Abstract
BACKGROUND AND OBJECTIVES:
New US Down syndrome (DS) BMI growth charts were recently published, but their utility in identifying children with excess adiposity or increased cardiometabolic risk (CMR) remains unknown. We sought to compare the ability of the Centers for Disease Control and Prevention (CDC) BMI 85th percentile and DS-specific BMI 85th percentile to identify excess adiposity in children with DS.
METHODS:
Participants with DS aged 10 to 20 years were enrolled in a cross-sectional CMR study. Data from typically developing children enrolled in the Bone Mineral Density in Childhood Study (BMDCS) were used for comparison. Sensitivity and specificity were calculated to assess the CDC BMI 85th percentile in the BMDCS and DS groups, and the DS-specific BMI 85th percentile in the DS group, relative to fat mass index (FMI) ≥80th percentile, a threshold associated with increased CMR.
RESULTS:
Included were 121 DS participants (age 14.8 ± 3.3 years, 57% girls) and 7978 BMDCS reference data points (age 15.0 ± 3.0 years, 51.3% girls). The CDC BMI 85th percentile identified FMI ≥80th percentile with 96.9% sensitivity and 87.4% specificity in typically developing children. Similarly, the CDC BMI 85th percentile identified FMI ≥80th percentile with 100% sensitivity and 78.3% specificity in children with DS. In contrast, the sensitivity of the DS-specific BMI 85th percentile was only 62.3% (P < .0001), but was 100% specific.
CONCLUSIONS:
For children with DS ≥10 years, the CDC BMI growth chart 85th percentile is a better indicator of excess adiposity, than the new DS-specific BMI charts. Additional studies are needed to clarify the relationships of BMI and FMI with CMR in DS.
What’s Known on This Subject:
BMI growth charts were recently published for children with Down syndrome (DS), but how well the DS-specific BMI charts identify excess adiposity compared with standard Centers for Disease Control and Prevention (CDC) BMI charts in children with DS is unknown.
What This Study Adds:
CDC and DS-specific BMI charts were compared in their ability to predict elevated fat mass index in children with DS age ≥10 years. CDC BMI charts are a better indicator of excess adiposity in this group than DS-specific BMI charts.
BMI is used to screen for excess adiposity and cardiometabolic risk (CMR) in children and adults. Increased BMI is common in individuals with Down syndrome (DS),1 but the short stature and altered body proportions that characterize DS can also influence the BMI measure. The extent to which BMI captures excess body adiposity and associated CMR in this population with known altered body composition2 is not known. In 2011, the American Academy of Pediatrics recommended that providers use standard Centers for Disease Control and Prevention (CDC) or World Health Organization reference curves, including those for BMI, to monitor children with DS until new DS-specific growth charts were available.3 However, evidence was absent for determining thresholds for excess adiposity for children with DS using the CDC or World Health Organization charts.
Recently, new growth charts were developed for the US DS population and included growth charts for BMI.4 These BMI charts reflect the BMI distribution of contemporary children with DS in the United States, but their utility in identifying excess adiposity and increased risk of obesity-related complications remains unknown.
Fat mass index (FMI) is an accurate indicator of adiposity in children and adolescents.5 Using National Health and Nutrition Examination Survey data, Weber et al6 showed that the 80th percentile for FMI was the optimal threshold for identifying children at increased risk for metabolic syndrome, a clustering of conditions resulting from insulin resistance and obesity, which is associated with an increased risk of cardiovascular disease and type 2 diabetes. Determining whether the standard CDC BMI growth chart or the new DS-specific BMI growth chart more accurately identifies FMI ≥80th percentile in the DS population is an important consideration in developing screening recommendations for children and adolescents with DS.
The goals of this study were to compare the sensitivity and specificity of DS-specific and CDC-derived BMI z scores as predictors of 80th percentile FMI in a large sample of children with DS ages 10 to 20 years, and to compare the performance of CDC-derived BMI z scores in identifying body adiposity in the sample with DS to that of an unaffected reference group of healthy children.
Methods
DS Study Sample
Participants with DS were recruited as part of a larger cross-sectional observational study of CMR in DS from 2 institutions, The Children’s Hospital of Philadelphia (CHOP) and Children’s National Health System (CNHS).
Inclusion criteria were patients with a diagnosis of DS and an age of 10 to 20 years. Exclusion criteria included major organ system illness not related to DS (except diabetes mellitus), current or previous oncologic process, cyanotic or unstable congenital heart disease, pulmonary hypertension, pregnancy, genetic syndrome known to affect glucose tolerance, familial hypercholesterolemia, or current treatment with medications known to affect insulin sensitivity or lipids (other than diabetes agents in known diabetes mellitus). Subjects were included in the current study if they completed a whole-body dual-energy radiograph absorptiometry (DXA) scan and anthropometric exam.
Data were collected from February 2013 to August 2015. Protocols were approved by CHOP and CNHS institutional review boards and consent/assent was obtained from parents and participants, respectively, when appropriate.
Weight (kilograms) was measured by digital electronic scale (Scaletronix), calibrated daily, and stature (centimeters) was measured on a wall-mounted stadiometer (Holtain) with the participant in light clothing without shoes by trained research anthropometrists using standard techniques.
Whole-body DXA scans were obtained using a Hologic Horizon or Discovery bone densitometer (Hologic, Inc, Bedford, MA) at CHOP or a Discovery bone densitometer at CNHS. Quality control was performed using a Hologic Whole Body Phantom scanned 3 times per week on each device. We did not adjust for intermachine differences so that our results would be more generalizable to the clinical setting. All DXA scans were reviewed and analyzed at CHOP using Hologic Software, version 13.5.2 (Hologic).
Bone Mineral Density in Childhood Study Sample
Data from the Bone Mineral Density in Childhood Study (BMDCS) were used to allow for comparison of the DS group with unaffected, healthy children (BMDCS sample). Detailed study procedures and inclusion/exclusion criteria for the BMDCS have previously been described.7,8 To summarize, the BMDCS collected height, weight, and longitudinal DXA measurements at 5 different US centers for 2014 healthy children from multiple ethnic groups, aged 5 to 19 years at enrollment. Inclusion criteria included birth weight >2.3 kg and no evidence of precocious or delayed puberty. Exclusion criteria at enrollment included height, weight, or BMI <3rd or >97th percentile and a medical condition known to affect growth.7,8 Participants were followed for up to 7 years, and throughout that time, excursions in BMI <3rd or >97th percentiles occurred.
To correspond to the ages of DS subjects, the BMDCS sample used for comparison was limited to data obtained at ages 10 to 20 years. Only BMDCS participants with measures of whole-body fat mass and BMI were included for the current analyses (1725 participants contributed 7978 unique, DXA-derived body composition and anthropometric measures).
Statistical Analysis
All analyses used z scores to account for the expected age and sex differences in BMI and body composition measures, so that data across this large age range could be combined. Age- and sex-specific BMI z scores were generated for both the BMDCS and DS participants using the 2000 CDC reference data.9 For the DS group, DS-specific BMI z scores by age and sex were calculated using the recently published DS reference data.4 If age was >20.0 years, then age was adjusted to 20.0 years for these calculations. FMI (fat mass/height2) and lean BMI (LBMI; excluding bone mass/height2) were calculated (kilogram per square meter), and FMI and LBMI z scores were generated for both groups using previously published reference data.5
Study data were collected and managed using REDCapelectronic data capture tools hosted at The Children's Hospital of Philadelphia.10 Statistical analyses were performed with Stata version 13.1 (StataCorp, College Station, TX). Two-sample t tests (or nonparametric equivalent) were used to compare continuous data between the DS and BMDCS samples; statistical significance was defined as P < .05.
We used visual inspection to compare the DS BMI growth curves4 to the 2000 CDC BMI growth curves.9 Scatterplots displayed the distribution of FMI z scores to CDC-derived BMI z scores for the DS and BMDCS samples. Similar scatterplots were developed for LBMI z scores.
The test characteristics of sensitivity, specificity, positive predictive value, and negative predictive value were used to compare the ability of the CDC BMI 85th percentile in the BMDCS and DS groups and the DS-specific BMI 85th percentile in DS subjects to identify FMI ≥80th percentile (FMI z score ≥0.84). The 85th percentile (z score ≥1.04) for BMI was chosen because this is the threshold recommended for the identification of overweight status in children.11 The McNemar test was used to compare the sensitivity and specificity results of using the CDC or DS-specific BMI 85th percentile within the DS group. Cohen’s κ coefficient was also calculated for each group as an index of chance-corrected agreement to additionally compare the different growth chart categorical threshold abilities in identifying FMI ≥80th percentile.
Simple correlations were also performed between both CDC and DS-specific BMI z scores and FMI z scores in both groups. To test for 2-way interactions between FMI z scores and CDC-derived BMI z scores by DS status and by sex, linear regression models were developed with FMI z score as the dependent variable and interaction terms (DS by CDC BMI z score and sex by CDC BMI z score) as the independent variables. The correlations between FMI z scores and CDC BMI z scores were then stratified and examined by sex and DS status.
Results
Participant Characteristics
Descriptive characteristics of the DS (n = 121; 57% girls) and BMDCS (n = 1725; 7978 observations; 51.3% girls) participants are found in Table 1. CDC BMI z scores for DS subjects were significantly higher than for the BMDCS sample and were nearly 1 SD greater than the DS-specific BMI z scores. FMI and LBMI z scores were both significantly higher in the DS group than in the BMDCS sample.
TABLE 1.
Characteristics of DS Participants and BMDCS Reference Data
| DS Group | BMDCS Sample | P | |
|---|---|---|---|
| Sample, n | 121 | 7978 | |
| Age, y | 14.8 ± 3.3 | 15.0 ± 3.0 | .6 |
| Sex (% girls) | 57% | 51.3% | .2 |
| % African American | 20% | 22.8% | .4 |
| CDC BMI z score | 1.23 ± 0.97 | 0.31 ± 0.88 | <.0001 |
| % CDC BMI ≥85th | 61% | 22.1% | n/a |
| DS BMI z score | 0.34 ± 1.07 | n/a | n/a |
| % DS BMI ≥85th | 31% | n/a | n/a |
| % DS BMI ≥50th | 58% | n/a | n/a |
| FMI z score | 0.73 ± 0.91 | −0.25 ± 0.82 | <.0001 |
| % FMI ≥80th | 50% | 11.3% | n/a |
| LBMI z score | 0.53±1.06 | −0.17 ± 0.81 | <.0001 |
| CDC HAZ | −2.28±1.05 | 0.15 ± 0.86 | <.0001 |
Data presented as mean ± SD unless otherwise specified. n/a, not applicable.
Comparing Growth Charts
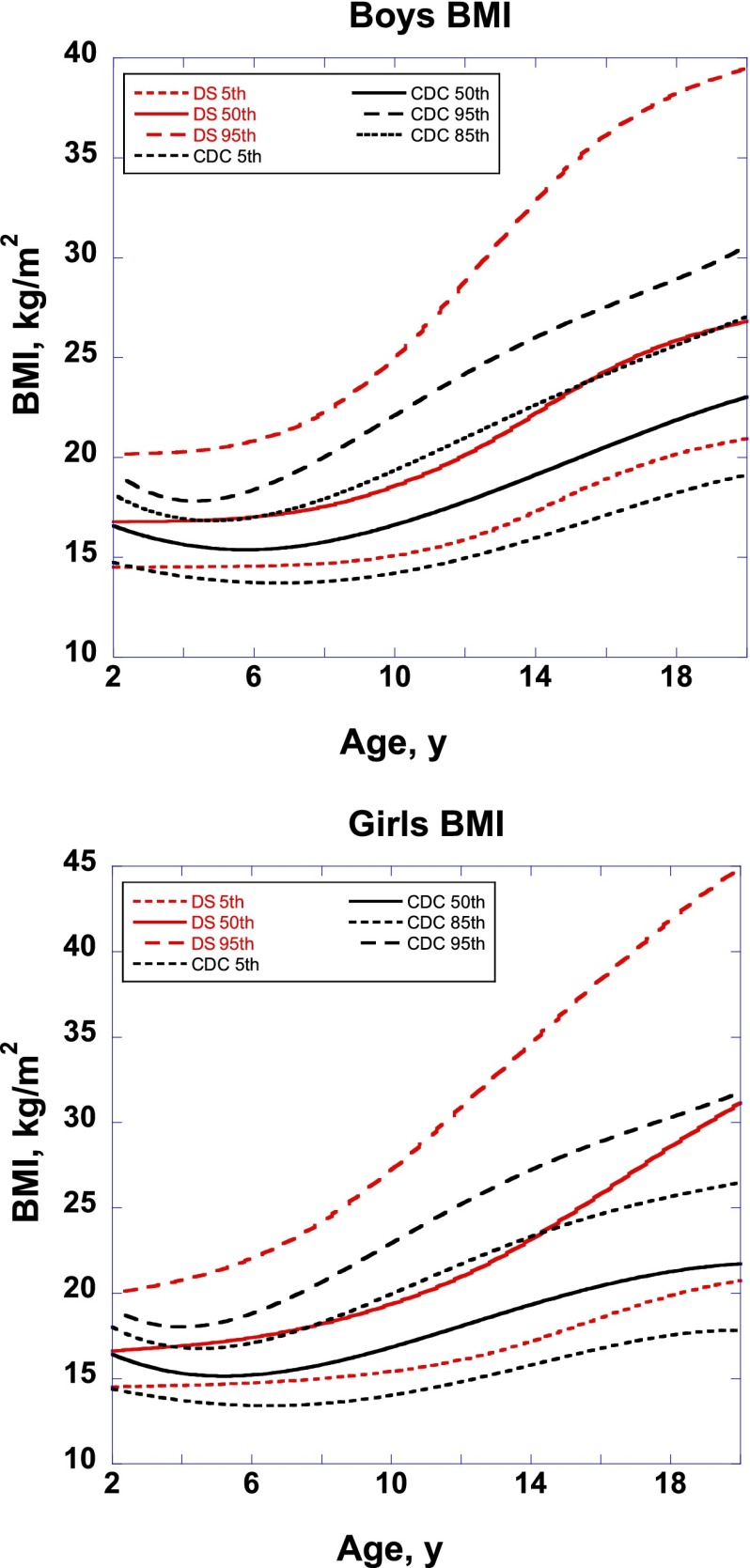
The overlay of DS-specific BMI growth charts on CDC BMI growth charts are shown in Fig 1. For both sexes, the DS-specific BMI 95th percentile was higher than the CDC BMI 95th percentile, even at the youngest ages. At almost all ages for boys, the DS-specific 50th percentile approximated the CDC 85th percentile. For girls, the DS-specific 50th percentile also approximated the CDC 85th percentile until about age 14 years, at which time the DS-specific 50th percentile exceeded the CDC 85th percentile and began to approach the CDC 95th percentile by age 20 years.
FIGURE 1.
Overlay of DS BMI growth charts and 2000 CDC BMI growth charts, by sex.
Detection of Excess Adiposity
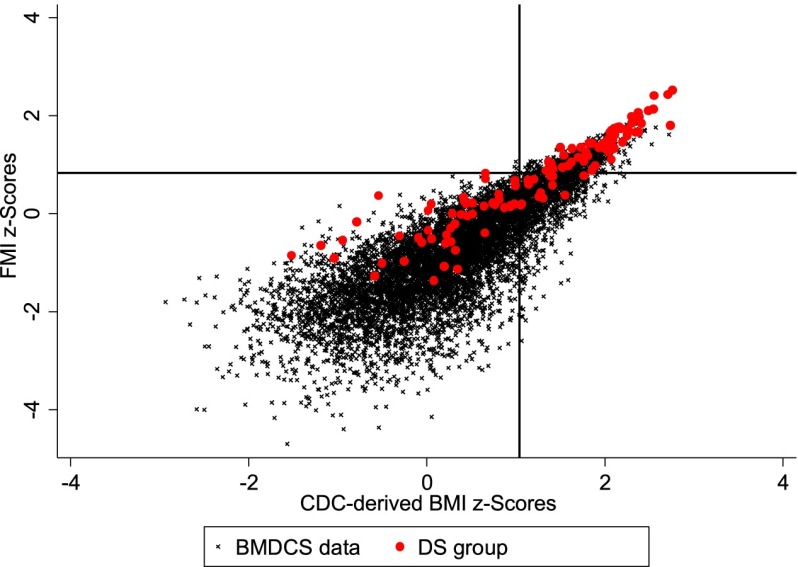
Scatterplots of FMI z scores by CDC BMI z scores for the BMDCS sample and DS subjects are shown in Fig 2. FMI z scores for children with DS were higher than those for the BMDCS sample at the lower end of the CDC 2000 BMI z scores, but the distributions overlapped at BMI z scores >2.
FIGURE 2.
FMI z scores by CDC-derived BMI z scores in the reference population and participants with DS. X-line approximates BMI 85th percentile (z = 1.04) and Y-line approximates FMI 80th percentile (z = 0.84).
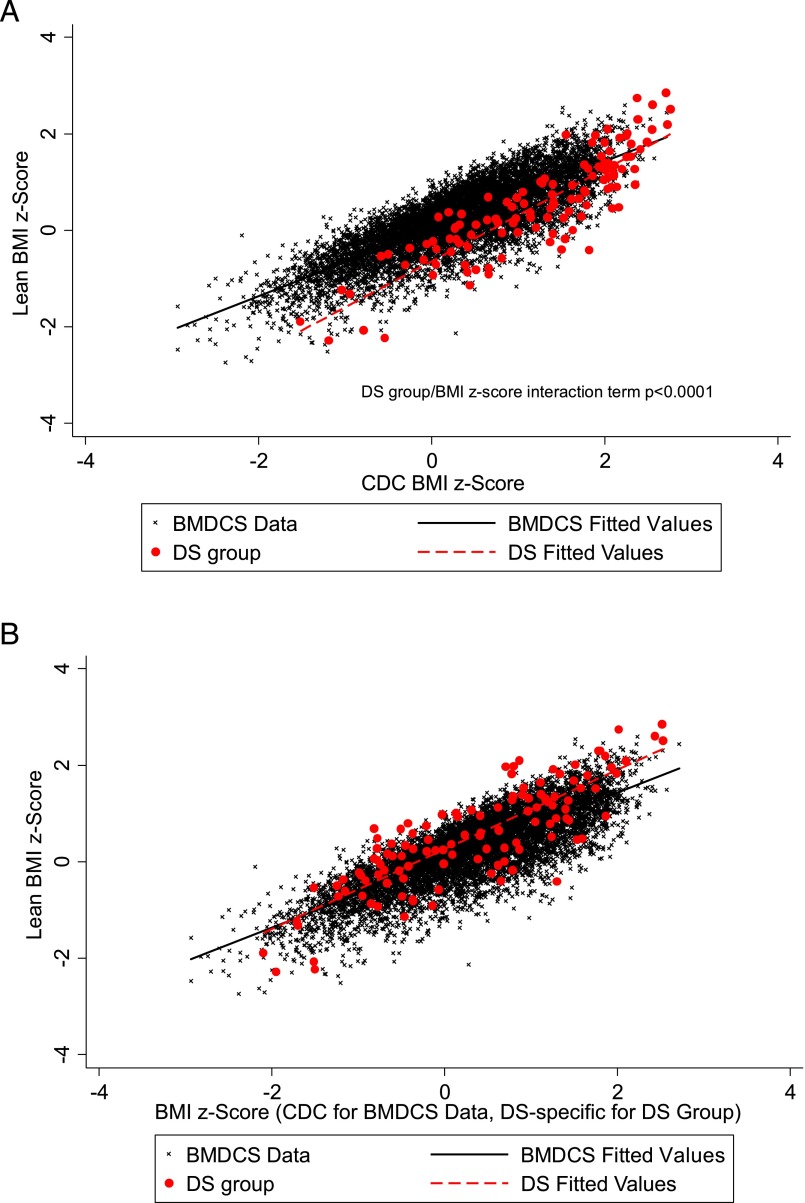
Scatterplots of LBMI z scores by BMI z scores for the BMDCS sample and DS subjects (using both CDC BMI z scores and DS-specific BMI z scores) are shown in Fig 3. For a given CDC BMI z score, LBMI z scores for children with DS were generally lower than those for the BMDCS sample at most CDC BMI z scores, but they overlapped for BMI z scores >2 (Fig 3A). When DS-specific BMI z scores were used, the relationship between LBMI z scores and BMI z scores in DS more closely approximated the relationship seen in the BMDCS sample (Fig 3B).
FIGURE 3.
A, LBMI z scores by CDC-derived BMI z scores in the reference population and participants with DS. B, LBMI z scores by CDC derived BMI z scores in the reference population and DS-specific BMI z scores for participants with DS.
Test characteristics for the detection of FMI ≥80th percentile in the BMDCS sample using the CDC 85th BMI percentile, DS subjects using the CDC 85th BMI percentile, and DS subjects using the DS-specific 85th BMI percentile are shown in Table 2. Because the CDC BMI 85th percentile appeared to visually approximate the DS-specific 50th percentile for almost all ages in the DS subjects, performance parameters were also calculated for the DS-specific 50th percentile (Table 2).
TABLE 2.
Detection of FMI ≥80th Percentile by Group
| BMDCS Sample (Using CDC 85% BMI) | DS Group (Using CDC 85% BMI) | DS Group (Using DS 85% BMI) | DS Group (Using DS 50% BMI) | |
|---|---|---|---|---|
| Sensitivity, % | 96.9 (95.5–97.9) | 100 (94.1–100) | 62.3 (49.0–74.4)* | 98.4 (91.2–100)** |
| Specificity, % | 87.4 (86.6–88.1) | 78.3 (65.8–87.9) | 100 (94.0–100)* | 83.3 (71.5–91.7)** |
| PPV, % | 49.4 (47.0–51.7) | 82.4 (71.8–90.3) | 100 (90.7–100) | 85.7 (75.3–92.9) |
| NPV, % | 99.5 (99.3–99.7) | 100 (92.5–100) | 72.3 (61.4–81.6) | 98.0 (89.6–100) |
| κ | 0.59 (0.57–0.62) | 0.79 (0.68–0.89) | 0.62 (0.49–0.75) | 0.82 (0.72–0.92) |
Data presented with 95% confidence intervals. NPV, negative predictive value; PPV, positive predictive value.
P < .001 compared with CDC 85th percentile for DS using McNemar test.
P = not significant compared with CDC 85th percentile for DS using McNemar test.
Although the DS-specific BMI 85th percentile was quite specific for the identification of FMI ≥80th percentile (100%), this threshold had much lower sensitivity compared with the sensitivity of the CDC 85th percentile in DS (62.3% vs 100%, P < .0001). Use of the DS-specific BMI 50th percentile for the identification of FMI ≥80th percentile showed no statistical difference in sensitivity and specificity when compared with the CDC BMI 85th percentile for the DS subjects. κ Coefficients for these 2 DS groups (0.79 and 0.82 for the CDC BMI 85th percentile and DS-specific BMI 50th percentile, respectively) showed significant overlap in 95% confidence intervals, but were both higher than that for the DS group using the DS-specific 85th percentile (Table 2).
These test characteristics were also calculated separately for boys and girls, but these sex-specific analyses did not significantly differ from those already reported (data not shown).
DS and Sex Differences
Simple correlation coefficients for the associations between both CDC and DS-specific BMI z scores and FMI z scores for the BMDCS and DS groups are shown in Table 3. Linear regression models to evaluate for 2-way interactions between FMI z scores and CDC-derived BMI z scores in the entire group revealed a marginally significant DS/BMI-z interaction (P = .06) and a significant sex/BMI-z interaction (P = .01). Sex-specific models identified a significant DS/BMI-z interaction in boys (P = .02; girls: P = .5). When correlations between FMI z scores and CDC BMI z scores were stratified into sex and DS-status groups (Table 3), girls with DS showed the highest correlation (r = 0.96, P < .0001), followed by boys with DS (r = 0.90, P < .0001), girls without DS (r = 0.89, P < .0001), and boys without DS (r = 0.82, P < .0001).
TABLE 3.
Correlation Coefficients for the Associations Between BMI z Scores and FMI z Scores
| Correlation Coefficient With FMI z Scores | |
|---|---|
| CDC BMI z score for the entire BMDCS group | 0.85 |
| CDC BMI z score for BMDCS girls | 0.89 |
| CDC BMI z score for BMDCS boys | 0.82 |
| CDC BMI z score for the entire DS group | 0.92 |
| CDC BMI z score for DS girls | 0.96 |
| CDC BMI z score for DS boys | 0.90 |
| DS-specific BMI z score for the entire DS group | 0.93 |
| DS-specific BMI z score for DS girls | 0.95 |
| DS-specific BMI z score for DS boys | 0.89 |
All P < .0001.
Discussion
Little is known about the true extent of CMR in the DS population. DS has traditionally been considered an “atheroma-free” condition, and individuals with DS were considered to be protected from atherosclerotic disease.12 More recent studies have disproved this model by identifying not only the presence of cardiovascular disease in DS,13 but also an increased risk for ischemic heart disease in DS.14 The contribution of obesity to these outcomes has not been investigated. Because BMI indexes weight relative to height, the shorter limbs and altered body proportions of people with DS could conceivably inflate BMI, and how well the BMI measure serves as an index of obesity in DS is unknown. Ideally, defining the BMI or BMI percentile threshold that best predicts CMR should be based on cardiometabolic outcomes. In the absence of such information, we used a FMI ≥80th percentile as an indicator of excess adiposity in children ages 10 to 20 years to evaluate the performance of the CDC and DS-specific BMI charts in identifying excess body adiposity in DS. The CDC BMI 85th percentile identified excess adiposity well in both the unaffected reference population and in children with DS, in whom it was more sensitive than the DS-specific BMI 85th percentile. The use of the DS-specific 50th percentile was similar in terms of test characteristics to that of the CDC 85th percentile for the DS subjects, but did not provide enough additional benefit to encourage its use. This finding provides the much-needed evidence to support the recommendation to use the CDC BMI charts for children with DS.
BMI charts are a widely used, easily accessible, and useful screening tool for identifying children at increased risk for health concerns. Based on our results, we suggest that the best way to screen for excess adiposity in DS is to monitor BMI using the established overweight and obese definitions based on the 2000 CDC BMI growth charts. The 50th percentile on the DS BMI charts may be similarly efficacious in identifying excess adiposity in children with DS, but using the DS-specific BMI curves and a 50th percentile threshold may lead to confusion among the medical community and families, and straightforward recommendations are needed.
In 2007, the American Academy of Pediatrics endorsed updated obesity screening recommendations for healthy children and adolescents to monitor yearly BMI plotted on standard growth charts and defined those with an age- and sex-specific BMI percentile ≥85th as overweight and ≥95th as obese.11 These BMI percentiles have excellent sensitivity for the identification of children most at risk for the health-related consequences of obesity and are easily applied in clinical practice. Among typically developing children, body fat distribution rather than the total body mass may have a greater impact on cardiovascular risk,15,16 but body composition measurement by methods such as DXA cannot practically be applied as a screening measure in clinical practice. Currently, BMI is the easiest and best tool available for the identification of overweight and obese individuals, because it can be performed in almost all clinical settings at a low cost. Indeed, Weber et al6 did not find improved detection of metabolic syndrome using FMI when compared with BMI.
Obesity is a significant problem in pediatrics, and having DS is associated with an increased risk for obesity.17 Previous reports estimate a prevalence of obesity as high as 30% to 50%,18 as well as increased adiposity compared with unaffected peers.19 Our inspection of the new DS BMI growth charts compared with the CDC BMI growth charts also indicated that at least half of children with DS today would be categorized as being overweight or obese (CDC BMI ≥85th percentile). Another striking result from our visual inspection of the DS BMI growth charts is the great amount of excess adiposity in older girls with DS. The charts indicate that by late adolescence, at least half of girls with DS will be diagnosed as obese, with many more categorized as overweight. Weight-related interventions are crucial to ensure the continued health and well-being of these children, with targeted interventions specifically geared toward older girls with DS being most critical at this time.
Our study also uncovered a number of additional body composition outcomes. Regardless of DS status, sex affects the relationship between FMI and BMI z scores, with higher correlations between FMI and BMI z scores in girls than in boys. Weber et al5 found that in children and adolescents, FMI increases with increasing age to a greater extent in girls than in boys, a finding that supports our results and indicates that fat mass contributes more to BMI in girls than in boys. Bandini et al20 showed that girls with DS had a higher correlation of BMI z score and percentage body fat by DXA than boys with DS. We also found that DS status had an influence on the FMI and BMI z score relationship in boys only: boys with DS have higher contributions of fat mass to BMI than boys without DS; the contribution of fat mass to BMI was more similar among girls with and without DS.
This study has many strengths. We produced one of the largest data sets of body composition in children with DS. By comparing them to a large, multicenter, multiethnic reference sample, we demonstrate support for currently used cut-offs for excess adiposity in children with and without DS. This study is also the first to critically evaluate the newly published US DS growth charts. Without these findings, providers of children with DS could have mistakenly used the 85th percentile on the DS-specific BMI charts to screen for obesity-related health outcomes. This use could result in missing many children with DS potentially clinically significant elevations in BMI. One important limitation is that these findings cannot be generalized to children under the age of 10 years. Another important limitation is the assumption that FMI as a marker for excess adiposity best reflects risk of current and future obesity-related complications in children with DS. Although we had the ability to categorize children and adolescents with DS as overweight or obese based on findings of excess adiposity, minimal data are available regarding the overall risk of cardiovascular disease or other obesity-related comorbidities in this population. Additional study is necessary to correlate these alterations in body composition with known, obesity-related complications in DS.
Conclusions
Our results indicate that for children and adolescents with DS, the 85th percentile on the CDC BMI growth charts is a better indicator of excess adiposity than the 85th percentile on the DS-specific BMI growth charts. Although the DS-specific charts are still an excellent method to compare patients with DS to their DS peers, the CDC BMI charts should be the preferred method for the early identification obesity in children with DS.
Acknowledgments
We thank the study participants and their families, as well as research coordinators Rachel Walega, Amber Lauff, and Priscilla Andalia and students Sarah Appeadu, Elizabeth Stulpin, Claire Trindle, Natalie Rosetti, Jeffrey Signora, Cassandra Zhi, Cedar Slovacek, Suzanne M. Arnott, Monica N. Salama, and Emily Eicheldinger for their diligent efforts. In addition, we thank the CHOP Clinical and Translational Research Center, the CNHS Center for Translational Science, the CHOP Pediatric Research Consortium, the CHOP Recruitment Enhancement Core, and the Bone Mineral Density in Childhood Study Group, without whom this study would not have been possible, for their contributions.
Glossary
- BMDCS
Bone Mineral Density in Childhood Study
- CDC
Centers for Disease Control and Prevention
- CHOP
The Children’s Hospital of Philadelphia
- CMR
cardiometabolic risk
- CNHS
Children’s National Health System
- DS
Down syndrome
- DXA
dual-energy radiograph absorptiometry
- FMI
fat mass index
- LBMI
lean BMI
Footnotes
Dr Hatch-Stein carried out the data analyses, drafted the initial manuscript, and revised the manuscript; Dr Zemel conceptualized and designed the study and reviewed and revised the manuscript; Ms Prasad coordinated and supervised data collection at 1 of the 2 sites and critically reviewed the manuscript; Dr Kalkwarf conceptualized and designed the study and critically reviewed the manuscript; Dr Pipan conceptualized and designed the study, recruited subjects, and reviewed and revised the manuscript; Dr Magge conceptualized and designed the study and reviewed and revised the manuscript; Dr Kelly conceptualized and designed the study and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants R01HD071981 (Drs Kelly and Magge), T32DK63688-11 A1 (Dr Hatch-Stein), the Endocrine Society (Dr Hatch-Stein), and N01-HD-1-3228, -3329, -3330, -3331, -3332, -3333. It was also supported by grants UL1RR024134 (National Center for Research Resources), UL1TR000003 (The Children’s Hospital of Philadelphia), and UL1TR000075 (Children’s National Health System) from the National Institutes of Health, National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hsieh K, Rimmer JH, Heller T. Obesity and associated factors in adults with intellectual disability. J Intellect Disabil Res. 2014;58(9):851–863 [DOI] [PubMed] [Google Scholar]
- 2.González-Agüero A, Ara I, Moreno LA, Vicente-Rodríguez G, Casajús JA. Fat and lean masses in youths with Down syndrome: gender differences. Res Dev Disabil. 2011;32(5):1685–1693 [DOI] [PubMed] [Google Scholar]
- 3.Bull MJ; Committee on Genetics . Health supervision for children with Down syndrome. Pediatrics. 2011;128(2):393–406 [DOI] [PubMed] [Google Scholar]
- 4.Zemel BS, Pipan M, Stallings VA, et al. Growth Charts for Children With Down Syndrome in the United States. Pediatrics. 2015;136(5). Available at: www.pediatrics.org/cgi/content/full/136/5/e1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98(1):49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber DR, Leonard MB, Shults J, Zemel BS. A comparison of fat and lean body mass index to BMI for the identification of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2014;99(9):3208–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92(6):2087–2099 [DOI] [PubMed] [Google Scholar]
- 9.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002(246):1–190 [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barlow SE; Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 12.Murdoch JC, Rodger JC, Rao SS, Fletcher CD, Dunnigan MG. Down’s syndrome: an atheroma-free model? BMJ. 1977;2(6081):226–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raina T, McGrath E, Gunn J. Myocardial infarction in a patient with down syndrome: a case report and review of the literature. Clin Cardiol. 2011;34(2):87–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill DA, Gridley G, Cnattingius S, et al. Mortality and cancer incidence among individuals with Down syndrome. Arch Intern Med. 2003;163(6):705–711 [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health. 2013;13:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peltz G, Aguirre MT, Sanderson M, Fadden MK. The role of fat mass index in determining obesity. Am J Hum Biol. 2010;22(5):639–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermon C, Alberman E, Beral V, Swerdlow AJ. Mortality and cancer incidence in persons with Down’s syndrome, their parents and siblings. Ann Hum Genet. 2001;65(Pt 2):167–176 [DOI] [PubMed] [Google Scholar]
- 18.Day SM, Strauss DJ, Shavelle RM, Reynolds RJ. Mortality and causes of death in persons with Down syndrome in California. Dev Med Child Neurol. 2005;47(3):171–176 [DOI] [PubMed] [Google Scholar]
- 19.Harris N, Rosenberg A, Jangda S, O’Brien K, Gallagher ML. Prevalence of obesity in International Special Olympic athletes as determined by body mass index. J Am Diet Assoc. 2003;103(2):235–237 [DOI] [PubMed] [Google Scholar]
- 20.Bandini LG, Fleming RK, Scampini R, Gleason J, Must A. Is body mass index a useful measure of excess body fatness in adolescents and young adults with Down syndrome? J Intellect Disabil Res. 2013;57(11):1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]