Abstract
BACKGROUND AND OBJECTIVES:
Poorly designed labels and packaging are key contributors to medication errors. To identify attributes of labels and dosing tools that could be improved, we examined the extent to which dosing error rates are affected by tool characteristics (ie, type, marking complexity) and discordance between units of measurement on labels and dosing tools; along with differences by health literacy and language.
METHODS:
Randomized controlled experiment in 3 urban pediatric clinics. English- or Spanish-speaking parents (n = 2110) of children ≤8 years old were randomly assigned to 1 of 5 study arms and given labels and dosing tools that varied in unit pairings. Each parent measured 9 doses of medication (3 amounts [2.5, 5, and 7.5 mL] and 3 tools [1 cup, 2 syringes (0.2- and 0.5-mL increments)]), in random order. Outcome assessed was dosing error (>20% deviation; large error defined as > 2 times the dose).
RESULTS:
A total of 84.4% of parents made ≥1 dosing error (21.0% ≥1 large error). More errors were seen with cups than syringes (adjusted odds ratio = 4.6; 95% confidence interval, 4.2–5.1) across health literacy and language groups (P < .001 for interactions), especially for smaller doses. No differences in error rates were seen between the 2 syringe types. Use of a teaspoon-only label (with a milliliter and teaspoon tool) was associated with more errors than when milliliter-only labels and tools were used (adjusted odds ratio = 1.2; 95% confidence interval, 1.01–1.4).
CONCLUSIONS:
Recommending oral syringes over cups, particularly for smaller doses, should be part of a comprehensive pediatric labeling and dosing strategy to reduce medication errors.
What’s Known on This Subject:
Despite studies showing that >40% of parents make errors dosing liquid medications, there has been limited focus to date on identifying specific attributes of pediatric labels and dosing tools that could be improved to reduce the likelihood of error.
What This Study Adds:
Significant reductions in dosing errors would probably result if parent oral syringe use was promoted over dosing cups, especially when smaller doses are recommended. Avoidance of teaspoon alone on medication labels may also be helpful in decreasing errors.
Over the past decade, growing attention has been paid to the problem of unintentional medication errors resulting from suboptimal drug labeling and medication packaging.1–7 Although considerable progress has been attained in making labeling improvements for adult medications,3,8–11 to date there has been limited work incorporating a pediatric perspective, despite studies documenting parent dosing error rates of ≥40%.12–16 Lack of evidence regarding best practices has been a barrier to establishing standards related to the labeling and dosing of pediatric medications.17
Unlike most prescription drugs taken by adults, pediatric medications are unique in their reliance on liquid formulations.18 With oral liquid medicines, parents must choose an appropriate tool with which to measure and administer medicine to their children.14 In addition, a range of measurement units (eg, milliliter, teaspoon, tablespoon), along with their associated abbreviations, are used as part of instructions on labels and dosing tools, contributing to confusion and multifold errors.7,14,19–21
To promote dosing accuracy, both the American Academy of Pediatrics (AAP) and the US Food and Drug Administration (FDA) recommend that parents use dosing tools with standard markings (eg, oral syringes, droppers, dosing cups) rather than nonstandard kitchen spoons, which vary widely in size and shape.19,21–24 However, no national guidelines exist regarding which type of tool should be provided to families. Oral syringes are considered the gold standard when accuracy is critical.14,25–27 Cups are most frequently included with over-the-counter (OTC) products.28 Several studies have found that cups are associated with higher rates of parent errors, but they were limited in scope with respect to the range of dose amounts tested and aspects such as complexity of tool markings.14,27,29
Unit of measurement discordance has become an issue of concern for prescription and OTC medicines.19,29 One study of top-selling OTC pediatric products found that nearly 90% had a mismatch in units between the label and dosing tool,28 before a 2009 FDA guidance for industry was issued.22 A study of prescribed products found that more than a third of the time, the label did not contain the same units as the prescription.13 Recently, the AAP issued a policy statement endorsing a move to a milliliter-exclusive system and avoidance of terms such as teaspoon and tablespoon,7 a stance consistent with that of other organizations, including the FDA and the American Academy of Family Physicians,20,30–32 but there are concerns that such a move could result in greater confusion because parents may be comfortable dosing using teaspoon and tablespoon terms and unfamiliar with milliliter units7,13; the United States has had a long-standing dependence on nonmetric units.33
In this study, we sought to fill gaps in evidence about best practices for the labeling and dosing of pediatric liquid medications. Specifically, we examined the extent to which rates of parent dosing errors are affected by discordance in unit pairing on the label and tool and by dosing tool characteristics (ie, type, marking complexity). We hypothesized that unit concordance would be associated with fewer errors and that parents would measure most accurately with syringes. We also sought to examine differences in impact by parent health literacy and language, because low health literacy and limited English proficiency are factors known to place children at risk for error.23,34–36
Methods
Participants, Recruitment, and Randomization
This was a randomized controlled experiment to examine the degree to which specific attributes of medication labels and dosing tools affect parent errors in dosing liquid medicines. As part of the SAFE Rx for Kids (Safe Administration for Every Prescription for Kids) study, subjects were enrolled from pediatric outpatient clinics at Bellevue (New York, NY), Gardner Packard Children’s Health Care Center (Stanford, CA), and Children’s Healthcare of Atlanta at Hughes Spalding (Atlanta, GA). Institutional review board approval was obtained from each site.
During clinic hours when enrollment took place, research assistants (RAs) consecutively assessed parents and caregivers to determine eligibility. Inclusion criteria were parent or legal guardian ≥18 years old with a child ≤8 years old, presenting for nonemergency care, who was English or Spanish-speaking, usually administers medications, and had no previous participation in a medication-related study. Exclusion criteria included visual acuity worse than 20/50 (Rosenbaum), hearing impairment, and parent or child too ill to participate. Participants provided written, informed consent.
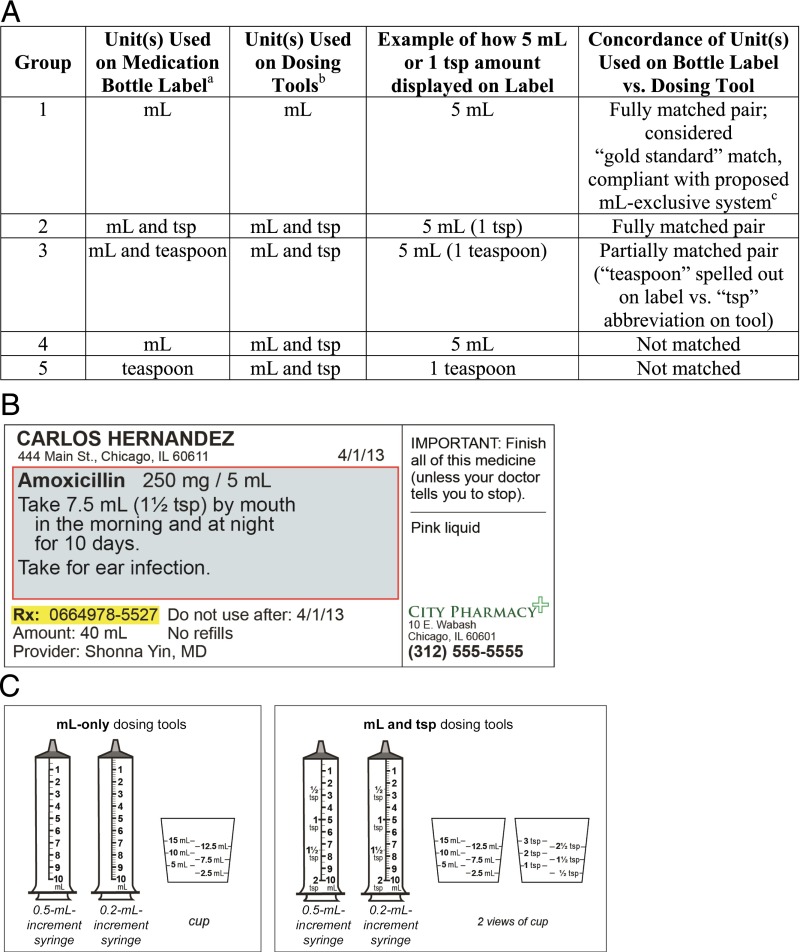
Upon enrollment, subjects were randomly assigned to 1 of 5 groups. Groups differed by the pairing of units used on the bottle label and tool: mL–mL (group 1), mL and tsp–mL and tsp (group 2), mL and teaspoon–mL and tsp (group 3), mL–mL and tsp (group 4), and teaspoon–mL and tsp (group 5) (Fig 1A). Because a move to a milliliter-only system has been recommended by numerous organizations, group 1 was considered the gold standard scenario. Randomization was conducted via a random number generator, blocked by site, in sets of 100 (20 per group). The lead project coordinator (J.J.J.) generated the allocation sequence; RAs at each site were blinded to group until after subjects were enrolled. Once the dosing assessment was initiated, it was not possible for the RA or participant to remain blinded, because it was clear from the labels and tools being presented which group the participant had been assigned to.
FIGURE 1.
Medication labels and dosing tools tested. A, Comparison of randomization group characteristics. Unit label and dosing tool pairings were chosen because they represent the most common current standard practices used to display dose amounts on medication labels and dosing tools. The combination of units on labels and dosing tools applied to 3 different dosing tools given to each person (2 oral syringes [1 0.2-mL increment and 1 0.5-mL increment] and 1 cup); each subject measured 3 doses with the 3 tools, for a total of 9 doses. aExample of group 2 medication label is shown in Fig 1B. Teaspoon units on English-language medication labels were translated into Spanish, consistent with recommended pharmacy practices. The abbreviation tsp was displayed as cdta on Spanish-language medication bottle labels, and teaspoon was displayed as cucharadita on the Spanish-language medication bottle labels. bDosing tools had units marked in English only, as is standard practice in the United States. For dosing tools, mL and tsp tools are most commonly used and were therefore included for the majority of groups. See Fig 1C. cThe milliliter-only system is endorsed by the AAP, the Centers for Disease Control and Prevention, and other national organizations. B, Example of group 2 medication label (English). C, Dosing tools tested.
Assessments
All assessments were performed on the day of enrollment. Interviews were conducted by trained RAs in English or Spanish (caregiver preference). Dosing assessments were conducted, followed by a survey to assess sociodemographics and health literacy. A $20 gift card incentive was provided.
Dosing Accuracy
Trained RAs presented each caregiver with a series of bottle labels and tools, which caregivers looked at to respond to questions and demonstrate dosing (Fig 1). Each caregiver was asked to measure 3 amounts (2.5, 5, and 7.5 mL) by using 3 tools (9 total trials). The 3 tools were 2 syringes (10-mL capacity; 1 with 0.2-mL and 1 with 0.5-mL increment markings) and 1 dosing cup (30-mL capacity). A random number generator was used to randomize the order in which caregivers were presented with each tool type and dose amount. Custom-designed tools (Comar, Buena, NJ) were used so that tools tested across groups varied only by markings.
Caregivers were presented with the 9 sets of label and tool pairs, one at a time. Labels were in English or Spanish (caregiver preference). Caregivers were allotted as much time as they wanted to read each label and were instructed, “Please use this [DOSING TOOL HANDED TO PARENT] to show me how much medicine the label tells you to give the child each time you give the medicine.” For each trial, caregivers were given a standard medication bottle, filled to the same level; the medication used had a viscosity similar to common children’s medication suspensions.
Dosing error was the primary outcome variable; magnitude of error was determined by an established protocol.14 The weight of the measured dose (tool weight containing parents’ measured dose minus preassessment tool weight) was compared with a reference weight (eg, for 5-mL dose, the average weight of 5 mL measured by 10 pediatricians using an oral syringe was determined). A pharmacy-grade electronic digital prescription class II scale (Torbal DRX-4; Fulcrum Inc, Clifton, NJ) was used.
The primary criterion used to determine whether an error was made was whether the measured amount fell within 20% of the label amount.12–14,16,37 To look at errors of greater magnitude, we also performed analyses for large errors, using a cutoff point of 2 times above the tested dose.
Sociodemographic Data, Health Literacy, and Child Health Status
Sociodemographic data assessed included child (age, gender) and parent (age, relationship to child, income, country of birth, race or ethnicity, language, education) characteristics. Parent health literacy was assessed with the Newest Vital Sign.38 Child’s chronic disease status and medication use were assessed via questions adapted from the Children With Special Health Care Needs screener.39
Statistical Analyses
Statistical analyses were performed in SAS software version 9.4 (SAS Institute, Inc, Cary, NC). We used χ2, analysis of variance, and Kruskall–Wallis tests to compare parent characteristics between randomization groups. For dosing accuracy, analyses were performed to compare error rates (with cutoffs of >20% deviation and >2 times the dose) by randomization group and tool type (ie, syringe with 0.2- or 0.5-mL-increment markings, cup). Findings were analyzed by assigned group (all parents received assigned label–tool pairings). Multiple logistic regression with generalized estimating equations was used to account for repeated measures (9 trials per subject). In addition to group and tool type, covariates selected a priori for inclusion in adjusted analyses were key study variables of dose amount, dosing order, and label language. In addition, characteristics found to be statistically different between groups were included (ie, health literacy). Stratified analyses and interaction tests were performed by health literacy and by language.
Sample Size Calculation
We conservatively estimated a sample size of 420 patients per arm, or 2100 total subjects, based on known rates of dosing errors from previous studies, which typically range from 10% to 50% depending on the tool used.12,14,27 This sample size would allow us to detect an absolute difference of ∼10% with 80% power for our hypotheses related to unit pairings and tool type.
Results
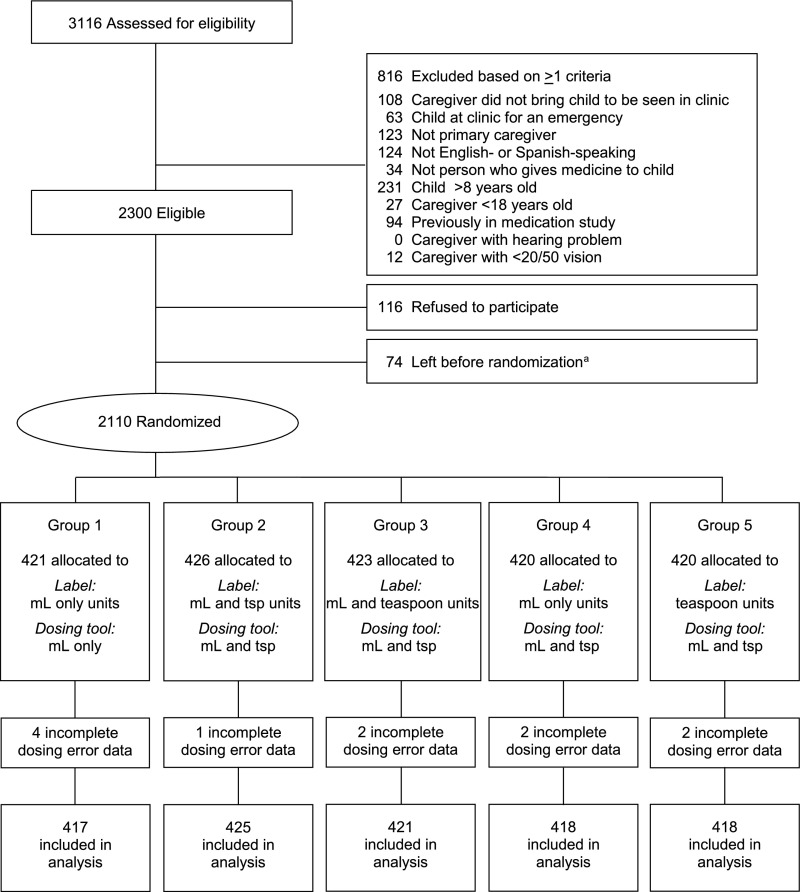
Between August 26, 2013 and December 18, 2014, 2110 parents enrolled in the study and were randomly assigned to 1 of the 5 groups (Fig 2). Dosing assessments were completed for 2099 parents (Table 1).
FIGURE 2.
Study enrollment flowchart. aRan out of time after signing consent.
TABLE 1.
Characteristics of Study Population (n = 2099)a
| Entire Population | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P | |
|---|---|---|---|---|---|---|---|
| Label Unit | mL | mL and tsp | mL and teaspoon | mL | teaspoon | ||
| Dosing Tool Unit | mL | mL and tsp | mL and tsp | mL and tsp | mL and tsp | ||
| N = 417 | N = 425 | N = 421 | N = 418 | N = 418 | |||
| Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | Mean (SD) or n (%) | ||
| Child characteristics | |||||||
| Age, y, mean (SD) | 2.0 (2.2) | 2.1 (2.2) | 2.3 (2.3) | 2.0 (2.1) | 2.0 (2.1) | 1.9 (2.1) | .2 |
| Gender, n (%) female | 987 (47.0) | 206 (49.4) | 210 (49.4) | 196 (46.6) | 181 (43.3) | 194 (46.4) | .4 |
| Chronic medical problem treated with medication, n (%)b | 352 (16.8) | 74 (17.7) | 64 (15.1) | 64 (15.2) | 76 (18.2) | 74 (17.7) | .6 |
| Parent characteristics | |||||||
| Age, y, mean (SD) | 30.0 (7.3) | 29.9 (7.1) | 30.2 (7.5) | 29.1 (7.1) | 29.8 (7.5) | 29.5 (7.5) | .3 |
| Gender, n (%) female | 1930 (91.9) | 384 (92.1) | 397 (93.4) | 391 (92.9) | 384 (91.9) | 374 (89.5) | .3 |
| Relationship to child, n (%) mother | 1881 (89.6) | 376 (90.2) | 384 (90.4) | 379 (90.0) | 375 (89.7) | 367 (87.8) | .8 |
| Marital status single, n (%)c | 803 (38.7) | 160 (38.8) | 166 (39.6) | 157 (37.7) | 167 (40.3) | 153 (37.0) | .9 |
| Income, n (%) | .02 | ||||||
| <$10 000 | 497 (23.7) | 113 (27.1) | 99 (23.3) | 85 (20.2) | 104 (24.9) | 96 (23.0) | |
| $10 000–$19 999 | 554 (26.4) | 94 (22.5) | 114 (26.8) | 144 (34.2) | 107 (25.6) | 95 (22.7) | |
| $20 000–$39 999 | 583 (27.8) | 109 (26.1) | 122 (28.7) | 107 (25.4) | 111 (26.6) | 134 (32.1) | |
| ≥$40 000 | 255 (12.1) | 55 (13.2) | 47 (11.1) | 48 (11.4) | 56 (13.4) | 49 (11.7) | |
| Unknown or missing | 210 (10.0) | 46 (11.0) | 43 (10.1) | 37 (8.8) | 40 (9.6) | 44 (10.5) | |
| Country of birth: non-US born, n (%)d | 1031 (49.5) | 203 (49.2) | 230 (54.4) | 201 (48.2) | 201 (48.2) | 196 (47.3) | .3 |
| Race or ethnicity, n (%)e | .9 | ||||||
| Hispanic | 1140 (54.8) | 224 (54.4) | 227 (53.8) | 225 (54.2) | 241 (57.8) | 223 (53.9) | |
| Non-Hispanic | |||||||
| White, non-Hispanic | 79 (3.8) | 14 (3.4) | 14 (3.3) | 15 (3.6) | 18 (4.3) | 18 (4.3) | |
| Black, non-Hispanic | 695 (33.4) | 141 (34.2) | 146 (34.6) | 134 (32.3) | 134 (32.1) | 140 (33.8) | |
| Other, non-Hispanic | 166 (8.0) | 33 (8.0) | 35 (8.3) | 41 (9.9) | 24 (5.8) | 33 (8.0) | |
| Language Spanish, n (%)f | 736 (35.1) | 157 (37.6) | 158 (37.2) | 134 (31.8) | 145 (34.7) | 142 (34.0) | .4 |
| Education, n (%)g | .9 | ||||||
| Less than high school graduate | 638 (30.7) | 132 (32.0) | 137 (32.5) | 118 (28.3) | 128 (30.8) | 123 (29.7) | |
| High school graduate or equivalent | 674 (32.4) | 127 (30.8) | 141 (33.4) | 138 (33.1) | 138 (33.3) | 130 (31.4) | |
| Higher than high school graduate | 769 (37.0) | 154 (37.3) | 144 (34.1) | 161 (38.6) | 149 (35.9) | 161 (38.9) | |
| Health literacy, n (%)h | .04 | ||||||
| Low | 740 (36.0) | 148 (36.5) | 139 (33.4) | 142 (34.6) | 157 (38.1) | 154 (37.2) | |
| Marginal | 843 (41.0) | 166 (40.9) | 191 (45.9) | 167 (40.7) | 175 (42.5) | 144 (34.8) | |
| Adequate | 475 (23.1) | 92 (22.7) | 86 (20.7) | 101 (24.6) | 80 (19.4) | 116 (28.0) | |
| Site characteristics | |||||||
| Emory | 690 (32.9) | 137 (32.9) | 140 (32.9) | 138 (32.8) | 137 (32.8) | 138 (33.0) | .97 |
| New York University | 701 (33.4) | 141 (33.8) | 141 (33.2) | 140 (33.3) | 139 (33.3) | 140 (33.5) | |
| Stanford | 708 (33.7) | 139 (33.3) | 144 (33.9) | 143 (34.0) | 142 (34.0) | 140 (33.5) |
Characteristics not different between enrolled subjects and those eligible who did not enroll (P > .05 for all).
Missing for 56 children overall (16 in group 1, 11 in group 2, 12 in group 3, 5 in group 4, and 12 in group 5).
Missing for 25 parents (5 in group 1, 6 in group 2, 5 in group 3, 4 in group 4, and 5 in group 5).
Missing for 15 parents (4 in group 1, 2 in group 2, 4 in group 3, 1 in group 4, and 4 in group 5).
Missing for 19 parents (5 in group 1, 3 in group 2, 6 in group 3, 1 in group 4, and 4 in group 5).
Language of survey administration.
Missing for 18 parents (4 in group 1, 3 in group 2, 4 in group 3, 3 in group 4, 4 in group 5).
Health literacy measured with the Newest Vital Sign (low = score 0–1, marginal = 2–3, adequate = 4–6). Data missing for 41 subjects who did not complete the Newest Vital Sign (11 in group 1, 9 in group 2, 11 in group 3, 6 in group 4, and 4 in group 5).
Dosing Accuracy
Nearly all parents (99.3%) measured ≥1 dose that was not the exact amount. Overall, 84.4% of parents made ≥1 dosing error (>20% deviation) in their 9 trials, with parents making errors in 25.3% of trials on average (mean [SD] number of errors = 2.3 [2.0]). Overdosing was present in 68.0% of errors. There were more errors with 2.5- and 7.5-mL dose amounts, compared with 5-mL dose amounts (2.5 vs 5 mL adjusted odds ratio [aOR] = 4.2; 95% CI, 3.8–4.6; 7.5 vs 5 mL aOR = 1.4; 95% CI, 1.2–1.5). Test order was associated with error, with a clear trend toward fewer errors as parents went through the trials. Overall, 21.0% made ≥1 large error (>2 times the dose).
Unit of Measurement Pairing
Group 5 was the only group that was associated with more errors than the milliliter-only group 1 (aOR = 1.2; 95% CI, 1.01–1.4) (Table 2). Similar findings were seen with large errors (aOR = 1.4; 95% CI, 0.97–1.9). No group by health literacy interaction was found, but a group by language interaction was seen (P = .006) (Table 3).
TABLE 2.
Dosing Error by Dosing Tool Type and Randomization Group (n = 2058)
| Dosing Error (>20% Deviation) | Large Dosing Error (>2 Times the Dose) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Trials With Errors/Parenta | Pb | aORc | 95% CI | P | % Trials With Large Errors/Parenta | Pb | aORc | 95% CI | P | |||
| Group | Label Unit | Tool Unit | ||||||||||
| Unit of measurement pairing on label versus dosing tool | ||||||||||||
| 1 | mL | mL | 25.3 | .002 | 1.0 | Ref | Ref | 2.9 | .08 | 1.0 | Ref | Ref |
| 2 | mL and tsp | mL and tsp | 22.8 | — | 0.9 | 0.7–1.04 | .1 | 2.7 | — | 1.0 | 0.7–1.4 | .9 |
| 3 | mL and teaspoon | mL and tsp | 22.9 | — | 0.9 | 0.7–1.03 | .1 | 3.0 | — | 1.1 | 0.8–1.6 | .7 |
| 4 | mL | mL and tsp | 25.4 | — | 1.0 | 0.8–1.2 | .8 | 3.5 | — | 1.2 | 0.8–1.7 | .4 |
| 5 | teaspoon | mL and tsp | 29.6 | — | 1.2 | 1.01–1.4 | .04 | 3.6 | — | 1.4 | 0.97–1.9 | .08 |
| Dosing tool type | ||||||||||||
| Cup | 43.0 | <.001 | 4.6 | 4.2–5.1 | <.001 | 5.8 | <.001 | 3.8 | 3.1–4.7 | <.001 | ||
| Syringe (0.2-mL increment, 10-mL capacity) | 16.7 | — | 1.0 | 0.96–1.1 | .4 | 1.8 | — | 1.0 | 0.8–1.3 | .9 | ||
| Syringe (0.5-mL increment, 10-mL capacity) | 16.2 | — | 1.0 | Ref | Ref | 1.8 | — | 1.0 | Ref | Ref | ||
Ref, referent.
Percentage of trials with errors per parent.
Type 3 χ2 from full model.
Full model adjusting for randomization group, tool type, dose amount, dosing order, language, and health literacy.
TABLE 3.
Dosing Error by Dosing Tool Type and Randomization Group, Stratified by Health Literacy and Language (n = 2058)
| % Trials With Errors/Parenta | Dosing Error (>20% deviation) | ||||||
|---|---|---|---|---|---|---|---|
| Pb | aORc | 95% CI | P | ||||
| By Health Literacy | |||||||
| Low health literacy (n = 740) | |||||||
| Unit of measurement pairing on label vs dosing tool | |||||||
| Group | Label Unit | Tool Unit | |||||
| 1 | mL | mL | 32.4 | .03 | 1.0 | Ref | Ref |
| 2 | mL and tsp | mL and tsp | 28.5 | 0.8 | 0.6–1.1 | .2 | |
| 3 | mL and teaspoon | mL and tsp | 30.2 | 0.9 | 0.7–1.2 | .4 | |
| 4 | mL | mL and tsp | 31.9 | 1.0 | 0.7–1.3 | .9 | |
| 5 | teaspoon | mL and tsp | 38.7 | 1.3 | 0.98–1.7 | .07 | |
| Dosing tool type | |||||||
| Cup | 48.9 | <.001 | 3.4 | 3.0–3.9 | <.001 | ||
| Syringe (0.2-mL increment, 10-mL capacity) | 24.9 | 1.1 | 0.98–1.2 | .1 | |||
| Syringe (0.5-mL increment, 10-mL capacity) | 23.6 | 1.0 | Ref | Ref | |||
| Marginal health literacy (n = 843) | |||||||
| Unit of measurement pairing on label vs dosing tool | |||||||
| Group | Label Unit | Tool Unit | |||||
| 1 | mL | mL | 21.8 | .2 | 1.0 | Ref | Ref |
| 2 | mL and tsp | mL and tsp | 21.5 | 0.9 | 0.7–1.2 | .6 | |
| 3 | mL and teaspoon | mL and tsp | 22.2 | 0.97 | 0.7–1.3 | .8 | |
| 4 | mL | mL and tsp | 24.5 | 1.1 | 0.9–1.5 | .4 | |
| 5 | teaspoon | mL and tsp | 28.6 | 1.3 | 0.95–1.7 | .1 | |
| Dosing tool type | |||||||
| Cup | 43.1 | <.001 | 5.6 | 4.8–6.6 | <.001 | ||
| Syringe (0.2-mL increment, 10-mL capacity) | 13.8 | 1.0 | 0.9–1.2 | .8 | |||
| Syringe (0.5-mL increment, 10-mL capacity) | 13.7 | 1.0 | Ref | Ref | |||
| Adequate health literacy (n = 475) | |||||||
| Unit of measurement pairing on label vs dosing tool | |||||||
| Group | Label Unit | Tool Unit | |||||
| 1 | mL | mL | 20.3 | .2 | 1.0 | Ref | Ref |
| 2 | mL and tsp | mL and tsp | 16.3 | 0.9 | 0.6–1.3 | .5 | |
| 3 | mL and teaspoon | mL and tsp | 14.0 | 0.7 | 0.5–1.01 | .1 | |
| 4 | mL | mL and tsp | 14.6 | 0.7 | 0.5–1.05 | .1 | |
| 5 | teaspoon | mL and tsp | 18.8 | 0.9 | 0.7–1.4 | .7 | |
| Dosing tool type | |||||||
| Cup | 33.6 | <.001 | 6.5 | 5.0–8.5 | <.001 | ||
| Syringe (0.2-mL increment, 10-mL capacity) | 8.2 | 0.9 | 0.7–1.2 | .4 | |||
| Syringe (0.5-mL increment, 10-mL capacity) | 8.8 | 1.0 | Ref | Ref | |||
| By Language | |||||||
| English (n = 1334) | |||||||
| Unit of measurement pairing on label vs dosing tool | |||||||
| Group | Label Unit | Tool Unit | |||||
| 1 | mL | mL | 25.6 | 0.2 | 1.0 | Ref | Ref |
| 2 | mL and tsp | mL and tsp | 22.0 | 0.8 | 0.7–1.02 | .07 | |
| 3 | mL and teaspoon | mL and tsp | 21.3 | 0.8 | 0.6–0.96 | .02 | |
| 4 | mL | mL and tsp | 23.7 | 0.9 | 0.7–1.06 | .2 | |
| 5 | teaspoon | mL and tsp | 25.2 | 0.9 | 0.7–1.1 | .4 | |
| Dosing tool type | |||||||
| Cup | 42.0 | <.001 | 5.2 | 4.5–5.9 | <.001 | ||
| Syringe (0.2-mL increment, 10-mL capacity) | 14.2 | 1.0 | 0.9–1.1 | .8 | |||
| Syringe (0.5-mL increment, 10-mL capacity) | 14.3 | 1.0 | Ref | Ref | |||
| Spanish (n = 724) | |||||||
| Unit of measurement pairing on label vs dosing tool | |||||||
| Group | Label Unit | Tool Unit | |||||
| 1 | mL | mL | 24.9 | <.001 | 1.0 | Ref | Ref |
| 2 | mL and tsp | mL and tsp | 24.1 | 1.0 | 0.8–1.3 | .9 | |
| 3 | mL and teaspoon | mL and tsp | 26.5 | 1.1 | 0.8–1.4 | .7 | |
| 4 | mL | mL and tsp | 28.7 | 1.2 | 0.9–1.6 | .2 | |
| 5 | teaspoon | mL and tsp | 38.3 | 2.0 | 1.5–2.6 | <.001 | |
| Dosing tool type | |||||||
| Cup | 44.7 | <.001 | 3.9 | 3.3–4.5 | <.001 | ||
| Syringe (0.2-mL increment, 10-mL capacity) | 20.8 | 1.1 | 0.98–1.2 | .1 | |||
| Syringe (0.5-mL increment, 10-mL capacity) | 19.5 | 1.0 | Ref | Ref | |||
Ref, referent.
Percentage of trials with dosing errors per parent.
Type 3 χ2 from full model.
Full model adjusting for randomization group, tool type, dose amount, dosing order, language, and health literacy. Models by health literacy adjusting for all except health literacy; models for language adjusting for all except language.
Dosing Tools
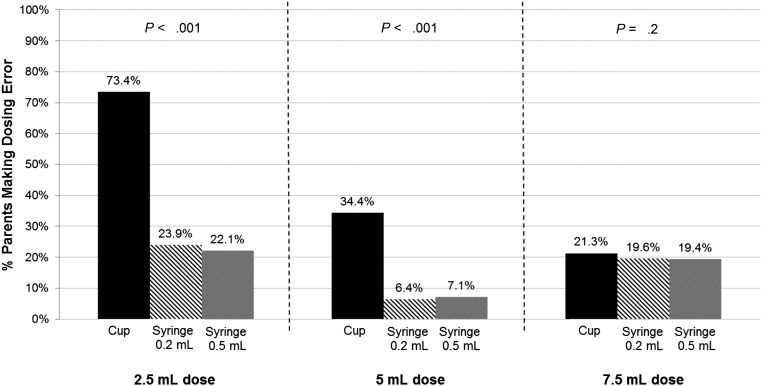
There was no significant difference in error rates with syringes that had 0.2-mL vs 0.5-mL-increment markings. More errors were seen with cups than syringes (cup vs 0.5-mL-increment syringe aOR = 4.6; 95% CI, 4.2–5.1 [Table 2]); differences in error rates were greatest for 2.5- and 5-mL doses (Fig 3). For large errors (>2 times the dose), the odds of error with cups remained higher than with syringes (aOR = 3.8; 95% CI, 3.1–4.7).
FIGURE 3.
Dosing errors by tool type across the 3 doses tested.
The odds of making an error with a cup versus syringe varied by health literacy (P < .001 for interaction) (Table 3). The odds of making an error by tool type also varied by language, with cup versus syringe differences more prominently seen for English-speaking parents, although both language groups had fewer errors with syringes (P < .001 for interaction).
Discussion
This study is the first to rigorously examine, within an experimental study, whether altering specific label and dosing tool attributes can reduce parent liquid medication dosing error rates. Overall, we found high dosing error rates. Little variation in errors was observed by unit pairings tested, although parents who received teaspoon-only labels with milliliter and teaspoon dosing tools made significantly more errors than those receiving milliliter-only labels and tools. Use of dosing cups greatly increased the risk of errors, especially with smaller dose amounts. Although the strength of associations differed somewhat by health literacy and language, our study clearly identified certain improvements that could be made to labels and tools to enhance dosing accuracy for parents across groups.
Overall, >80% of parents made ≥1 dosing error (>20% deviation), and >20% made ≥1 large error (>2 times the dose). Previous studies have demonstrated high error rates with liquid medications.12,13,15,16 A range of definitions for error have been used in the literature, with some relying on specific deviations in amount (eg, 0.2 mL),21 whereas others use percentage deviations (eg, 10%, 20%).16,37 We defined an error as >20% deviation, because we hoped to identify strategies that could be universally applied as part of a public health approach, recognizing that some medications have a narrow therapeutic window.15 For some medications, errors within an even smaller range (<20% deviation) may be clinically significant; additional intervention strategies may be important to reduce errors for these high-risk medications, including more intensive teaching or coaching.
Dosing error rates varied little by the unit pairings on the label and tool we studied. Use of teaspoon only on the label when paired with an mL and tsp tool was associated with a slightly higher error rate and was the only mismatch found to differ significantly from the milliliter-only group. Even in the milliliter-only group, parents made errors in 1 of 4 trials on average. These findings suggest that additional strategies beyond moving to milliliter-exclusive dosing, as supported by a 2015 AAP Policy Statement,7 will probably be needed for the greatest reduction in parent dosing error rates. Although no statistically significant difference by health literacy was seen, there was a trend for unit mismatches being most confusing for those with lower literacy. The impact of unit mismatch also varied significantly by language. Spanish parents faced a difficult mismatch in group 5, with cucharadita shown on the label and tools with mL and tsp.
In our study, cups were associated with >4 times the odds of error compared with syringes; similar findings were seen with large errors. Previous studies have demonstrated the superiority of syringes to cups when a 5-mL dose was tested14,27; our study is unique in that we examined a range of doses. One reason why cups may be inferior to syringes is that the same distance along the side of the tool represents a greater volume for cups than for syringes (eg, for cups, 1 mm might represent 0.8 mL; for syringes, 1 mm might represent 0.1 mL).27 In addition, when a cup is not held at eye level, it may appear to be filled to a particular marking when it is not.14 Even with syringes, however, a significant number of parents made dosing errors, suggesting that more intensive education by physicians, pharmacists, and other staff may be needed; use of strategies such as pictures or drawings, teachback, or showback, and demonstration may be beneficial.40
Interestingly, although there was a comparable reduction in absolute risk for error of 24% to 30% across literacy and language groups, a significant percentage of low-literacy (1 in 4) and Spanish-speaking parents (1 in 5) still made errors with syringes. These findings suggest that for these at-risk populations, a policy change to replace cups with syringes will probably not be sufficient.
We also found that the odds of error for syringes versus cups varied by dose, with tool type having the greatest impact with smaller doses. Our findings suggest that it may be beneficial to recommend the use of different tool types depending on the dose amount. The 2015 AAP policy statement on milliliter-exclusive dosing recommends provision of standardized tools with milliliter markings, preferably syringes, with cups and spoons with calibrated markings considered acceptable alternatives; no recommendations based on dose amount were provided.7 Our findings indicate that particularly when smaller doses are prescribed, providers may want to encourage parent use of syringes by providing them with a syringe to take home; cups may be acceptable for larger doses. Because parents may not use tools provided to them, counseling and general education about the importance and proper use of standard dosing tools remain important.
Interestingly, parents made more errors with dose amounts of 2.5 and 7.5 mL overall, compared with 5 mL, suggesting that whole numbers may be better understood. Additional study is needed to explore the potential benefit of limiting doses to whole number amounts.
Notably, the simplification of syringes with fewer markings was not associated with a difference in errors. It may be that parents benefit so much from using a syringe over a cup that the added benefit of simplification of markings is not discernible. Few studies have examined the implications of variations in markings in depth; for this study, we were able to look at only 2 variations. It remains possible that other strategies to simplify markings (eg, inclusion of only markings specific to recommended doses) could influence error rates.
This study has the following limitations. Errors were identified via a hypothetical assessment and might not reflect how parents actually dose at home. Parents measured medications as part of 9 trials, and test order was associated with error, consistent with a learning effect; however, the order in which each trial was conducted was randomized, and order was adjusted for in models. To minimize subject burden, a limited range of doses were tested, and only cups and oral syringes were tested. Tools were marked only in English, reflecting current standard practices. Not all potential unit pairings were included; we selected 5 common pairings. We did not include pairings involving mismatches of greater discordance such as a teaspoon label with a milliliter tool, because it is well established that complete mismatches should be avoided. Only 1 label design format was used. Our study focused on measurement, and not on other issues involved in the administration of medications to a child (eg, spillage). This study was conducted with English- and Spanish-speaking parents who brought their children to 3 university-affiliated pediatric clinic sites serving predominantly low-income families; results may not be generalizable.
Conclusions
Findings from this study can be used to build on existing AAP policies related to milliliter-only dosing and provision of standardized dosing tools,7,24 to promote the safe use of pediatric liquid medications. Our findings suggest that health care providers should encourage oral syringe use for the measurement of liquid medications, particularly when small doses are recommended; this change would probably benefit all families, regardless of health literacy and language. The types of unit of measurement discordance between labels and tools we studied appeared to have a limited impact on error rates, although our findings support avoidance of using teaspoon alone on labels. Notably, even when syringes were used with concordant milliliter-only labels and tools, parents made 1 or 2 errors on average across the 9 trials in this experiment. Future studies are needed to examine additional strategies (eg, pictograms, tool size) to reduce errors and to test strategies in real-world settings.
Acknowledgments
We thank our research staff, including Purvi Tailor Raythatha, BSc (Pharm). We also thank the staff of the pediatric outpatient clinics at Bellevue Hospital Center, Gardner Packard Children’s Health Care Center, and Children’s Healthcare of Atlanta at Hughes Spaulding for their support. We thank Comar for helping our team develop customized medication dosing tools for this project.
Glossary
- AAP
American Academy of Pediatrics
- aOR
adjusted odds ratio
- CI
confidence interval
- FDA
US Food and Drug Administration
- OTC
over the counter
- RA
research assistant
Footnotes
Dr Yin conceptualized and designed the study, analyzed and interpreted the data, drafted the initial manuscript, critically revised the manuscript for important intellectual content, and provided study supervision; Drs Parker, Sanders, and Bailey helped conceptualize and design the study, were involved in the analysis and interpretation of the data, critically revised the manuscript for important intellectual content, and provided study supervision; Drs Dreyer, Mendelsohn, and Wolf helped conceptualize and design the study, analyzed and interpreted the data, critically revised the manuscript for important intellectual content, and provided study supervision; Ms Patel, Ms Jimenez, and Ms Maness participated in the design of the study and assisted in acquisition, analysis, and interpretation of the data and drafting of the manuscript; Dr Kim participated in the design of the study, analyzed and interpreted the data, and critically revised the manuscript for important intellectual content; Ms Jacobson, Ms Hedlund, Ms Smith, and Dr McFadden participated in the design of the study, assisted in acquisition, analysis, and interpretation of the data, and critically revised the manuscript for important intellectual content; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01854151).
FINANCIAL DISCLOSURE: Drs Bailey, Parker, and Wolf and Ms Jacobson have served as consultants to and received grant funding from Merck, Sharp and Dohme for work unrelated to this study.
FUNDING: Supported by the National Institutes of Health (NIH) National Institute of Child Health and Human Development (grant R01HD070864). Dr Yin is supported by Health Resources & Services Administration grant 12-191-1077, Academic Administrative Units in Primary Care. Dr Sanders is supported by a US Food and Drug Administration Centers of Excellence in Regulatory Science and Innovation grant (University of California, San Francisco–Stanford Centers of Excellence in Regulatory Science and Innovation Award 13). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Institute of Medicine The Safe Use Initiative and Health Literacy: Workshop Summary. Washington, DC: National Academy Press; 2010 [PubMed] [Google Scholar]
- 2.Institute of Medicine Standardizing Medication Labels: Confusing Patients Less: Workshop Summary. Washington, DC: National Academy Press; 2008 [Google Scholar]
- 3.Wolf MS, Davis TC, Curtis LM, et al. Effect of standardized, patient-centered label instructions to improve comprehension of prescription drug use. Med Care. 2011;49(1):96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Physicians Foundation Improving Prescription Drug Container Labeling in the United States: A Health Literacy and Medication Safety Initiative. 2007. Available at: https://www.nationalacademies.org/hmd/∼/media/Files/Activity%20Files/PublicHealth/HealthLiteracy/Commissioned-Papers/Improving%20Prescription%20Drug%20Container%20Labeling%20in%20the%20United%20States.pdf
- 5.Institute of Medicine Preventing Medication Errors. Washington, DC: National Academy Press; 2006 [Google Scholar]
- 6.Bix L, Bello NM, Auras R, Ranger J, Lapinski MK. Examining the conspicuousness and prominence of two required warnings on OTC pain relievers. Proc Natl Acad Sci USA. 2009;106(16):6550–6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul IM, Neville K, Galinkin JL, et al. . Metric units and the preferred dosing of orally administered liquid medications. Pediatrics. 2015;135(4):784–787 [Google Scholar]
- 8.US Pharmacopeia Prescription Container Labeling. 2011. Available at: www.usp.org/sites/default/files/usp_pdf/EN/USPNF/M5531.pdf
- 9.Wolf MS, Davis TC, Bass PF, et al. Improving prescription drug warnings to promote patient comprehension. Arch Intern Med. 2010;170(1):50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey SC, Wolf MS, Lopez A, et al. Expanding the Universal Medication Schedule: a patient-centred approach. BMJ Open. 2014;4(1):e003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Council for Prescription Drug Programs Universal Medication Schedule White Paper. Scottsdale, AZ: NCPDP; 2013. [Google Scholar]
- 12.Yin HS, Dreyer BP, van Schaick L, Foltin GL, Dinglas C, Mendelsohn AL. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch Pediatr Adolesc Med. 2008;162(9):814–822 [DOI] [PubMed] [Google Scholar]
- 13.Yin HS, Dreyer BP, Ugboaja DC, et al. Unit of measurement used and parent medication dosing errors. Pediatrics. 2014;134(2). Available at: www.pediatrics.org/cgi/content/full/134/2/e354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin HS, Mendelsohn AL, Wolf MS, et al. Parents’ medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164(2):181–186 [DOI] [PubMed] [Google Scholar]
- 15.Frush KS, Luo X, Hutchinson P, Higgins JN. Evaluation of a method to reduce over-the-counter medication dosing error. Arch Pediatr Adolesc Med. 2004;158(7):620–624 [DOI] [PubMed] [Google Scholar]
- 16.Simon HK, Weinkle DA. Over-the-counter medications. Do parents give what they intend to give? Arch Pediatr Adolesc Med. 1997;151(7):654–656 [DOI] [PubMed] [Google Scholar]
- 17.DeWalt DA. Ensuring safe and effective use of medication and health care: perfecting the dismount. JAMA. 2010;304(23):2641–2642 [DOI] [PubMed] [Google Scholar]
- 18.Kaushal R, Jaggi T, Walsh K, Fortescue EB, Bates DW. Pediatric medication errors: what do we know? What gaps remain? Ambul Pediatr. 2004;4(1):73–81 [DOI] [PubMed] [Google Scholar]
- 19.Shah R, Blustein L, Kuffner E, Davis L. Communicating doses of pediatric liquid medicines to parents/caregivers: a comparison of written dosing directions on prescriptions with labels applied by dispensed pharmacy. J Pediatr. 2014;164(3):596–601, e591 [DOI] [PubMed] [Google Scholar]
- 20.Institute for Safe Medication Practices ISMP Quarterly Action Agenda. October–December 2011. Available at: www.ismp.org/Newsletters/acutecare/articles/A1Q12Action.asp. Accessed November 29, 2013
- 21.McMahon SR, Rimsza ME, Bay RC. Parents can dose liquid medication accurately. Pediatrics. 1997;100(3 pt 1):330–333 [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health Human Services Guidance for Industry: Dosage Delivery Devices for Orally Ingested OTC Liquid Drug Products. 2011. Available at: www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM188992.pdf. Accessed November 1, 2013
- 23.Leyva M, Sharif I, Ozuah PO. Health literacy among Spanish-speaking Latino parents with limited English proficiency. Ambul Pediatr. 2005;5(1):56–59 [DOI] [PubMed] [Google Scholar]
- 24.Yaffe SJ, Bierman CW, Cann HM, et al. Inaccuracies in administering liquid medication. Pediatrics. 1975;56(2):327–328 [PubMed] [Google Scholar]
- 25.Madlon-Kay DJ, Mosch FS. Liquid medication dosing errors. J Fam Pract. 2000;49(8):741–744 [PubMed] [Google Scholar]
- 26.McKenzie M. Administration of oral medications to infants and young children. US Pharm. 1981;6:55–67 [Google Scholar]
- 27.Sobhani P, Christopherson J, Ambrose PJ, Corelli RL. Accuracy of oral liquid measuring devices: comparison of dosing cup and oral dosing syringe. Ann Pharmacother. 2008;42(1):46–52 [DOI] [PubMed] [Google Scholar]
- 28.Yin HS, Wolf MS, Dreyer BP, Sanders LM, Parker RM. Evaluation of consistency in dosing directions and measuring devices for pediatric nonprescription liquid medications. JAMA. 2010;304(23):2595–2602 [DOI] [PubMed] [Google Scholar]
- 29.Litovitz T. Implication of dispensing cups in dosing errors and pediatric poisonings: a report from the American Association of Poison Control Centers. Ann Pharmacother. 1992;26(7-8):917–918 [DOI] [PubMed] [Google Scholar]
- 30.National Council for Prescription Drug Programs NCPDP Recommendations and Guidance for Standardizing the Dosing Designations on Prescription Container Labels of Oral Liquid Medications. 2014. Available at: www.ncpdp.org/ncpdp/media/pdf/wp/dosingdesignations-oralliquid-medicationlabels.pdf
- 31.American Academy of Family Physicians Preferred Unit of Measurement for Liquid Medications. 2011. Available at: www.aafp.org/about/policies/all/preferred-unit.html. Accessed December 5, 2013
- 32.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry Safety Considerations for Container Labels and Carton Labeling Design to Minimize Medication Errors. 2013. Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm349009.pdf. Accessed December 5, 2013
- 33.Vawter SM, De Forest RE. The international metric system and medicine. JAMA. 1971;218(5):723–726 [PubMed] [Google Scholar]
- 34.Yin HS, Dreyer BP, Foltin G, van Schaick L, Mendelsohn AL. Association of low caregiver health literacy with reported use of nonstandardized dosing instruments and lack of knowledge of weight-based dosing. Ambul Pediatr. 2007;7(4):292–298 [DOI] [PubMed] [Google Scholar]
- 35.Yin HS, Forbis SG, Dreyer BP. Health literacy and pediatric health. Curr Probl Pediatr Adolesc Health Care. 2007;37(7):258–286 [DOI] [PubMed] [Google Scholar]
- 36.Bradshaw M, Tomany-Korman S, Flores G. Language barriers to prescriptions for patients with limited English proficiency: a survey of pharmacies. Pediatrics. 2007;120(2). Available at: www.pediatrics.org/cgi/content/full/120/2/e225 [DOI] [PubMed] [Google Scholar]
- 37.Kozer E, Scolnik D, Macpherson A, et al. Variables associated with medication errors in pediatric emergency medicine. Pediatrics. 2002;110(4):737–742 [DOI] [PubMed] [Google Scholar]
- 38.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bethell CD, Read D, Stein RE, Blumberg SJ, Wells N, Newacheck PW. Identifying children with special health care needs: development and evaluation of a short screening instrument. Ambul Pediatr. 2002;2(1):38–48 [DOI] [PubMed] [Google Scholar]
- 40.Yin HS, Dreyer BP, Moreira HA, et al. Liquid medication dosing errors in children: role of provider counseling strategies. Acad Pediatr. 2014;14(3):262–270 [DOI] [PMC free article] [PubMed] [Google Scholar]