Abstract
Background/Aim:
Over the past two decades, several advances have been made in the management of patients with hepatocellular carcinoma (HCC) and portal vein tumor thrombosis (PVTT). Yttrium-90 (90Y) radioembolization has recently been made a treatment option for patients with HCC and PVTT. However, there is still a need to systematicly evaluate the outcomes of 90Y radioembolization for HCC and PVTT. We aimed to assess the safety and effectiveness of 90Y radioembolization for HCC and PVTT. We performed a systematic review of clinical trials, clinical studies, and abstracts from conferences that qualified for analysis.
Materials and Methods:
PubMed, EMBASE, Cochrane Database of Systematic Review, CINAHL, and the “gray” literature (Google Scholar) were searched for all reports (1991-2016) related to 90Y radioembolization for HCC and PVTT.
Results:
A total of 14 clinical studies and three abstracts from conferences including 722 patients qualified for the analysis. The median length of follow-up was 7.2 months; the median time to progression was 5.6 months, and median disease control rate was 74.3%. Radiological response data were reported in five studies, and the median reported value of patients with complete response, partial response, stable disease, and progressive disease were 3.2%, 16.5%, 31.3%, and 28%, respectively. The median survival was 9.7 months for all patients, including the median overall survival (OS) were 12.1, 6.1 months of Child-Pugh class A and B patients, and the median OS were 6.1, 13.4 months of main and branch PVTT patients, respectively. The common toxicities were fatigue, nausea/vomiting, abdominal pain, mostly not requiring medical intervention needed no medication intervention.
Conclusions:
90Y radioembolization is a safe and effective treatment for HCC and PVTT.
Key Words: Hepatocellular carcinoma, portal vein tumor thrombosis, radioembolization, toxicity, yttrium-90
Portal vein tumor thrombosis (PVTT) occurs in a substantial portion of hepatocellular carcinoma (HCC) patients and in approximately 10%–40% of patients at diagnosis.[1,2] PVTT has a profound adverse effect on prognosis, with the median survival time of patients who have unresectable HCC with PVTT being significantly reduced (2–4 months) compared with those without PVTT (10–24 months).[1,3] The presence of PVTT also limits the treatment options, with HCC treatment guidelines often considering PVTT a contraindication for transplantation, curative resection, and transarterial chemoembolization (TACE).[4,5] Although the presence of PVTT poses a challenging treatment dilemma,[1] many treatments of HCC with PVTT have been reported, including surgical,[2] TACE,[4,5,6,7,8,9] external beam radiotherapy,[10] gamma-knife radiosurgery,[4] TACE combined with endovascular implantation of an iodine-125 seed strand,[11] and transarterial radioembolization.[12] However, the optimal treatment for patients with HCC and PVTT remains largely controversial.[7] Yttrium-90 (90 Y) radioembolization is a locoregional liver-directed therapy that involves transcatheter delivery of particles embedded with the radioisotope 90Y. In addition to obliteration of the arterial blood supply, the 90Y results in a 50–150 Gy dose of radiation to the tumor tissue, which results in tumor necrosis, including HCC and PVTT.[13] It was reported that 90Y radioembolization is a safe and effective treatment for patients with HCC and PVTT.[6] However, there is still a need to systematically evaluate the outcomes of this treatment modality.
The purpose of this study was to comprehensively review the safety and effectiveness of 90Y radioembolization for HCC and PVTT.
MATERIALS AND METHODS
Search strategy
PubMed, EMBASE, Cochrane Database of Systematic Review, CINAHL and the “gray” literature (Google Scholar) were searched from January 1, 1991 (the first commercial availability of the 90Y products) to January 25, 2016, for the full text describing 90Y in the treatment of HCC and PVTT (keywords: (Liver cancer or liver tumor or primary liver cancer or hepatocellular carcinoma); (portal vein tumor thrombosis or portal vein thrombosis); and (yttrium-90 or 90Y or TheraSphere or SIR-Spheres) and English language). We retrieved potentially relevant articles and reviewed their reference lists to find studies that our search strategy may have missed. Clinical trials, clinical studies, and abstracts from conferences that qualified for analysis were included in this review.
The primary objective of this study was to determine the overall survival (OS), and the secondary objectives were to identify the radiological response and clinical toxicity.
Inclusion criteria
The inclusion criteria were (1) clinical trials, clinical studies, or abstracts from conferences; (2) describing 90Y in the treatment of PVTT; (3) at least include the primary objective: OS. Exclusion criteria were as follows: (1) Review articles, animal studies, laboratory investigations, case reports, and case series; (2) any duplicated clinical studies. All the clinical trials, clinical studies, and abstracts from conferences were reviewed for qualification according to our study criteria.
Data extraction
A standardized data extraction database was created by tabulating the following information: First author, year of publication, prospective or retrospective design, quality criteria, number of patients, patient characteristics, assessment criteria (European Association for the Study of the Liver [EASL]; Response Evaluation Criteria in Solid Tumors [RECIST]; World Health Organization [WHO]), time to progression (TTP), disease control rate, radiological response, OS, and clinical toxicity. Two members of the research team conducted the literature search independently to verify data accuracy and completeness, with a third one resolving any discrepancy. Studies were classified into three levels of evidence as follows: Level I, randomized controlled trials (RCTs); level II, non-RCTs or well-designed cohort studies; and level III, observational studies, as described by the U.S. Preventive Services Task Force.
Definitions
OS was calculated in months for all subjects from the date of radioembolization treatment to the date of death or last follow-up. Disease control rate was defined as the percentage of patients who have achieved complete response (CR), partial response (PR), and stable disease (SD) to 90Y radioembolization.[14]
RESULTS
The initial search yielded 225 English reports from January 1, 1991, to January 25, 2016. An additional search using Google Scholar found no additional relevant articles. Of the 225 reports, a total of 208 reports were excluded due to unqualified studies (n = 182), review articles (n = 21), and duplicated clinical studies (n = 5) [Figure 1]. Ultimately, a total of 17 reports, including 14 studies (five prospective and nine retrospective) and three abstracts from conferences were included in this study (Kokabi N, et al.;[15] Mazzaferro V, et al.;[16] Memon K, et al.;[17] Woodall CE, et al.;[18] Kulik LM, et al.;[19] Salem R, et al.;[20] Garin E, et al.;[21] Sangro B, et al.;[22] Inarrairaegui M, et al.;[23] Salem R, et al.;[24] Tsai AL, et al.;[25] Hilgard P, et al.;[26] Akinwande O, et al.;[27] Lee VH, et al.;[28] Biederman DM, et al.;[29] Kim YH, et al.;[30] El Fouly A, et al.[31]). The quality of evidence was as follows: level II, n = 6, and level III, n = 11.
Figure 1.
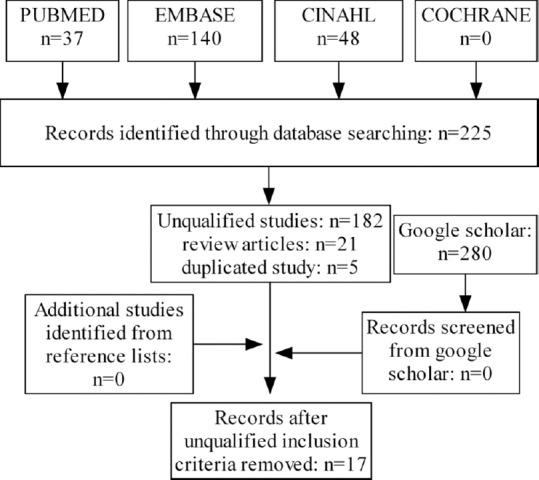
Flow diagram of literature search
Table 1 summarizes the patient demographic characteristics of the included reports. A total of 722 cases were included in the final analysis. The median value of the percentage of male patients was 80.5% (range 70%–94.2%) with a median age of 64 years (range 56–67 years). The median percentage of the reported main PVTT was 42.1% (range 0%–100%), and the extrahepatic metastases were present in a median of 12% cases (range 0%–31.9%). The median percentage of Child-Pugh class A, B, C were 67% (range 20%–92.7%), 33% (range 7.3%–62%), and 0 (0%–27%), respectively. The median value of the radioactivity delivered was 2.6 GBq (range 1.4–3.1 GBq). Of the 722 cases, glass microspheres were used in 540 cases, resin microspheres were used in 160 cases, and the other 22 cases were unspecified.
Table 1.
Summary of the characteristics of the included studies
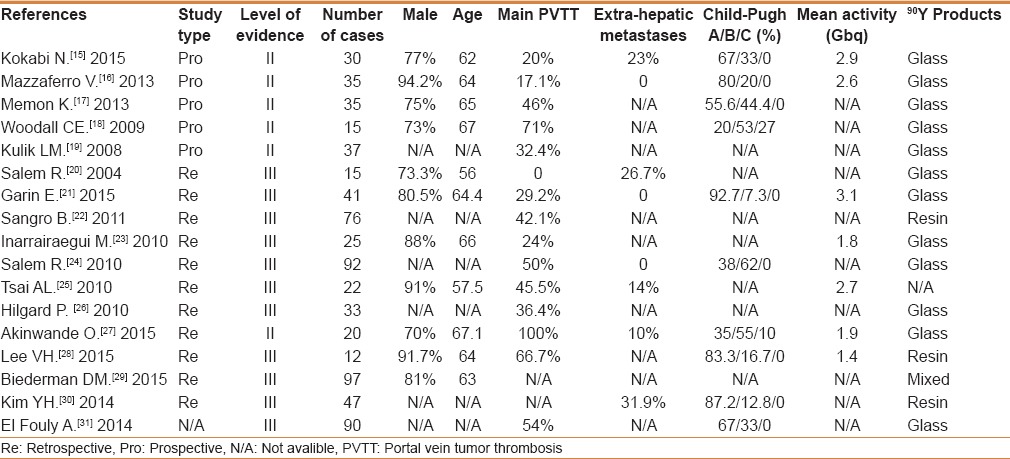
Table 2 summarizes the effectiveness of 90Y treatment of the included studies. The median length of follow-up was 7.2 months (range 6–36 months), the median TTP was 5.6 months (range 4.9–9 months), and the median disease control rate was 74.3% (range 16.7%–94%). Radiological response data were reported in five studies; the median reported value of patients with CR, PR, SD, and progressive disease (PD) were 3.2% (range 0%–12.2%), 16.5% (range 5%–73.2%), 31.3% (range 5%–64%), and 28% (range 0%–80%), respectively. OS results were reported in all studies, with the median survival being 9.7 months (range 3–23.7 months).
Table 2.
Summary of the outcomes of the included studies
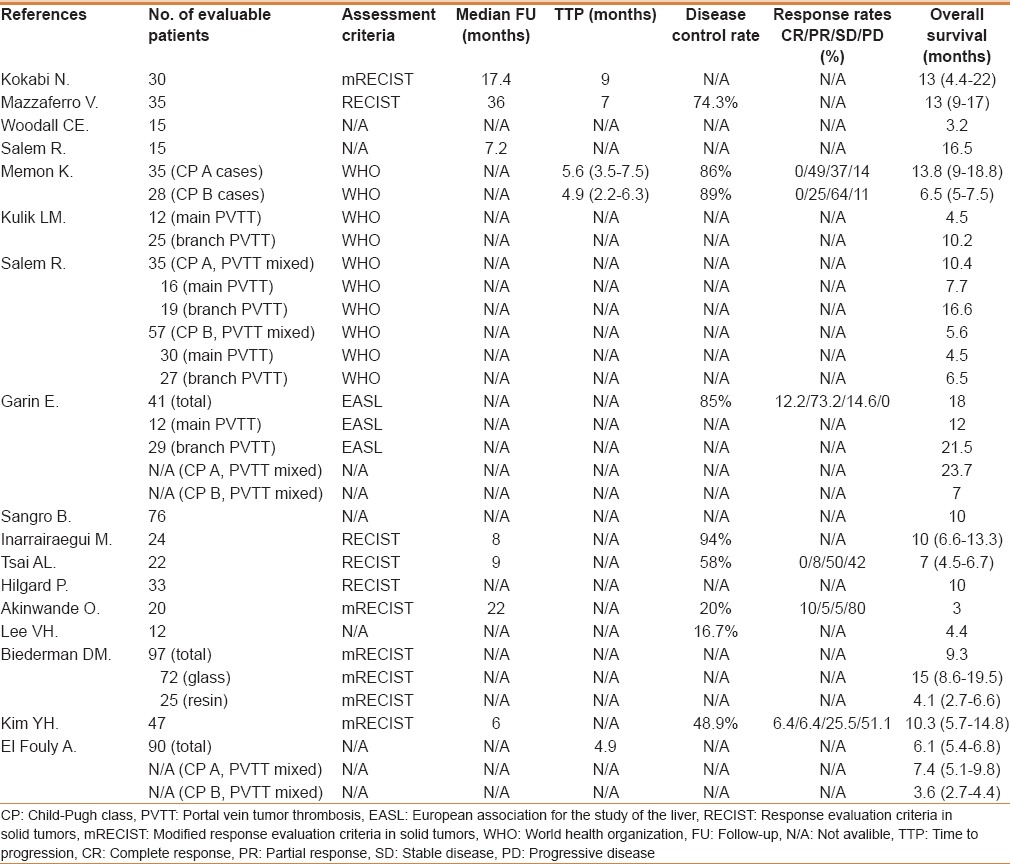
The Child–Pugh class A (n = 70) and B (n = 85) patients were reported in four studies (only two studies identified the total patients number), with median OS being 12.1 months (7.4–23.7 months) of Child-Pugh class A patients, and 6.1 months (3.6–7 months) of Child–Pugh class B patients [Table 2]. The main PVTT (n = 70) and branch PVTT (n = 100) patients were reported in three studies, with median OS being 6.1 months (4.5–12 months) of main PVTT patients, and 13.4 months (6.5–21.5 months) of branch PVTT patients [Table 2].
Table 3 presents a thorough overview of the toxicity associated with 90Y radioembolization. Although toxicities were not available for most of the included studies, the common toxicities were fatigue (range 2.9%–67%), abdominal pain (range 2.9%–57%), and nausea/vomiting (range 5.7%–28%).
Table 3.
Summary the toxicity of patients in the included studies

DISCUSSION
This review demonstrated the median disease control rate as 74.3%, and median survival as 9.7 months following 90Y radioembolization for HCC and PVTT patients. The median OS were 12.1 and 6.1 months of Child-Pugh class A and B patients, respectively, and the median OS were 6.1 and 13.4 months of main and branch PVTT patients, respectively.
90Y radioembolization treatment, a form of intraarterial brachytherapy, is a technique in which glass or resin particles are labeled with 90Y. The radioisotope 90Y is a pure β-emitter with no primary gamma radiation. HCC and PVTT are fed mainly by hepatic arterial system rather than portal venous blood. Therefore, the dominant arterial flow of malignant tissue allows the delivery of high doses of radiation to tumors while keeping the exposure of the healthy liver at minimum with selective 90Y microsphere distribution. It was reported that 90Y internal radiation therapy can provide tumor doses as high as 50-150 Gy, in contrast to traditional whole liver external beam radiation where the radiation dose has been limited to 30 Gy to prevent adjacent organ injury.[32,33] To date, the published reports documented in this sample demonstrate that 90Y treatment can be used safely in patients with portal circulation compromised at the level of the first-order portal branches.[12,17,34] Also, Tsai et al.[10] reported that 90Y treatment is tolerated in patients with HCC and major PVTT, and the median OS (7 months) is promising.
Most of the studied patients had end-stage disease, reflected by the high proportion of patients with Child-Pugh class B or C and main PVTT. The median disease control rate in the review of studies was 74.3%, with a median TTP of 5.6 months, and median survival of 9.7 months. It demonstrates that 90Y radioembolization is an effective treatment for HCC and PVTT. 90Y may also lead to tumor downstaging, which might allow for subsequent surgical resection or radiofrequency ablation.
From this study, the median OS of patients in Child-Pugh class A was longer than patients in Child-Pugh class B (12.1 vs 6.1 months); and the median OS of patients in branch PVTT was longer than that in main PVTT patients (13.4 vs 6.1 months). It shows that tumor progression was slower in patients with Child-Pugh class A and branch PVTT than patients with Child-Pugh class B and main PVTT.
Although embolization with 90Y does results in permanent vascular blockade with glass or resin particles, the smaller caliber of the particles (20-30 μm glass, 20-60 μm resin) impart the theoretical advantage of a lesser degree of macroscopic arterial occlusion. It was reported that the 90Y treatment exerts less of an ischemic effect compared with TACE (threefold lower incidence of postembolization syndrome).[35]
Overall, 90Y treatment is a well-tolerated procedure, especially when compared with TACE. The most frequently encountered toxicities are symptoms of the post-radioembolization syndrome, including fatigue, abdominal pain, nausea/vomiting, and fever. Although the incidence of symptoms of post-radioembolization syndrome was not reported in most of the included studies, the common toxicities were abdominal pain, nausea/vomiting, and fatigue. Fortunately, postembolization syndrome can be managed with medication and the symptoms generally subside within a week post-radioembolization.
Study limitation
This study was limited by the small number and quality of included studies, as only 14 studies and three abstracts were included and the overall evidence was level II or III by the U.S. Preventive Services Task Force, and all the included studies were not specifically focused on and lacked details related to the etiological factors (especially hepatitis B- and hepatitis C-related HCC). Also, the number of patients and the observation time was short in the included studies, all of which may bias the results. Furthermore, only four studies mentioned the OS of different Child-Pugh class (A/B) and three studies mentioned the OS of different PVTT location (main/branch), which may also bias the results. Lastly, we cannot prove which 90Y product (glass/resin) is better due to the small studied sample of only 160 cases that used resin microsphere.
CONCLUSIONS
90Y radioembolization is a safe and effective treatment for HCC and PVTT. However, this study lacks adequate power to draw definitive conclusions based on the presented data. A larger cohort might allow more meaningful conclusions and more robust survival data.
Financial support and sponsorship
This study was supported by High-level Medical Talents Training Project of Changzhou (NO. 2016CZBJ009 and NO. 2016CZLJ007). Social Development Fund of Jiangsu Science and Technology (NO. BE2016658). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung TK, Lai CL, Wong BC, Fung J, Yuen MF. Clinical features, biochemical parameters, and virological profiles of patients with hepatocellular carcinoma in Hong Kong. Aliment Pharmacol Ther. 2006;24:573–83. doi: 10.1111/j.1365-2036.2006.03029.x. [DOI] [PubMed] [Google Scholar]
- 3.Schöniger-Hekele M, Müller C, Kutilek M, Oesterreicher C, Ferenci P, Gangl A. Hepatocellular carcinoma in central Europe: Prognostic features and survival. Gut. 2001;48:103–9. doi: 10.1136/gut.48.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jelic S, Sotiropoulos GC. ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):59–64. doi: 10.1093/annonc/mdq166. [DOI] [PubMed] [Google Scholar]
- 6.Pirisi M, Avellini C, Fabris C, Scott C, Bardus P, Soardo G, et al. Portal vein thrombosis in hepatocellular carcinoma: Age and sex distribution in an autopsy study. J Cancer Res Clin Oncol. 1998;124:397–400. doi: 10.1007/s004320050189. [DOI] [PubMed] [Google Scholar]
- 7.Lin DX, Zhang QY, Li X, Ye QW, Lin F, Li LL. An aggressive approach leads to improved survival in hepatocellular carcinoma patients with portal vein tumor thrombus. J Cancer Res Clin Oncol. 2011;137:139–49. doi: 10.1007/s00432-010-0868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 9.Hawkins MA, Dawson LA. Radiation therapy for hepatocellular carcinoma: From palliation to cure. Cancer. 2006;106:1653–63. doi: 10.1002/cncr.21811. [DOI] [PubMed] [Google Scholar]
- 10.Lu XJ, Dong J, Ji LJ, Xiao LX, Ling CQ, Zhou J. Tolerability and efficacy of gamma knife radiosurgery on hepatocellular carcinoma with portal vein tumor thrombosis. Oncotarget. 2016;7:3614–22. doi: 10.18632/oncotarget.6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang M, Fang Z, Yan Z, Luo J, Liu L, Zhang W, et al. Transarterial chemoembolisation (TACE) combined with endovascular implantation of an iodine-125 seed strand for the treatment of hepatocellular carcinoma with portal vein tumour thrombosis versus TACE alone: A two-arm, randomised clinical trial. J Cancer Res Clin Oncol. 2014;140:211–9. doi: 10.1007/s00432-013-1568-0. [DOI] [PubMed] [Google Scholar]
- 12.Pracht M, Edeline J, Lenoir L, Latournerie M, Mesbah H, Audrain O, et al. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: Preliminary results. Int J Hepatol. 2013;2013:827649. doi: 10.1155/2013/827649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bienert M, McCook B, Carr BI, Geller DA, Sheetz M, Tutor C, et al. 90Y microsphere treatment of unresectable liver metastases: Changes in 18F-FDG uptake and tumour size on PET/CT. Eur J Nucl Med Mol Imaging. 2005;32:778–87. doi: 10.1007/s00259-004-1752-1. [DOI] [PubMed] [Google Scholar]
- 14.Sznol M. Reporting disease control rates or clinical benefit rates in early clinical trials of anticancer agents: Useful endpoint or hype? Curr Opin Investig Drugs. 2010;11:1340–1. [PubMed] [Google Scholar]
- 15.Kokabi N, Camacho JC, Xing M, El-Rayes BF, Spivey JR, Knechtle SJ, et al. Open-label prospective study of the safety and efficacy of glass-based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis. Cancer. 2015;121:2164–74. doi: 10.1002/cncr.29275. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: A phase 2 study. Hepatology. 2013;57:1826–37. doi: 10.1002/hep.26014. [DOI] [PubMed] [Google Scholar]
- 17.Memon K, Kulik L, Lewandowski RJ, Mulcahy MF, Benson AB, Ganger D, et al. Radioembolization for hepatocellular carcinoma with portal vein thrombosis: Impact of liver function on systemic treatment options at disease progression. J Hepatol. 2013;58:73–80. doi: 10.1016/j.jhep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodall CE, Scoggins CR, Ellis SF, Tatum CM, Hahl MJ, Ravindra KV, et al. Is selective internal radioembolization safe and effective for patients with inoperable hepatocellular carcinoma and venous thrombosis? J Am Coll Surg. 2009;208:375–82. doi: 10.1016/j.jamcollsurg.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71–81. doi: 10.1002/hep.21980. [DOI] [PubMed] [Google Scholar]
- 20.Salem R, Lewandowski R, Roberts C, Goin J, Thurston K, Abouljoud M, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma in patients with portal vein thrombosis. J Vasc Interv Radiol. 2004;15:335–45. doi: 10.1097/01.rvi.0000123319.20705.92. [DOI] [PubMed] [Google Scholar]
- 21.Garin E, Rolland Y, Edeline J, Icard N, Lenoir L, Laffont S, et al. Personalized dosimetry with intensification using 90Y-loaded glass microsphere radioembolization induces prolonged overall survival in hepatocellular carcinoma patients with portal vein thrombosis. J Nucl Med. 2015;56:339–46. doi: 10.2967/jnumed.114.145177. [DOI] [PubMed] [Google Scholar]
- 22.Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: A European evaluation. Hepatology. 2011;54:868–78. doi: 10.1002/hep.24451. [DOI] [PubMed] [Google Scholar]
- 23.Iñarrairaegui M, Thurston KG, Bilbao JI, D'Avola D, Rodriguez M, Arbizu J, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2010;21:1205–12. doi: 10.1016/j.jvir.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Tsai AL, Burke CT, Kennedy AS, Moore DT, Mauro MA, Dixon RD, et al. Use of yttrium-90 microspheres in patients with advanced hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2010;21:1377–84. doi: 10.1016/j.jvir.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741–9. doi: 10.1002/hep.23944. [DOI] [PubMed] [Google Scholar]
- 27.Akinwande O, Kim D, Edwards J, Brown R, Philips P, Scoggins C, et al. Is radioembolization ((90) Y) better than doxorubicin drug eluting beads (DEBDOX) for hepatocellular carcinoma with portal vein thrombosis? A retrospective analysis. Surg Oncol. 2015;24:270–5. doi: 10.1016/j.suronc.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee VH, Leung DK, Luk MY, Tong CC, Law MW, Ng SC, et al. Yttrium-90 radioembolization for advanced inoperable hepatocellular carcinoma. Onco Targets Ther. 2015;20(8):3457–64. doi: 10.2147/OTT.S92473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biederman DM, Tabori NE, Titano JJ, Pierobon ES, Fischman AM, Patel RS, et al. Outcomes of yttrium-90 therapy in the treatment of hepatocellular carcinoma (HCC) with portal vein thrombosis (PVT): Resin-based vs. Glassbased microspheres. Conference: 40th Annual Scientific Meeting of the Society of Interventional Radiology. J Vasc Interv Radiol. 2015;26(Suppl 1):S109–10. [Google Scholar]
- 30.Kim YH, Kim GM, Chun HJ, Kwon DI, Won JY, Lee KH, et al. Radioembolization for Portal Vein Tumor Thrombosis Using Yttrium-90 Resin Microspheres: General Experiences in Korea. Conference: Cardiovascular and Interventional Radiological Society of Europe. Cardiovasc Intervent Radiol. 2014;37(Suppl 1):S252. [Google Scholar]
- 31.El Fouly A, Dechene A, Best J, Mueller S, Lauenstein T, Bockisch A, et al. Selective internal radiotherapy with yttrium-90 microspheres for hepatocellular carcinoma with portal vein thrombosis. Conference: 49th Annual Meeting of the European Association for the Study of the Liver, International Liver Congress 2014, London United Kingdom. J Hepatol. 2014;60(Suppl 1):S409. [Google Scholar]
- 32.Sarfaraz M, Kennedy AS, Cao ZJ, Sackett GD, Yu CX, Lodge MA, et al. Physical aspects of yttrium-90 microsphere therapy for nonresectable hepatic tumors. Med Phys. 2003;30:199–203. doi: 10.1118/1.1538235. [DOI] [PubMed] [Google Scholar]
- 33.Dancey JE, Shepherd FA, Paul K, Sniderman KW, Houle S, Gabrys J, et al. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. Nucl Med. 2000;41:1673–81. [PubMed] [Google Scholar]
- 34.Kokabi N, Camacho JC, Xing M, Qiu D, Kitajima H, Mittal PK, et al. Apparent diffusion coefficient quantification as an early imaging biomarker of response and predictor of survival following yttrium-90 radioembolization for unresectable infiltrative hepatocellular carcinoma with portal vein thrombosis. Abdom Imaging. 2014;39:969–78. doi: 10.1007/s00261-014-0127-8. [DOI] [PubMed] [Google Scholar]
- 35.Goin JE, Dancey JE, Roberts CA, Sickles CJ, Leung DA, Soulen MC. Comparison of postembolization syndrome in the treatment of patients with unresectable hepatocellular carcinoma: Transcatheter chemo-embolization versus yttrium-90 glass microspheres. World J Nucl Med. 2004;3:49–56. [Google Scholar]


