Abstract
Background/Aim:
The aim of this study was to assess the role of serum pigment epithelium-derived factor (PEDF) and matrix metalloproteinase-9 (MMP-9) in progression of liver cirrhosis and development of hepatocellular carcinoma (HCC).
Patients and Methods:
Serum levels of PEDF and MMP-9 were tested in 212 patients with liver cirrhosis and in a control group of 30 healthy volunteers. HCC was diagnosed in 45 of the 212 patients studied (21%).
Results:
Serum PEDF and MMP-9 were higher in the study group than that in the control group (P < 0.001). In patients with alcoholic or mixed (alcoholic and viral hepatitis-related) cirrhosis, serum PEDF was higher than that in other patients (13970.2 ± 13406.9 ng/ml vs. 8563.5 ± 9602.7 ng/ml, P = 0.008). In patients with viral hepatitis-related cirrhosis, significantly higher PEDF levels were recorded in those with HCC (13429.1 ± 12045.8) than that in patients without HCC (6660.1 ± 7927.1; P = 0.04). There was a trend for higher serum MMP-9 in patients with HCC (5778.7 ± 12426.6 vs. 1389.8 ± 1944.7 in those without HCC; P = 0.07). Significant negative correlation between serum MMP-9 and serum alpha-fetoprotein in patients with HCC was observed (r = −0.54; P = 0.04).
Conclusion:
Serum PEDF and MMP-9 could be auxiliary markers in diagnosis of HCC, especially in patients with low alpha-fetoprotein level. Alcohol consumption can affect serum PEDF.
Key Words: Fibrosis markers, hepatocellular carcinoma, liver cirrhosis
The pathogenesis of hepatocellular carcinoma (HCC) is a multifactorial process which has not been fully explained so far. Early diagnosis is still difficult; this neoplasm is frequently diagnosed in the advanced phase. Serum α-fetoprotein (AFP) and ultrasound imaging (US) for many years have been the gold standard of HCC screening. However, sensitivity and specificity of serum AFP in HCC diagnosis are relatively low,[1,2] therefore, it was excluded from the American Association for the Study of Liver Diseases (AASLD) guidelines for HCC screening in 2010[3] and from European Association for the Study of the Liver (EASL) guidelines in 2012,[4] whereas others still recommend testing AFP in addition to US.[5,6,7] There is a need for new, more sensitive, and reliable diagnostic tests. There are several molecular markers of HCC currently under investigation including cellular markers, cellular cycle regulators, markers of apoptosis, telomerase activity, adhesive molecules, markers of extracellular matrix degradation, markers of angiogenesis, growth factors and their receptors, oncofetal and glycoprotein antigens, enzymes and isoenzymes, and genetic markers.[8]
Pigment epithelium-derived factor (PEDF) is an endogenously produced glycoprotein with a molecular weight of 50 kD, occurring commonly in various organs and exhibiting diverse biological activity. It belongs to the superfamily of serine protease inhibitors. PEDF reaches high concentrations in serum, which can be used for diagnostic purposes.[9] Repeatedly confirmed effect of this molecule is inhibition of angiogenesis.[10] It is believed that PEDF has neuroprotective properties,[11] acts as an antioxidant and anti-inflammatory agent,[12] inhibits cellular proliferation and supports cell differentiation, and protects against tumor metastasis.[13] Liver is the major site of PEDF synthesis.[14] PEDF is an important factor in many liver diseases.[15] The mechanism of its action remains largely unclear. Antifibrogenic activity of intrahepatic PEDF has been demonstrated.[16] PEDF is secreted by fat cells as well, playing a role in the development of insulin resistance, metabolic syndrome, and liver steatosis.[17,18,19,20,21,22]
Matrix metalloproteinase-9 (MMP-9) is a zinc-dependent enzyme also called gelatinase B, with a molecular weight of 92 kD. It degrades extracellular matrix proteins, particularly collagen IV, which is a component of basement membrane. This enzyme plays an important role in inflammation, tissue remodeling process, as well as promotes tumor cell migration and metastasis.[23,24] The main sources of matrix metalloproteinases in the damaged liver are stellate cells with endothelium and inflammatory cells.[25] It was found that serum or plasma MMP-9 may be a useful marker of the processes that occur in the tissues.[26] Elevated MMP-9 levels were observed in colorectal cancer and breast cancer,[27] stomach cancer,[28] bladder cancer, acute leukemia, rheumatoid arthritis, melanoma, and HCC.[29] The activity of MMP-9 is checked on several levels: transcription – control of cytokines, activation of the proenzyme – enzyme cascade, including serine proteases and other metalloproteinases, finally – regulation by specific tissue inhibitors of metalloproteinases (TIMP). The data on the serum MMP-9 as a marker of fibrosis are controversial.[30]
In this study, we investigated the usefulness of serum PEDF and MMP-9 in evaluating the progression of liver cirrhosis and development of HCC.
PATIENTS AND METHODS
The study group consisted of 212 patients with cirrhosis from the database e-Hepar; 127 men and 85 women, aged from 28 to 86 years, with a mean age of 54.6 years. The study also included a control group of 30 healthy volunteers; 12 men and 18 women, aged from 28 to 52 years, with a mean age 38.3 years. Informed consent was obtained from each patient included in the study. Serum PEDF and MMP-9 were determined by ELISA Kits (PEDF – ChemiKine, Chemicon International; MMP-9 – Platinum ELISA, eBioscience). Standard biochemical serum tests were carried out, including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alpha-fetoprotein (AFP). The severity of liver cirrhosis was assessed using the Child-Pugh score. Statistical analysis was performed using Statistica; Spearman's rank correlation test and the Mann–Whitney U test were used. P value of < 0.05 was considered to be statistically significant.
Analyzing the etiology of liver cirrhosis in the study group, pure viral hepatitis-related cirrhosis was identified in 124 patients, pure alcoholic cirrhosis in 27 patients, cirrhosis of mixed etiology (viral hepatitis-related and alcoholic) in 43 patients, and cirrhosis of other or unknown etiology in 18 patients. From 167 patients infected with hepatotropic viruses, 126 patients were infected with hepatitis C virus (HCV), 33 with hepatitis B virus (HBV), and 8 patients were co-infected with HBV and HCV.
HCC was diagnosed in 45 of the 212 patients studied (21%). These were patients with viral hepatitis-related liver cirrhosis (36 patients) and cirrhosis of mixed etiology (8 patients). None of the patients with alcoholic liver disease were diagnosed with HCC. Among patients with HCC, 40 were infected with HCV, 4 patients were diagnosed with HBV, and 1 patient was infected with both viruses.
RESULTS
The concentration of both PEDF and MMP-9 was significantly higher in patients with cirrhosis than in the control group (for PEDF, respectively, 11000.7 ± 11367.7 ng/ml vs. 417.3 ± 266.5 ng/ml, P < 0.001; for MMP-9 1863.5 ± 4692.8 ng/ml vs. 94.9 ± 21.6 ng/ml, P < 0.001). There were no significant differences in levels of PEDF or MMP-9 between the groups A, B, and C according to the Child-Pugh classification [Figures 1 and 2]. There were significant differences in the levels of PEDF, depending on the etiology of cirrhosis. In patients with alcoholic or mixed (alcoholic and viral hepatitis-related) cirrhosis, serum PEDF was higher than in other patients (13970.2 ± 13406.9 ng/ml vs. 8563.5 ± 9602.7 ng/ml, P = 0.008) [Figure 3].
Figure 1.
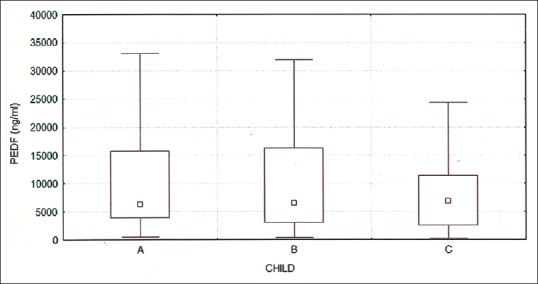
Serum PEDF in groups A, B, and C according to the Child-Pugh; P = NS
Figure 2.
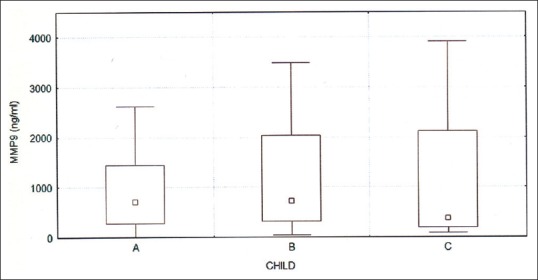
Serum MMP-9 in groups A, B, and C according to the Child-Pugh; P = NS
Figure 3.
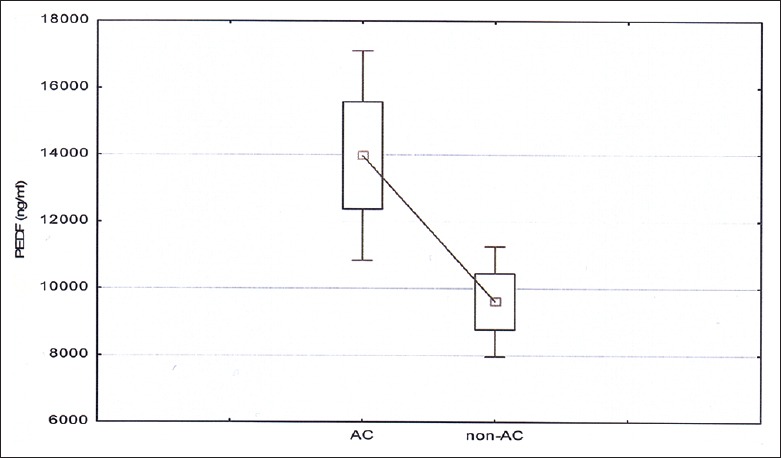
Serum PEDF depending on the alcoholic etiology of cirrhosis; P = 0.008. AC, alcoholic cirrhosis; non-AC, non-alcoholic cirrhosis.
In further analysis of the group of patients with viral hepatitis-related cirrhosis, there were significantly higher PEDF levels recorded in patients with HCC (13429.1 ± 12045.8) than that in patients without HCC (6660.1 ± 7927.1; P = 0.04) [Figure 4].
Figure 4.
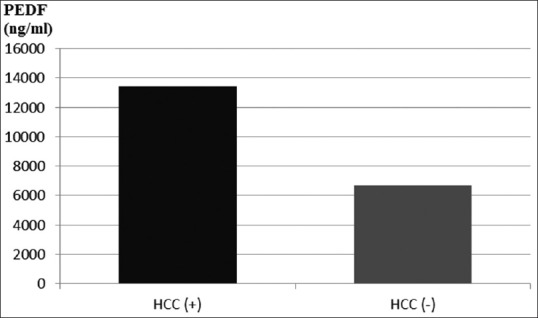
Serum PEDF in patients with viral hepatitis-related cirrhosis, depending on the presence of HCC; P = 0.04
Similarly, there was also a trend for higher serum MMP-9 in patients with HCC (5778.7 ± 12426.6 vs. 1389.8 ± 1944.7 in those without HCC; P = 0.07). The analysis was performed in all patients, regardless of the etiology of cirrhosis [Figure 5].
Figure 5.
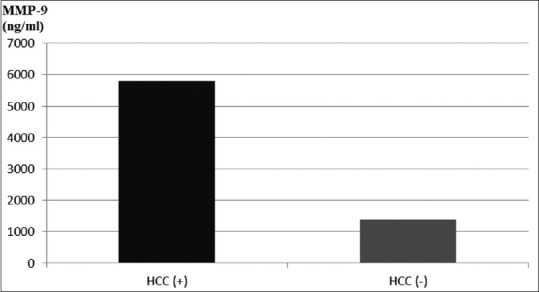
Serum MMP-9 in patients with cirrhosis, depending on the presence of HCC; P = 0.07
By examining correlations of various factors associated with the disease to the value of serum PEDF, a negative correlation between serum PEDF and ALT level (r = −0.18, P = 0.06) was found. This correlation was particularly visible and statistically significant in patients without HCC (r = −0.27; P = 0.03). Significant negative correlation between serum MMP-9 and serum AFP in patients with HCC was observed (r = −0.54; P = 0.04).
DISCUSSION
In this study, serum PEDF and MMP-9 were determined in patients with cirrhosis of different etiologies, and their value in the diagnosis of HCC was assessed. PEDF, which has antiangiogenic and antiproliferative activity, is considered to be a protective factor, reducing the risk of HCC development.[31] In contrast, MMP-9 is an enzyme whose activity promotes tumor growth and invasion.[32] In our study, we have shown that both of these markers are elevated in patients with cirrhosis, especially in patients with HCC. There are few reports describing changes in serum levels of PEDF in patients with cirrhosis; decreased level of PEDF in liver tissue was reported in these patients.[16,33] Elevated serum PEDF was described in patients with liver steatosis.[18,20] As MMP-9 is the enzyme involved in the degradation of PEDF in the liver, it is postulated that there is a relationship of these two markers in certain liver diseases.[33,34] There was a decrease of PEDF in liver tissue of humans and animals with alcoholic liver disease with concomitant increase of MMP-9.[33] There is a hypothesis that PEDF inhibits the development of cirrhosis by direct inactivation of the stellate cells and the induction of apoptosis[16] and by stimulating apoptosis in the HCC cells.[35,36]
Some studies have confirmed elevated levels of MMP-9 in patients with chronic liver disease.[29,37] Studies have also shown a correlation of serum MMP-9 with the progression of liver fibrosis,[37] as well as transaminase levels,[37,38] which was not confirmed in our study. Other authors, in contrast to us, found reduction in serum MMP-9 in patients with chronic hepatitis C and cirrhosis.[39] Many studies have demonstrated higher MMP-9 levels in plasma[22] and in liver tissue[40,41] in patients with HCC. In tumor tissue, higher expression of this enzyme was detected than in adjacent liver tissue.[40,41,42] Hayasaka et al.[29] determined the sensitivity (53%) and specificity (89%) of plasma MMP-9 for the detection of HCC. It was also found that MMP-9 correlated with the progression of cancer: Vascular invasion,[28,29,42] a low tumor cell differentiation,[42] capsule infiltration,[40] the incidence of metastasis,[28] overall stage of HCC, and with poor prognosis.[42,43] The value of simultaneous determination of MMPs and tissue inhibitors of MMP (TIMPs) is emphasized. The ratios of MMP: TIMP and MMP-9:MMP-2 are important in the diagnosis and prognosis of HCC.[44,45]
In our study, we found that serum MMP-9 correlates negatively with serum AFP. Other studies do not confirm these results.[29,46] Our results can be partially explained by a possible diversity of histopathological forms of HCC in the study group. Carcinoma with low cell differentiation may not produce AFP. In this case, the determination of MMP-9 would increase the likelihood of the diagnosis of HCC.
In the study group, we found significantly higher level of serum PEDF in patients who abuse alcohol (alcoholic or mixed cirrhosis) than that in patients with viral hepatitis-related cirrhosis. Sogawa et al.[47] also confirmed that PEDF levels may be an independent exponent of excessive alcohol consumption. A negative correlation of PEDF and ALT level in our study suggests that PEDF is not a marker of inflammation in the liver.
These are only preliminary studies regarding the potential role of serum PEDF and MMP-9 as adjunctive diagnostic biochemical markers in HCC. The limitations of these tests is that their cut-offs are not defined. Further studies are needed to better characterize the suitability of these tests in clinical practice.
In conclusion, serum PEDF and MMP-9 may aid in the diagnosis of HCC, especially in patients with low AFP, where the diagnosis is questionable. Alcohol consumption can affect the level of serum PEDF. Serum PEDF and MMP-9 are higher in cirrhosis, however, their level does not differentiate the stages of cirrhosis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Grant number 12710 from The National Centre for Research and Development, Ministry of Science and Higher Education, Poland.
REFERENCES
- 1.Chen JG, Parkin DM, Chen QG, Lu JH, Shen QJ, Zhang BC, et al. Screening for liver cancer: Results of a randomised controlled trial in Qidong, China. J Med Screen. 2003;10:204–9. doi: 10.1258/096914103771773320. [DOI] [PubMed] [Google Scholar]
- 2.Paul SB, Gulati MS, Sreenivas V, Madan K, Gupta AK, Mukhopadhyay S, Acharya SK. Evaluating patients with cirrhosis for hepatocellular carcinoma: Value of clinical symptomatology, imaging and alpha-fetoprotein. Oncology. 2007;72(Suppl 1):117–23. doi: 10.1159/000111717. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. 2015. Mar, [Last accessed on August 08, 2016]. Available from: URL: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/ [PubMed]
- 6.World Health Organization. Guidelines for the Screening, Care, and Treatment of Persons with Hepatitis C Infection. 2014. Apr, [Last accessed on August 08, 2016]. Available from: URL: http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/ [PubMed]
- 7.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–74. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singhal A, Jayaraman M, Dhanasekaran DN, Kohli V. Molecular and serum markers in hepatocellular carcinoma: Predictive tools for prognosis and recurrence. Crit Rev Oncol Hematol. 2012;82:116–40. doi: 10.1016/j.critrevonc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Petersen SV, Valnickova Z, Enghild JJ. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: Purification and characterization. Biochem J. 2003;374:199–206. doi: 10.1042/BJ20030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson DW, Volpert OV, Gillis P, Crawford SE, Xu H, Benedict W, et al. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–8. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 11.Bilak MM, Corse AM, Bilak SR, Lehar M, Tombran-Tink J, Kuncl RW. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J Neuropathol Exp Neurol. 1999;58:719–28. doi: 10.1097/00005072-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor. FASEB J. 2006;20:323–5. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 13.Hoshina D, Abe R, Yamagishi SI, Shimizu H. The role of PEDF in tumor growth and metastasis. Curr Mol Med. 2010;10:292–5. doi: 10.2174/156652410791065327. [DOI] [PubMed] [Google Scholar]
- 14.Tombran-Tink J, Mazuruk K, Rodriguez IR, Chung D, Linker T, Englander E, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotropic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- 15.Yamagishi SI, Matsui T, Kawaguchi T, Sata M. Patophysiological role of pigment epithelium-derived factor (PEDF) in hepatic disorders. Curr Med Chem. 2010;17:1995–2000. doi: 10.2174/092986710791233670. [DOI] [PubMed] [Google Scholar]
- 16.Ho TC, Chen SL, Shih SC, Wu JY, Han WH, Cheng HC, et al. Pigment epithelium-derived factor is an intrinsic antifibrosis factor targeting hepatic stellate cells. Am J Pathol. 2010;177:1798–811. doi: 10.2353/ajpath.2010.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Famulla S, Lamers D, Hartwig S, Passlack W, Horrighs A, Cramer A, et al. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int J Obes. 2011;35:762–72. doi: 10.1038/ijo.2010.212. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz Y, Eren F, Ayyildiz T, Colak Y, Kurt R, Senates E, et al. Serum pigment epithelium-derived factor levels are increased in patients with biopsy-proven nonalcoholic fatty liver disease and independently associated with liver steatosis. Clin Chim Acta. 2011;412:2296–9. doi: 10.1016/j.cca.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Stejskal D, Karpísek M, Svesták M, Hejduk P, Sporová L, Kotolová H. Pigment epithelium-derived factor as a new marker of metabolic syndrome in Caucasian population. J Clin Lab Anal. 2010;24:17–9. doi: 10.1002/jcla.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi S, Adachi H, Abe A, Yashiro T, Enomoto M, Furuki K, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–50. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 21.Borg ML, Andrews ZB, Duh EJ, Zechner R, Meikle PJ, Watt MJ. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–66. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chung C, Doll JA, Gattu AK, Shugrue C, Cornwell M, Fitchev P, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–8. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–33. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 24.Hurst NG, Stocken DD, Wilson S, Keh C, Wakelam MJ, Ismail T. Elevated serum matrix metalloproteinase 9 (MMP-9) concentration predicts the presence of colorectal neoplasia in symptomatic patients. Br J Cancer. 2007;97:971–7. doi: 10.1038/sj.bjc.6603958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahara T, Furui K, Yata Y, Jin B, Zhang LP, Nambu S, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology. 1997;26:1521–9. doi: 10.1002/hep.510260620. [DOI] [PubMed] [Google Scholar]
- 26.Zucker S, Hymowitz M, Conner C, Zarrabi HM, Hurewitz AN, Matrisian L, et al. Measurement of matrix metalloproteinases and tissue inhibitors of metalloproteinases in blood and tissues. Clinical and experimental applications. Ann N Y Acad Sci. 1999;878:212–27. doi: 10.1111/j.1749-6632.1999.tb07687.x. [DOI] [PubMed] [Google Scholar]
- 27.Zucker S, Lysik RM, Zarrabi MH, Moll U. M (r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993;53:140–6. [PubMed] [Google Scholar]
- 28.Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ, Chi NH, et al. Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res. 2007;13:2054–60. doi: 10.1158/1078-0432.CCR-06-2299. [DOI] [PubMed] [Google Scholar]
- 29.Hayasaka A, Suzuki N, Fujimoto N, Iwama S, Fukuyama E, Kanda Y, et al. Elevated plasma levels of matrix metalloproteinase-9 (92-kd type IV collagenase/gelatinase B) in hepatocellular carcinoma. Hepatology. 1996;24:1058–62. doi: 10.1053/jhep.1996.v24.pm0008903375. [DOI] [PubMed] [Google Scholar]
- 30.Bruno CM, Valenti M, Bertino G, Ardiri A, Consolo M, Mazzarino CM, et al. Altered pattern of circulating matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinase-2 in patients with HCV-related chronic hepatitis. Relationship to histological features. Panminerva Med. 2009;51:191–6. [PubMed] [Google Scholar]
- 31.Ek ET, Dass CR, Choong PF. PEDF: A potential molecular therapeutic target with multiple anti-cancer activities. Trends Mol Med. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Korn WM. Moving toward an understanding of the metastatic process in hepatocellular carcinoma. World J Gastroenterol. 2001;7:777–8. doi: 10.3748/wjg.v7.i6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung C, Shugrue C, Nagar A, Doll JA, Cornwell M, Gattu A, et al. Ethanol exposure depletes hepatic pigment epithelium-derived factor, a novel lipid regulator. Gastroenterology. 2009;136:331–40. doi: 10.1053/j.gastro.2008.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollina EA, Legesse-Miller A, Haley EM, Goodpaster T, Randolph-Habecker J, Coller HA. Regulating the angiogenic balance in tissues. Cell Cycle. 2008;7:2056–70. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai LJ, Ho TC. Pigment epithelial-derived factor inhibits c-FLIP expression and assists ciglitazone-induced apoptosis in hepatocellular carcinoma. Anticancer Res. 2011;31:1173–80. [PubMed] [Google Scholar]
- 36.Broadhead ML, Dass CR, Choong PF. Cancer cell apoptotic pathways mediated by PEDF: Prospects for therapy. Trends Mol Med. 2009;15:461–7. doi: 10.1016/j.molmed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Capone F, Guerriero E, Sorice A, Maio P, Colonna G, Castello G, et al. Characterisation of metalloproteinases, oxidative status and inflammation levels in the different stages of fibrosis in HCV patients. Clin Biochem. 2012;45:525–9. doi: 10.1016/j.clinbiochem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Helaly GF. Differences in circulating MMP-9 levels with regard to viral load and AST: ALT ratio between chronic hepatitis B and C patients. Br J Biomed Sci. 2011;68:38–42. doi: 10.1080/09674845.2011.11732840. [DOI] [PubMed] [Google Scholar]
- 39.Badra G, Lotfy M, El-Refaie A, Obada M, Abdelmonem E, Kandeel S, et al. Significance of serum matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in chronic hepatitis C patients. Acta Microbiol Immunol Hung. 2010;57:29–42. doi: 10.1556/AMicr.57.2010.1.3. [DOI] [PubMed] [Google Scholar]
- 40.Arii S, Mise M, Harada T, Furutani M, Ishigami S, Niwano M, et al. Overexpression of matrix metalloproteinase 9 gene in hepatocellular carcinoma with invasive potential. Hepatology. 1996;24:316–22. doi: 10.1053/jhep.1996.v24.pm0008690399. [DOI] [PubMed] [Google Scholar]
- 41.Kim KR, Bae JS, Choi HN, Park HS, Jang KY, Chung MJ, et al. The role of serum response factor in hepatocellular carcinoma: An association with matrix metalloproteinase. Oncol Rep. 2011;26:1567–72. doi: 10.3892/or.2011.1421. [DOI] [PubMed] [Google Scholar]
- 42.Nart D, Yaman B, Yilmaz F, Zeytunlu M, Karasu Z, Kiliç M. Expression of matrix metalloproteinase-9 in predicting prognosis of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2010;16:621–30. doi: 10.1002/lt.22028. [DOI] [PubMed] [Google Scholar]
- 43.El Tayebi HM, Salah W, El Sayed IH, Salam EM, Zekri AR, Zayed N, et al. Expression of insulin-like growth factor-II, matrix metalloproteinases, and their tissue inhibitors as predictive markers in the peripheral blood of HCC patients. Biomarkers. 2011;16:346–54. doi: 10.3109/1354750X.2011.573095. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Moneim SS, Abdou Mostafa EF, Osama A. Circulating matrix metalloproteinase-1 (MMP-1) and tissue inhibitor metalloproteinase-1 (TIMP-1) as serum markers of liver fibrosis in patients with chronic viral hepatitis. Arab J Gastroenterol. 2008;9:39–43. [Google Scholar]
- 45.Yeh HC, Lin SM, Chen MF, Pan TL, Wang PW, Yeh CT. Evaluation of serum matrix metalloproteinase (MMP)-9 to MMP-2 ratio as a biomarker in hepatocellular carcinoma. Hepatogastroenterology. 2010;57:98–102. [PubMed] [Google Scholar]
- 46.Ghada F, Helaly GF. Impact of hepatitis B viral load on serum IL-6, MMP-9 and alpha-fetoprotein levels and their correlation with liver injury in chronic hepatitis B infection. Cytokine. 2008;43:251. [Google Scholar]
- 47.Sogawa K, Kodera Y, Satoh M, Kawashima Y, Umemura H, Maruyama K, et al. Increased serum levels of pigment epithelium-derived factor by excessive alcohol consumption-detection and identification by a three-step serum proteome analysis. Alcohol Clin Exp Res. 2011;35:211–7. doi: 10.1111/j.1530-0277.2010.01336.x. [DOI] [PubMed] [Google Scholar]


