −40 | .06b |
| Writtend | −15 | −29 | .046c | |
| Daily living | −27 | −28 | −47 | .02b,c |
| Personal | −26 | −27 | −45 | .02b,c |
| Domesticd | −12 | −16 | −40 | .003b,c |
| Communityd | −12 | −24 | −39 | .01b |
| Socialization | −9 | −25 | −40 | <.001b,e |
| Interpersonal relationships | −11 | −25 | −38 | .002b |
| Play and leisure | −11 | −18 | −37 | .001b,c |
| Coping skillsd | −1 | −25 | −32 | .01b,e |
| Motor functioning | −23 | −27 | −46 | .046b |
| Gross | −26 | −22 | −34 | .21 |
| Fine | −19 | −24 | −40 | .13 |
P values are from an analysis of variance test.
P < .05 from a t-test comparing the 0 to <3 and ≥6 age groups.
P < .05 from a t-test comparing the 3 to <6 and ≥6 age groups.
Missing for more than half of subjects in youngest age group because domestic, community, and coping skills subdomains are not administered to children <1 y of age and written subdomain is not administered to children <3 y of age.
P < .05 from a t-test comparing the 0 to <3 and 3 to <6 age groups.
To further characterize age-related differences and determine whether any domains were selectively spared or impaired, differences in magnitudes of declines among domains were compared. For the youngest group, declines were significantly smaller for socialization compared with other domains (communication P = .005, daily living P < .001, motor functioning P < .001). For older children, declines were smaller in communication compared with daily living (P = .03). No differences were noted between domains in preschool children.
For all groups, approximately half had overall adaptive behavior composite scores ≥70 at follow-up (infant/toddlers, 15/28 [54%]; preschoolers, 13/24 [54%]; older, 14/33 [42%]). For the younger groups, approximately half had composite scores within 1 SD of their baselines (infant/toddlers, 14/28 [50%]; preschoolers, 11/24 [46%]), whereas this occurred in approximately one-fifth of the oldest group (7/33 [21%]). Similarly, fewer in the older group had domain scores that remained within 1 SD of their baselines (for infants/toddlers, preschoolers, and older children respectively: communication [46%, 46%, 24%], daily living [39%, 46%, 18%], socialization [61%, 42%, 30%], and motor [46%, 50%, 43%]).
Cognitive test performance is presented in Tables 4 and 5. On Mullen scales, most obtained scores that were either the lowest possible or were well below average ranges for overall composite and individual scales (Table 4). Because the lowest reported Mullen score is 49 and many children performed very poorly, developmental quotients (developmental age/chronologic age × 100) were calculated to more fully understand the range of outcomes as deviations from normal expectations; 31% had developmental quotients <25 for all 4 scales (Supplemental Table 8, Supplemental Figure 2)
TABLE 4.
Mullen Scales of Early Learning Composite and Scale Scores for Children <6 y Old at Follow-up (n = 42)
| Score Range | Early Learning Composite | Visual Receptiona | Fine Motora | Receptive Languagea | Expressive Languagea |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Lowest possible score | 18 (43) | 15 (36) | 19 (45) | 15 (36) | 20 (48) |
| 50–69 (well below average) | 9 (21) | 7 (17) | 6 (14) | 9 (21) | 9 (21) |
| 70–84 (below average) | 7 (17) | 7 (17) | 5 (12) | 6 (14) | 4 (10) |
| 85–115 (average) | 1 (2) | 7 (17) | 8 (19) | 9 (21) | 6 (14) |
| >115 (above average) | 7 (17) | 6 (14) | 4 (10) | 3 (7) | 3 (7) |
Scores were transformed to correspond to a scale with mean 100 and SD 15.
TABLE 5.
WASI Full-Scale IQ Composite and Subtest Scores for Children ≥6 y at Follow-Up
| Score Range | Full Scale IQ Composite, n = 17 | Vocabulary,a n = 17 | Matrix Reasoning,a n = 18 |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 55–69 (well below average) | 4 (24) | 5 (29) | 3 (17) |
| 70–84 (below average) | 4 (24) | 4 (24) | 2 (11) |
| 85–115 (average) | 9 (53) | 7 (41) | 13 (72) |
| >115 (above average) | 0 (0) | 1 (6) | 0 (0) |
Eighteen additional subjects were not eligible for testing because they were reported to have no means of functional communication.
One child was Spanish speaking and therefore was administered only Matrix Reasoning.
Scores were transformed to correspond to a scale with mean 100 and SD 15.
Nineteen older children were eligible for cognitive testing, based on VABS-II scores, and 18 of the 19 participated; approximately half performed in the average range and the others performed below to well below average (Table 5). More children displayed average or above performance on nonverbal than verbal reasoning (72% vs 47%).
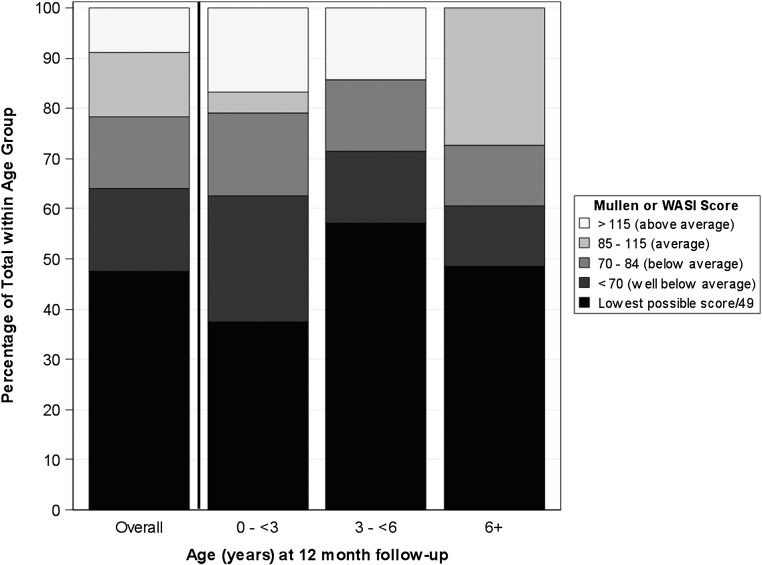
To examine cognitive functioning across the age range, performance based on cognitive composite scores (early learning composite from Mullen or 2-subtest composite from the WASI) were examined. Forty-seven percent were either not eligible for testing on the WASI or obtained the lowest possible Mullen score, 17% obtained above the lowest possible score, but >2 SDs below the means, 14% obtained scores between >1 and ≤2 SDs below means, 13% within 1 SD of means, and 9% >1 SD above means. Figure 1 depicts percentage of children within each range for the overall cognitive composite.
FIGURE 1.
Cognitive composite standard scores.
Relationships Among Outcome Measures
VABS-II overall scores and domain scores were strongly correlated with early learning composites and each Mullen scale; correlations ranged from 0.77 to 0.91 (Supplemental Table 9). In contrast, correlations between VABS-II overall and domain scores with WASI composite and subtest scores were moderate at best (VABS-II motor domain versus WASI composite, r = 0.51, P = .04; VABS-II motor domain versus WASI matrix reasoning, r = 0.51, P = .03; no other significant correlations).
Predictors of Neurobehavioral Decline
Table 6 displays results of univariate and multivariate regression analyses that examined predictors of neurobehavioral outcome, defined as absolute change from baseline to follow-up VABS-II scores. Older age at CA and higher baseline VABS-II scores influenced magnitude of VABS-II declines. No other demographic variables predicted outcome. Neither CA etiology nor treatment group was associated VABS-II change. In a multivariate model, when controlling for baseline VABS-II, older age at OH-CA remained associated with greater decline in functioning.
TABLE 6.
Predictors of VABS-II Overall Behavior Composite Change from Baseline to 12-mo Follow-up
| Univariate | Multivariable, R2 = 0.24 | ||||
|---|---|---|---|---|---|
| Parameter Estimate (95% CI) | R2 | P | Parameter Estimate (95% CI) | P | |
| Child/Family variables | |||||
| Age, y (continuous) | −1.84 (−2.92 to −0.75) | 0.12 | .001 | −1.31 (−2.36 to −0.25) | .02 |
| Boys | 2.19 (−13.10 to 17.49) | 0.001 | .78 | ||
| Caregiver’s highest level of education | 0.03 | .59 | |||
| Some high school or less | [reference] | ||||
| High school graduate or GED | 2.13 (−18.04 to 22.29) | ||||
| Vocational school or some college | 13.44 (−7.48 to 34.37) | ||||
| College degree | 13.76 (−8.19 to 35.70) | ||||
| Graduate or doctoral degree | 6.86 (−15.99 to 29.71) | ||||
| Family functioninga | 6.75 (−9.46 to 22.96) | 0.008 | .41 | ||
| Baseline VABS-II | −0.85 (−1.25 to −0.46) | 0.18 | <.001 | −0.71 (−1.12 to −0.31) | <.001 |
| CA characteristics | |||||
| No. of epinephrine dosesb | −1.02 (−3.83 to 1.79) | 0.007 | .47 | ||
| CA etiology (respiratory, cardiac, or other) | 0.05 | .12 | |||
| Respiratory | [reference] | ||||
| Cardiac | 19.18 (0.91 to 37.45) | ||||
| Other/Unknown | 2.75 (−16.71 to 22.22) | ||||
| Hypothermia | 1.81 (−12.06 to 15.69) | 0.001 | .80 | ||
CI, confidence interval.
Missing for 1 subject.
Missing for 3 subjects.
Discussion
This is the first detailed, prospective study of long-term neurobehavioral outcomes in pediatric OH-CA survivors who were comatose after resuscitation. Results revealed significant declines in all domains of caregiver-reported neurobehavioral functioning, including communication, daily living, socialization, and motor skills. Older children sustained greatest declines from baseline functioning. Most children displayed significant deficits on performance-based cognitive testing. Older age at OH-CA and higher baseline VABS-II were predictive of decline in neurobehavioral functioning. Other demographic and CA characteristics, including targeted temperature treatment group, were not predictive of outcomes.
Strengths of this study are the prospective design, relatively large sample size compared with previous reports, broad age range, high follow-up rate, and detailed outcome measures that assess multiple domains of functioning, including caregiver report and objective performance. Our sample was restricted to a well-characterized and rarely studied group of children who were comatose within the first several hours after resuscitation (pain localization or responsiveness to commands were THAPCA-OH exclusion criteria). Although our results can help clinicians tasked with early prognostication to better understand the range of neurobehavioral outcomes in children at highest risk for neurobehavioral morbidity after OH-CA, results cannot be generalized to all OH-CA survivors.
Our results reveal considerable neurobehavioral morbidity, including significant declines in all domains of neurobehavioral functioning. Although many children displayed severe to profound impairment, we found a range of outcomes with half functioning broadly within normal limits (within 2 SDs of the mean) based on the VABS-II and a third functioning similarly well on cognitive testing. To our knowledge, with the exception of the THAPCA-OH trial outcome report,9 only 1 other study has examined long-term outcome in children who are comatose after resuscitation after CA. In that study of 25 children who remained comatose for at least 24 hours after CA, 23 had profound cognitive and motor impairment at least 1 year later.1 In the THAPCA-OH population, maximum duration of coma could not be evaluated, because children received sedative and paralytic agents during the study intervention period for temperature management.
A major strength of VABS-II is that it assesses multiple domains. We speculated that domains could be selectively affected or spared at different ages or possibly in different treatment groups. Our results indicate functioning was adversely affected with significant declines across all domain and all subdomain scores (mean declines of 23 to 35 standard score points representing mean change of −22% to −33%). Qualitatively, performance was most impaired for motor and daily-living skills. This pattern is consistent with a recent study of school-aged children who were assessed by using the original VABS after very severe traumatic brain injury (all requiring rehabilitation and many unable to participate in performance-based cognitive testing). In that study, children unable to participate in standardized testing were impaired in all domains. Similar to our findings, greatest impairment was noted on motor and daily living skills domains and least in socialization.19
Older children had greatest declines in VABS-II functioning. Literature exploring the relationship between age at brain injury and neurobehavioral outcome has yielded inconsistent findings; however, older age at the time of CA and follow-up were also associated with worse outcomes in a recent study that examined long-term neuropsychological outcomes in pediatric CA.3 Although there is evidence to suggest that children who sustain early diffuse brain injuries are more vulnerable to ongoing impairment than those injured later,20–22 there may be >1 critical developmental period associated with heightened risk for poor outcome.23 Thus, older children may be more vulnerable to the impact of brain injury associated with OH-CA than younger children. However, the testing measures may have been more sensitive to detection of change in older children (ie, the VABS-II may have a “floor effect” for very young children), as fewer items are required to obtain a score within each subdomain in the youngest compared with older children.
Moreover, many functional skills measured by the VABS-II are not expected to be present in young children and therefore age-corrected VABS-II scores may appear less impaired in younger relative to older children with the same severity of neurobehavioral impairments. For example, the youngest children had a significantly smaller decline on the socialization domain compared with the other 3 domains. Socialization items designed for the youngest children focus on simple interactions (eg, shows interest in surroundings by looking around, smiles when approached) and few functional skills need be present to obtain age-appropriate scores in this domain. Young children with severe brain injury who are capable of interaction with the environment may obtain VABS-II socialization scores that reflect smaller declines from baseline compared with changes in other domains of functioning or compared with declines in older children in whom more complex socialization skills can be measured. Longer, prospective studies would be necessary to adequately assess the impact of OH-CA on socialization skills.
Higher baseline VABS-II scores were associated with greater decline. We speculate that new deficits were more readily discerned in children who were functioning the best before OH-CA. No family or CA characteristics influenced decline in functioning. Contrary to studies of outcome after other types of pediatric brain injury, family factors commonly associated with better outcome, including higher parental education/socioeconomic status24–27 and stronger family functioning25,28 were not protective; however, neurobehavioral impairments were less severe. In our study population, family factors likely did not modify outcome due to the severity of neurobehavioral morbidity.
Performance on a composite measure of cognitive functioning for all survivors enrolled in the THAPCA-OH trial was previously reported.9 In this analysis focusing only on survivors who displayed broadly normal baseline functioning, 36% had cognitive composite scores within 2 SDs of normal means, whereas 47% were either not eligible for WASI testing or obtained lowest possible Mullen scores. A recent study examining cognitive outcome after pediatric CA found much better outcomes with a group mean IQ score of 87, and only 6% too low functioning for testing.3 This study also found visual to be more impaired than verbal reasoning; in contrast, in our study, more children ≥6 years performed in the average range on the visual than the verbal reasoning WASI subtest. Several key differences preclude direct comparison between these studies. van Zellem et al3 included both OH-CA and in-hospital CA cases, time of follow-up was much longer (median 5.6 years), and only a subset of children were comatose after resuscitation.
Our results need to be considered in the context of several limitations. Accuracy of baseline functioning may have been limited, because families were asked to complete the VABS-II questionnaire during a time of crisis within 24 hours of their child’s OH-CA. It was particularly challenging to accurately assess baseline functioning in young infants. Data collection in the THAPCA-OH protocol did not include some sources of variation in patient characteristics and treatment that could influence outcome (eg, neuroimaging abnormalities, seizure burden, duration of coma, medical comorbidities, medications, rehabilitation services received). Moreover, given the limited number of older children eligible for testing, we could not examine functioning in specific neuropsychological domains (eg, executive functions, memory).
Conclusions
In this population of children who incurred OH-CA and were comatose after resuscitation, there was substantial neurobehavioral morbidity 1 year later. Older age was associated with worse outcomes, whereas CA and family variables were not.
Acknowledgments
We acknowledge the contributions of THAPCA-OH Trial Group collaborators (see Supplemental Information Appendix for THAPCA Trial Group Collaborators) and the families who participated in the THAPCA-OH trial.
Glossary
- CA
cardiac arrest
- FAD
Family Assessment Device
- Mullen
Mullen Scales of Early Learning
- OH-CA
out-of-hospital cardiac arrest
- PCPC
Pediatric Cerebral Performance Category
- POPC
Pediatric Overall Performance Category
- THAPCA-OH
Therapeutic Hypothermia after Pediatric Cardiac Arrest, Out-of-Hospital
- VABS-II
Vineland Adaptive Behavior Scales-Second Edition
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Dr Slomine oversaw all Vineland Adaptive Behavior Scales-Second Edition data collection, and contributed to manuscript preparation and editing; Drs Silverstein and Christensen contributed to study design and manuscript editing; Mr Page conducted all statistical analyses; Dr Holubkov oversaw all statistical analyses; Dr Dean participated in the study design, oversaw all data collection, and served as principal investigator for the Therapeutic Hypothermia after Pediatric Cardiac Arrest data coordinating center; Dr Moler designed the study and served as principal investigator for the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials; and all authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00878644).
FUNDING: Primary support for the conduct of the Therapeutic Hypothermia after Pediatric Cardiac Arrest, Out-of-Hospital Trial was funding from National Institutes of Health U01HL094345 (Dr Moler) and U01HL094339 (Dr Dean). Additional support from the following federal grants contributed to the planning of the Therapeutic Hypothermia after Pediatric Cardiac Arrest Trials: Eunice Kennedy Shriver National Institute of Child Health and Development, Bethesda, MD, HD044955 (Dr Moler) and HD050531 (Dr Moler). In part, support was from the participation of the following research networks: Pediatric Emergency Care Applied Research Network from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Pediatric Critical Care Research Network from cooperative agreements U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012, and U01HD049934. At several centers, clinical research support was supplemented by the following grants or cooperative agreements: UL1TR000003, P30HD040677, P30HD062171, U07MC09174, UL1 RR 024986, and UL1 TR 000433. Funded by the National Institutes of Health (NIH).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kriel RL, Krach LE, Luxenberg MG, Jones-Saete C, Sanchez J. Outcome of severe anoxic/ischemic brain injury in children. Pediatr Neurol. 1994;10(3):207–212 [DOI] [PubMed] [Google Scholar]
- 2.Maryniak A, Bielawska A, Walczak F, et al. Long-term cognitive outcome in teenage survivors of arrhythmic cardiac arrest. Resuscitation. 2008;77(1):46–50 [DOI] [PubMed] [Google Scholar]
- 3.van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41(6):1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Zellem L, Utens EM, Legerstee JS, et al. Cardiac arrest in children: Long-term health status and health-related quality of life. Pediatr Crit Care Med. 2015;16(8):693–702 [DOI] [PubMed] [Google Scholar]
- 5.Li G, Tang N, DiScala C, Meisel Z, Levick N, Kelen GD. Cardiopulmonary resuscitation in pediatric trauma patients: survival and functional outcome. J Trauma. 1999;47(1):1–7 [DOI] [PubMed] [Google Scholar]
- 6.Suominen PK, Sutinen N, Valle S, Olkkola KT, Lönnqvist T. Neurocognitive long term follow-up study on drowned children. Resuscitation. 2014;85(8):1059–1064 [DOI] [PubMed] [Google Scholar]
- 7.Horisberger T, Fischer E, Fanconi S. One-year survival and neurological outcome after pediatric cardiopulmonary resuscitation. Intensive Care Med. 2002;28(3):365–368 [DOI] [PubMed] [Google Scholar]
- 8.Moler FW, Meert K, Donaldson AE, et al. ; Pediatric Emergency Care Applied Research Network . In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Crit Care Med. 2009;37(7):2259–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moler FW, Silverstein FS, Holubkov R, et al. ; THAPCA Trial Investigators . Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372(20):1898–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young KD, Gausche-Hill M, McClung CD, Lewis RJ. A prospective, population-based study of the epidemiology and outcome of out-of-hospital pediatric cardiopulmonary arrest. Pediatrics. 2004;114(1):157–164 [DOI] [PubMed] [Google Scholar]
- 11.Epstein B, Baldwin L, Bishop D. The McMaster family assessment device. J Marital Fam Ther. 1983;9(2):171–180 [Google Scholar]
- 12.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121(1):68–74 [DOI] [PubMed] [Google Scholar]
- 13.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28(7):2616–2620 [DOI] [PubMed] [Google Scholar]
- 14.Zaritsky A, Nadkarni V, Hazinski MF, et al. Recommended guidelines for uniform reporting of pediatric advanced life support: the Pediatric Utstein Style. A statement for healthcare professionals from a task force of the American Academy of Pediatrics, the American Heart Association, and the European Resuscitation Council. Resuscitation. 1995;30(2):95–115 [DOI] [PubMed] [Google Scholar]
- 15.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales: Survey Forms Manual. 2nd ed. Minneapolis, MN: NCS Pearson; 2005 [Google Scholar]
- 16.Lux AL, Edwards SW, Hancock E, et al. ; United Kingdom Infantile Spasms Study . The United Kingdom Infantile Spasms Study (UKISS) comparing hormone treatment with vigabatrin on developmental and epilepsy outcomes to age 14 months: a multicentre randomised trial. Lancet Neurol. 2005;4(11):712–717 [DOI] [PubMed] [Google Scholar]
- 17.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: Psychological Corporation; 1999 [Google Scholar]
- 18.Mullen EM. Mullen Scales of Early Learning. Circle Pine, MN: American Guidance Service; 1995 [Google Scholar]
- 19.Recla M, Bardoni A, Galbiati S, et al. Cognitive and adaptive functioning after severe TBI in school-aged children. Brain Inj. 2013;27(7-8):862–871 [DOI] [PubMed] [Google Scholar]
- 20.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld J. Functional plasticity or vulnerability after early brain injury? Pediatrics. 2005;116(6):1374–1382 [DOI] [PubMed] [Google Scholar]
- 21.Anderson V, Catroppa C, Morse S, Haritou F, Rosenfeld JV. Intellectual outcome from preschool traumatic brain injury: a 5-year prospective, longitudinal study. Pediatrics. 2009;124(6). Available at: www.pediatrics.org/cgi/content/full/124/6/e1064 [DOI] [PubMed] [Google Scholar]
- 22.Karver CL, Wade SL, Cassedy A, et al. Age at injury and long-term behavior problems after traumatic brain injury in young children. Rehabil Psychol. 2012;57(3):256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson V, Spencer-Smith M, Wood A. Do children really recover better? Neurobehavioural plasticity after early brain insult. Brain. 2011;134(pt 8):2197–2221 [DOI] [PubMed] [Google Scholar]
- 24.Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: behavior and achievement. Neuropsychology. 2002;16(1):15–27 [DOI] [PubMed] [Google Scholar]
- 25.Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10(3):412–426 [DOI] [PubMed] [Google Scholar]
- 26.Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16(4):514–523 [DOI] [PubMed] [Google Scholar]
- 27.Ment LR, Vohr B, Allan W, et al. Change in cognitive function over time in very low-birth-weight infants. JAMA. 2003;289(6):705–711 [DOI] [PubMed] [Google Scholar]
- 28.McCarthy ML, MacKenzie EJ, Durbin DR, et al. ; Children’s Health After Trauma Study Group . Health-related quality of life during the first year after traumatic brain injury. Arch Pediatr Adolesc Med. 2006;160(3):252–260 [DOI] [PubMed] [Google Scholar]