Abstract
Advances in oncologic therapies have allowed many patients with breast cancer to achieve better outcomes and longer survival. However, this progress has been tempered by cardiotoxicity, associated with anticancer therapies, ranging from subclinical abnormalities to irreversible life-threatening complications, such as congestive heart failure or cardiomyopathy. In particular, exposure to chemotherapy (CHT), including anthracyclines and trastuzumab, can lead to cardiac dysfunction with short- or long-term consequences, among patients with breast cancer. The aim of this study is to highlight the potential role of commonly used cardiac medications in the prevention of anthracycline- and trastuzumab-mediated cardiotoxicity, in women with breast cancer, based on evidence from recent clinical trials. This overview is focused on the use of antihypertensive medications, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, outlining their cardioprotective effects in this patient population. In addition, the importance of biomarkers and modern imaging tests, as potential tools for detection and monitoring of cardiac dysfunction, induced by CHT, as well as some practical preventive and therapeutic strategies for cardio-oncology treatment teams, involved in the management of a growing number of women with breast cancer have been outlined. The content of this overview is based on a literature search of PubMed, within the last 5 years, mostly in relevance to the human epidermal growth factor receptor 2-positive patients with breast cancer, treated with anthracycline or trastuzumab therapy (in addition to surgery and/or radiation therapy [RT] regimen).
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, anthracycline, breast cancer, cardiotoxicity, trastuzumab
Breast cancer is the most commonly diagnosed malignancy in women worldwide.[1] In less developed countries, it is the leading cause of cancer death, and in developed countries, it represents the second (after lung cancer) cause of cancer death in females.[1] Although the overall 5-year survival rate of breast cancer has substantially increased over the past few decades, the cardiotoxic effects of many anticancer treatments now represent a serious concern, due to their negative impact on the patient outcomes and quality of life.[1,2] Recent research evidence indicates that the cardiovascular (CV) complications, including left ventricular (LV) dysfunction (LVD), myocardial ischemia (e.g., coronary heart disease [CHD] or myocardial infarction [MI]), arterial hypertension, atrial and ventricular arrhythmias, congestive heart failure (HF), cardiomyopathy (CM), and pericarditis-myocarditis syndrome, have been associated with commonly used anticancer chemotherapy (CHT) such as anthracyclines and the human epidermal growth factor receptor 2 (HER-2)-targeting agent trastuzumab (often associated with LVD and HF).[2,3] In addition, several CV adverse effects of adjuvant breast cancer systemic therapies can be associated with the use of radiotherapy (RT), endocrine therapy (e.g., tamoxifen and aromatase inhibitors), alkylating agents (e.g., cisplatin and cyclophosphamide), antimetabolites (e.g., methotrexate), microtubule-targeting agents (e.g., taxanes), antiangiogenic factors (e.g., bevacizumab), tyrosine kinase inhibitors, and the inhibitors of vascular endothelial growth factor (e.g., sorafenib or sunitinib).[2,3,4] In general, there are two models of the different types of CHT-induced cardiotoxicity: (1) a structural injury type, with damage to cardiomyocytes and (2) a dysfunction type, with functional impairment, limited to the time of exposure.[5] There is a great interest to prevent or reduce these negative cardiac consequences of otherwise effective oncology therapies. LV remodeling has been established as an early predictor of myocardial injury, progressing to cardiac dysfunction and overt HF. Although LV ejection fraction (LVEF) represents an important index of cardiac function, which can be evaluated via transthoracic echocardiography (ECHO), in large scale trials, biomarker measurements and clinical outcome measures can provide a more comprehensive insight to the patients’ cardiac condition.[6] Cardiac dysfunction due to antineoplastic therapy has usually been treated, according to the established cardiology guidelines for HF. However, such a treatment has often not been based on specific research evidence, relevant to cardiac disorders in the cancer patient population. Since the commonly used antihypertensive medications such as angiotensin-converting enzyme inhibitors (ACE-Is) angiotensin receptor blockers (ARBs), and beta blockers (BBs) can reduce mortality or reverse LV remodeling in patients with HF or asymptomatic LVD, their use has recently been explored in the population of patients with breast cancer undergoing anticancer therapies.[7,8,9] The aim of this article is to highlight the potential role of cardiac medications in the prevention of anthracycline- and trastuzumab-mediated cardiotoxicity, in patients with breast cancer, based on evidence from recent clinical trials. This overview is predominantly focused on the use of the commonly prescribed medications, such as ACE-Is, ARBs, and BB, outlining their cardioprotective effects in patients with breast cancer, exposed on CHT-induced cardiotoxicity. In addition, the importance of biomarkers and imaging tests as potential tools for detection and monitoring of the cardiac dysfunction induced by CHT and some practical preventive and therapeutic strategies for a growing number of women with breast cancer has been outlined. The content of this overview is based on a literature search of PubMed, using the search terms: Anthracycline, trastuzumab, cardiotoxicity, breast cancer, ACE-Is, BBs, and ARBs. The main search timeframe was setup for the last 5 years, mostly in relevance to the HER-2-positive patients with breast cancer, treated with anthracycline or trastuzumab therapy (in addition to surgery and/or RT regimen). This search was supplemented with some data from cross references (cited in the bibliography of relevant articles), addressing the effects of ACE-Is, BB, and ARB on cardiac condition in this patient population. After analyzing the most relevant PubMed publications, the selected data were concisely summarized.
Anthracycline - the Main Mechanisms of Cardiotoxicity and Potential Cardioprotective Strategies
In treating patients with breast cancer, anthracycline has been used as one of the classical chemotherapeutics, despite its dose-dependent cardiotoxicity.[5] It has been determined that anthracyclines, in addition to their anticancer effects, related to induction of DNA damage (via inhibition of topoisomerase 2 [Top2]-alpha in cancer cells) can impair DNA repair in cardiomyocytes (via inhibition of Top2-beta in cardiomyocytes).[10] In addition to these findings, some evidence exists that the reduction in cardiac regenerative capacity, and in consequence, the development of CM, among patients receiving anthracycline-based therapies, can be partially attributed to a premature senescence of circulating progenitor cells.[11,12] In the management of patients receiving anthracycline-based CHT, the most difficult therapeutic balance to achieve is to protect the heart, without decreasing the efficacy of the antineoplastic treatment. At present, several research studies have been exploring this field, and the recent meta-analysis by Kalam and Marwick, focused on studies in cancer survivors, revealed that the angiotensin antagonists, BB, and statins are effective in preventing the anthracycline-induced cardiotoxicity.[13] It should be highlighted that the early initiation, and the synergistic effects of ACE-I and BB, as a combination of medications with different mechanisms of action, can be beneficial for cardiac condition among many cancer patients. Moreover, it has been established that many actions of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, relevant to cardiac remodeling, are mediated via the activation of angiotensin II, which is being excessively produced upon the doxorubicin impact. It has been reported that a chronic treatment with the angiotensin 1 receptor antagonist, losartan, is possible to prevent the development of both contractile dysfunction and myocardial atrophy that has occurred in response to doxorubicin. These data suggest that the angiotensin II signaling pathway may play an important role in doxorubicin-induced Nox2 activation, although it is likely that other key upstream mediators are also involved. Since NADPH oxidase promotes pathologic cardiac remodeling, associated with doxorubicin CHT (via the NADPH-dependent semiquinone formation that plays a key role in cardiotoxic actions of anthracyclines), it is conceivable that an administration of cardiac medications, such as ARBs, which decrease the NADPH oxidase activity, among their other actions, might contribute to cardioprotective effects.[14]
Trastuzumab - its Reversible Cardiotoxicity and Strategies for Monitoring
Trastuzumab (herceptin) is a humanized monoclonal antibody, targeting the extracellular domain of the epidermal growth factor receptor subunit HER-2/ErbB2. Trastuzumab has been used for the treatment of breast cancer (overexpressing HER-2) and also for a metastatic gastric or gastroesophageal junction adenocarcinoma (expressing this receptor).[15] Trastuzumab has a cardiotoxicity profile that in some ways differs from anthracyclines, like for instance, it is characterized by the absence of histological abnormalities, and also, by the reversibility of LV function decline. In addition, it has been indicated that herceptin can impair the regenerative capacities of human resident cardiac stem cells.[16] It has been recommended that patients receiving adjuvant treatment with trastuzumab should have adequate cardiac monitoring, using transthoracic ECHO or radionuclide ventriculography multiple-gated acquisition (MUGA) scans at baseline, and then at 3, 6, and 9 months after starting this treatment. However, according to a recent large (over 2000 participants) population-based study of older patients with breast cancer, the rates of cardiac monitoring were low, and only about one-third of the participants was adequately monitored, including the patients with cardiac comorbidities, or advanced age, who were mostly prone to develop cardiotoxicity. This study results should influence the clinical practice by improving cardiac monitoring, especially since trastuzumab-related cardiotoxicity is reversible. Moreover, a higher physician's awareness of the key role of regular monitoring, as well as early detection of subclinical heart disease, and its subsequent management can prevent further progression of HF in this patient population.[17]
Recent Clinical Studies on Cardioprotection and Their Possible Implications for Practice
In patients with breast cancer, a primary cardioprotection encompasses all patients, who receive potentially cardiotoxic therapies, and a secondary cardioprotection involves selected high-CV risk patients, who present preclinical signs of cardiotoxicity (e.g., abnormal cardiac biomarkers).[2,3] In practice, the use of cardioprotectants differs, depending on these two clinical scenarios. Furthermore, according to a recent concept of primordial prevention, some cardioprotective strategies have been designed to address the specific CV needs, within a period of time immediately after the initial cancer diagnosis, but before the administration of potentially toxic antineoplastic therapy.[2,18]
According to the results of a recent meta-analysis by Yun et al., which was aimed to determine the efficacy of BB and angiotensin antagonists to prevent the early onset of anthracyclines-induced LVD and cardiac events, the use of both BB and angiotensin antagonists was associated with better LVEF preservation, and this benefit was prominent among patients treated with higher doses of anthracyclines.[7] This meta-analysis provides some support for the routine use of BBs and angiotensin antagonists among cancer patients undergoing treatment with anthracyclines. In addition, it indicates an urgent need for more controlled, long-term studies in this field to provide further recommendations for clinical practice in this area.[7] In response to this challenge, some recent clinical studies [Table 1] have been conducted, including the OVERCOME, the MANTICORE, and the PRADA trials that are discussed below.
Table 1.
Randomized controlled trials evaluating the role of heart failure pharmacotherapy for the prevention of chemotherapy-mediated cardiotoxicity
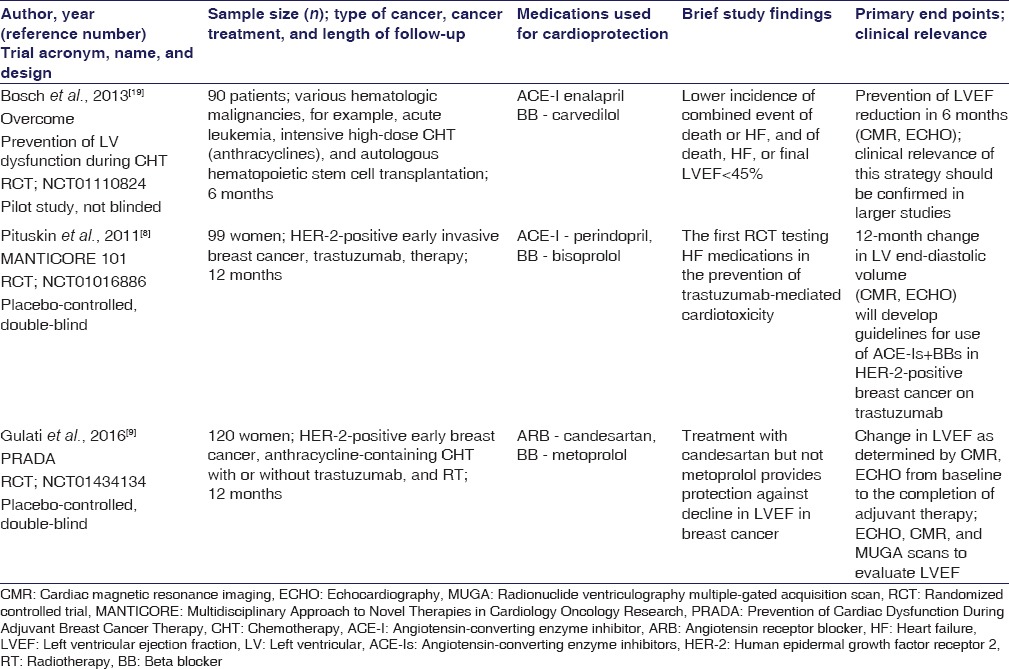
The OVERCOME Trial
The results of preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies (NCT01110824) have indicated that the combination therapy of ACE-I (enalapril) and BB (carvedilol) has prevented the LVEF (used as a measure of LV systolic dysfunction) reduction, during the 6 OVERCOME trial months of observation [Table 1]. Moreover, the OVERCOME study has revealed some benefits of antihypertensive pharmacotherapy, with regard to the combined secondary outcome measures (death or HF), among patients with malignancies, referred for intensive, high-dose CHT (including anthracyclines) and stem cell transplantation.[19] It seems likely that in some aspects, the OVERCOME trial has “paved the way” toward the desirable model of integrated cardio-oncology approach to patients with neoplastic diseases.
The MANTICORE Trial
Another important clinical study on cardioprotection is MANTICORE 101 - Breast (Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research) (NCT01016886). MANTICORE is the first double-blind, randomized controlled trial designed to prospectively evaluate the role of standard HF pharmacotherapy (including ACE-I-perindopril and BB-bisoprolol) for the prevention of trastuzumab-related cardiotoxicity among patients with HER-2-positive, invasive, early breast cancer [Table 1].[8] In this trial, multiparametric cardiac magnetic resonance imaging (CMR) was performed at baseline, and at 3, 12, and 24 months to evaluate LV remodeling (by measurement of changes in LV end-diastolic volume and LVEF).[8] It should be pointed out that this trial was terminated early, since a primary end point had been met, meaning that there had been no evident progression of the LVD, during the 1-year therapy with the ACE inhibitor and BB.[8] The final results of the 2-year follow-up assessment of the study participants are still pending; however, it is expected that the MANTICORE trial findings will help in the development of guidelines for prophylactic use of ACE-Is and BBs in HER-2-overexpresssing breast cancer patients treated with trastuzumab.
The PRADA Trial
The PRADA trial (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) (NCT01434134) is a recent study focused on exploring whether the cardiotoxicity could be prevented with the use of common cardiac medications (such as the ARB - candesartan and BB-metoprolol) during the anticancer treatment in a population of patients with HER-2-positive, early breast cancer [Table 1].[9] This randomized, placebo-controlled, double-blind trial was conducted in a homogeneous population of 120 women with breast cancer. It should be pointed out that the women with symptomatic CHF, valvular heart disease, CHD, or arrhythmias, as well as the ones receiving treatment with an ARB, BB, or ACE inhibitor within 1 month prior to the study initiation were excluded from this trial (to avoid bias).[9] The participants were randomized to receive candesartan (or matching placebo) or to receive metoprolol (or matching placebo). All the study patients also received anthracycline-containing CHT, with or without trastuzumab, and RT, for 10–61 weeks. To specifically examine whether or not blocking of the neurohormonal pathway can stop the cardiac dysfunction, and also, to assess whether the antihypertensive medications might have cardioprotective effects in this context, the cardiac function monitoring was conducted, by using serial transthoracic ECHO tests and CMR scans, both prior to starting the anticancer treatment and after its termination.[9] It should be highlighted that the cardiac MRI scan is the gold standard for quantifying LV remodeling and function. However, it is not easily accessible in the clinical practice, and thus, in the PRADA trial, both CMR and ECHO or radionuclide ventriculography MUGA scans were used to evaluate the LVEF, which was the study's primary end point. An assessment of the dynamic change in the LVEF has revealed a larger decline of LVEF in the placebo group, compared with the candesartan group. In addition, somewhat surprisingly, it has been shown that the decline of LVEF in the placebo group was almost identical to the one in the metoprolol group. This indicates that the candesartan exerts some cardio-protective actions in this patient population.[9] However, one of the puzzling issues of the PRADA trial has been the lack of cardioprotective effect of BBs. This can probably be explained by the fact that the BBs do not interfere with the direct cardiotoxic effect of the anthracyclines, and thus, they might not have an effect in the early treatment phase (examined in the PRADA patient population).[9] On the other hand, BBs might play some beneficial role in the development of cardiac dysfunction that can occur over a longer period of time. To elucidate these topics, further long-term follow-up studies are certainly merited. In summary, despite the small sample of patients, their low cardiac risk, and short follow-up period, the PRADA was the largest randomized trial, exploring the protective effect of the commonly used antihypertensive medications on cardiac dysfunction during therapy for breast cancer. Although a positive effect of candesartan has been revealed, further research trials focused on the ARB influence on the prevention of cardiotoxicity are necessary before a possible implementation of candesartan into practice for this specific purpose. Furthermore, defining the exact mechanisms of cardiotoxicity, induced by anticancer treatments and designing methodology to detect, monitor, and manage patients at high cardiac risk, represents the directions of further research in this area.[9]
Tools for the Detection and Monitoring of Chemotherapy-induced Cardiotoxicity - the Role of Cardiac Biomarkers
Current methods to detect patients at risk for cardiotoxicity from cancer therapy are still inadequate. It has been established that LVEF represents an important index of cardiac function. However, measurement of biomarkers can also provide some valuable details with regard to cardiac dysfunction, due to antineoplastic therapy.[6] In particular, cardiac troponins are biomarkers of CHT-induced cardiotoxicity, and the role of other biomarkers for the early detection of CHT-mediated cardiotoxicity has not been fully established yet. On the one hand, a highly sensitive cardiac troponin I (hsTnI) was predictive of a future decline in LVEF.[6] On the other hand, however, hsTnI did not increase the predictive value of global longitudinal strain for LVD in breast cancer patients.[6] A recent study has determined that early increases in cardiac TnI and myeloperoxidase levels offered some additional diagnostic information, with regard to the risk of cardiotoxicity, among breast cancer patients receiving doxorubicin and trastuzumab therapy. In contrast, other biomarkers, including N-terminal pro-B-type natriuretic peptide (Nt pro-BNP), high-sensitivity C-reactive protein (CRP), growth differentiation factor-15, placental growth factor, soluble fms-like tyrosine kinase receptor-1, or galectin-3 did not offer a significant diagnostic advantage in this group of patients.[20] However, prior to application of biomarkers’ measurement into clinical practice, independent validation of these findings is required.[20]
Cardiac Biomarkers and the Inter-relations between Cancer and Cardiovascular Disease
It should be emphasized that although the relation between certain CHT agents and cardiovascular disease (CVD) has been established, new evidence suggests that in addition to the well-recognized iatrogenic cardiotoxicity, some malignancies might contribute to cardiac deterioration via their own, intrinsic ways of action.[21] In particular, it has been reported that some patients with cancer can display elevated levels of Nt pro-BNP and high-sensitive troponin T (hsTnT), without clinical evidence of CVD.[21] A prospective analysis of associations between cardiac neurohormones or biomarkers (e.g., hsTnT) (their concentration in the blood) and cancer mortality, among oncology patients, who were not previously treated with any cardiotoxic antineoplastic regimen, has revealed that the Nt pro-BNP was the strongest predictor of all-cause mortality, while cardiac neurohormones, hsTnT, and copeptin were significantly correlated with mortality.[21]
The above results suggest that a possible subclinical (anatomical and functional) injury to myocardium can be associated with the development of neoplastic process itself. However, it is still unknown whether the effect on mortality is mostly connected with harmful local impact on the tumor microenvironment, or whether this is caused by systemic CV dysregulation. In general, the above findings support a need of diagnostic assessment and therapy for HF, among patients with cancer, beyond the preventing anticancer therapy-induced cardiotoxicity.[21] It is conceivable that long-term monitoring of patterns of the cardiac neurohormones or biomarkers, in the cancer patient population, might help explain whether the CVD risk increases steadily after a diagnosis of malignancy or whether it decreases when a patient is in recovery. Further research investigation in that area should contribute to designing practical implications focused on cardioprotective strategies for the more individualized management of patients with cancer.[21]
Predictors of Trastuzumab-induced Cardiotoxicity in Patients with Breast Cancer
Early indexes of LV systolic dysfunction (based on ECHO), together with biomarkers and noninvasive cardiac imaging scans, are useful for addressing the cardiac safety profile of trastuzumab therapy in an attempt to avoid the potential effects of HF. It has been reported that patients who have had cardiac troponin elevation during trastuzumab therapy were twice as likely to have no recovery of cardiac function in case of CHT-induced cardiotoxicity (e.g., CM). For this reason, it has been suggested that the baseline cardiac troponin (nonultrasensitive) should be measured, since its elevation can reflect CHT-mediated cardiac disease. In addition, such patients can present delayed gadolinium contrast enhancement on cardiac MRI scan.[22] Moreover, increased cardiac uptake on iodine-123-metaiodobenzylguanidine scintigraphy can help detect patients with reduced functional recovery from trastuzumab-induced cardiotoxicity.[23] Since these modern tests are not yet available in many clinical settings, physicians need to carefully follow their cancer patients, keeping in mind their increased risk of cardiac dysfunction. A study that analyzed the incidence of HF or CM after trastuzumab treatment for breast cancer has shown that the adjusted incidence of CM or HF was significantly increased in elderly women undergoing such therapy, compared with untreated patients or patients receiving CHT that did not contain trastuzumab or anthracycline.[24] A recent clinical trial on trastuzumab-associated cardiac events (at 8 years of median follow-up) has revealed that the incidence of LVEF decrease (by at least 10% to <50%) was 4% at 1 year and 7% at 2 years of trastuzumab treatment. Furthermore, the New York Heart Association functional Class III or IV HF was reported only in 0.8% of the study patients, and recovery of LV function to above 50% was reported in 80% of the trial patients, who had LVEF decline.[25] It should be pointed out that according to a meta-analysis by Chen et al., the patients receiving therapy with anthracyclines have not had a higher risk of developing cardiotoxicity over 3 years of follow-up. However, the combination of anthracyclines and trastuzumab accounted for a higher cardiac risk than the trastuzumab alone, as compared to the untreated or differently treated groups of breast cancer patients.[26] Furthermore, based on the results of a recent cohort study, the patients’ age has been reported as the main independent cardiac risk factor, which increased the probability of cardiotoxicity development (more than 11 folds) in patients aged 70 years or more, comparing with the ones below 50 years of age.[27] The second major predictor of cardiotoxicity was a history of CVD, which increased the cardiac risk more than 4 times.[27] Likewise, pre-existing CHD, arterial hypertension, and diabetes mellitus represented the most important risk factors for the development of HF among women over 70 years of age who received trastuzumab therapy.[28,29] Usually, the reduction in LVEF, assessed by two-dimensional ECHO in patients with arterial hypertension, did not decrease to values below 50%.[30] In addition, renal insufficiency (with a glomerular filtration rate below 78 ml/min/1.73 m2) has been identified as a new independent risk factor for trastuzumab-induced cardiotoxicity that increases the probability of adverse cardiac effects more than 3 folds.[31] In addition, it has been reported that the baseline LV diastolic dysfunction, after receiving the anthracycline-based CHT, represented a predictive factor of trastuzumab-induced cardiotoxicity in patients with breast cancer.[32] From a practical point of view, it needs to be highlighted that the integrative risk prediction model for HF and CM, after adjuvant trastuzumab therapy for breast cancer, has been developed and validated.[33] It should be noted that according to this index, the main predictors, such as the patient age, arterial hypertension, previous treatment with anthracycline, and history of CHD, can independently double the cardiac risk. Furthermore, studies based on the LVEF monitoring have confirmed the value of LV strain imaging, as an early marker of trastuzumab-induced cardiotoxicity.[34] Furthermore, the results of a small (42 women) prospective study, evaluating whether cardiac biomarkers (such as troponin T, CRP, and brain natriuretic peptide), tissue velocity imaging (TVI), strain imaging, and cardiac MRI can predict early LVD in HER-2-positive breast cancer patients, who received adjuvant trastuzumab therapy, have revealed that both TVI and strain imaging were able to identify preclinical changes in LV systolic function, prior to the detection of measurable changes in LVEF via ECHO.[35] In summary, there should be more focus on the utility of cardiac biomarkers, LV strain imaging, and cardiac MRI scans in predicting early LVD in patients with HER-2-positive breast cancer, treated with adjuvant trastuzumab therapy.
The Cardioprotective Role of Dexrazoxane in Patients with Metastatic Breast Cancer
Various strategies have been used in an attempt to decrease anthracycline-induced cardiotoxicity. One of them is dexrazoxane, a derivative of the metal-chelating agent ethylenediaminetetraacetic acid, that has been thought to attenuate anthracycline toxicity through iron chelation and to decrease the production of free radicals.[36] It should be underscored that dexrazoxane is the only United States Food and Drug Administration-approved medication for reducing the incidence and severity of CM associated with doxorubicin administration, among women with metastatic breast cancer. In particular, dexrazoxane therapy is recommended for patients, who received doses of doxorubicin >300 mg/m2 or doses of epirubicin >550 mg/m2 and who require further administration of these agents for advanced, anthracycline-sensitive cancers.[36] Furthermore, a meta-analysis on the prophylactic use of dexrazoxane, in patients receiving anthracyclines, confirmed a decrease in cardiac events, decreased HF risk, and increased CV event-free survival, compared to those who did not receive this medication.[13]
Although the exact cardioprotective molecular mechanism of dexrazoxane has not been fully elucidated, it may be related to the ability of dexrazoxane to prevent anthracycline binding to Top2 (a possible molecular target for primary prevention of anthracycline-induced cardiotoxicity).[37]
Future Perspectives for the Management of Cardiac Diseases in Cancer Patients - the role of Cardio-oncology
Due to the fact that cancer and CVD often coexist in the same patient, it should be highlighted that such patients will benefit from the combined clinical knowledge and experience of both oncology and cardiology specialists, enriched by the ongoing input from primary care physicians (who usually have established long-term relationships with patients) and pharmacists (who regularly monitor the CV risk, associated with antineoplastic treatments by conducting medication utilization reviews). Such a multidisciplinary team approach is very important in patients with breast cancer, treated with anthracycline- and trastuzumab-based CHT that often causes short- or long-term CV complications.[38] Cardio-oncology is a dynamically developing, new medical subspecialty that offers comprehensive approach (diagnostic and therapeutic) to patients with neoplastic diseases and CVD or CV risk.[38] The main goal of cardio-oncology is to reduce cardiac risks of anticancer treatments, via early detection and implementation of therapy (targeting iatrogenic cardiotoxic effects).[18,38] With regard to the patients with breast cancer, scheduled for CHT with anthracycline or trastuzumab, and especially those with CVD (or CV risk factors), a detailed CV workup (e.g., serial assessments of LV systolic function, cardiac biomarkers, and imaging tests), monitoring, and individualized therapeutic management (to optimize CV condition to achieve the best possible anticancer therapy results) should be conducted.[18,38,39,40] Furthermore, it is expected that some ongoing clinical trials will determine whether the CHT-induced CVD could be predicted (by using certain cardiac biomarkers or imaging scans) and reduced with therapeutic options, including ACE-Is, ARBs, BBs, aldosterone antagonists, or statins.[8,9,18,39,40]
Conclusion
Anthracyclines and trastuzumab have been associated with CV complications, and thus, reducing their cardiotoxic effects, without compromising anticancer efficacy, represents an important goal in the breast cancer patient population (especially among elderly patients or the ones with pre-existing CVD). Although some recommendations have been published in this field,[2,3,18,38,39] formal guidelines for the prevention and treatment of cancer therapy-induced cardiotoxicity are not yet available. At present, some important strategies[2,3,18,38,39] focused on cardioprotection in patients with breast cancer should include:
CV workup (baseline assessment/cardiology consultation) performed prior to the initiation of CHT (associated with cardiotoxic effects)
CV monitoring and follow-up beyond the completion of CHT, especially in patients on high doses of anthracyclines and the ones with CV comorbidities (e.g., CHD and HF)
Pre- and post-therapy LVEF evaluation, and in case of LVD, a possible discontinuation of CHT, with concurrent initiation of standard HF therapy
Evaluation by ECHO and cardiac biomarkers (e.g., troponins and BNP)
Standard HF treatment (in case of the development of CHT-induced HF).
Concurrently, the European Society for Medical Oncology Clinical Practice Guidelines for the management of cardiac dysfunction or high-CV risk recommend that the oncology patients with:
Symptomatic LVD (LVEF below 40%) should be treated with standard HF therapy, including an ACE-I (e.g., enalapril) in combination with a BB (e.g., carvedilol), unless specific contraindications exist; also, to prevent further decline of LVEF or aggravation of HF symptoms, an ACE-I should be considered, if LVEF is 40–50%[2,3,18]
Asymptomatic LVD (LVEF below 40%) should be treated with an ACE-I; in addition, a BB should also be considered in those who had prior MI.[2,3,18]
Continuous collaboration between oncologists, cardiologists, primary care physicians, and pharmacists engaged in the patient-centered, interdisciplinary therapeutic plan is essential. It should be emphasized that the benefit-risk ratio for any anticancer medication has to be analyzed in the context of an individual patient malignancy and CV condition together with regular monitoring of the patients’ clinical symptoms and parameters (e.g.: arterial blood pressure, heart rate, LVEF, renal function, and cardiac biomarkers).
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23(Suppl 7):vii155–66. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 3.Eschenhagen T, Force T, Ewer MS, de Keulenaer GW, Suter TM, Anker SD, et al. Cardiovascular side effects of cancer therapies: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011;13:1–10. doi: 10.1093/eurjhf/hfq213. [DOI] [PubMed] [Google Scholar]
- 4.Hong RA, Iimura T, Sumida KN, Eager RM. Cardio-oncology/onco-cardiology. Clin Cardiol. 2010;33:733–7. doi: 10.1002/clc.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900–2. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun S, Vincelette ND, Abraham I. Cardioprotective role of ß-blockers and angiotensin antagonists in early-onset anthracyclines-induced cardiotoxicity in adult patients: A systematic review and meta-analysis. Postgrad Med J. 2015;91:627–33. doi: 10.1136/postgradmedj-2015-133535. [DOI] [PubMed] [Google Scholar]
- 8.Pituskin E, Haykowsky M, Mackey JR, Thompson RB, Ezekowitz J, Koshman S, et al. Rationale and design of the Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101 – Breast): A randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer. 2011;11:318. doi: 10.1186/1471-2407-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati G, Heck SL, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): A 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J. 2016;37:1671–80. doi: 10.1093/eurheartj/ehw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 11.Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res. 2013;113:754–64. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piegari E, De Angelis A, Cappetta D, Russo R, Esposito G, Costantino S, et al. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res Cardiol. 2013;108:334. doi: 10.1007/s00395-013-0334-4. [DOI] [PubMed] [Google Scholar]
- 13.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: A systematic review and meta-analysis. Eur J Cancer. 2013;49:2900–9. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, McLaughlin D, Robinson E, Harvey AP, Hookham MB, Shah AM, et al. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with doxorubicin chemotherapy. Cancer Res. 2010;70:9287–97. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arteaga CL, Sliwkowski MX, Osborne CK, Perez EA, Puglisi F, Gianni L. Treatment of HER2-positive breast cancer: Current status and future perspectives. Nat Rev Clin Oncol. 2011;9:16–32. doi: 10.1038/nrclinonc.2011.177. [DOI] [PubMed] [Google Scholar]
- 16.Barth AS, Zhang Y, Li T, Smith RR, Chimenti I, Terrovitis I, et al. Functional impairment of human resident cardiac stem cells by the cardiotoxic antineoplastic agent trastuzumab. Stem Cells Transl Med. 2012;1:289–97. doi: 10.5966/sctm.2011-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavez-MacGregor M, Niu J, Zhang N, Elting LS, Smith BD, Banchs J, et al. Cardiac Monitoring During Adjuvant Trastuzumab-Based Chemotherapy Among Older Patients With Breast Cancer. J Clin Oncol. 2015;33:2176–83. doi: 10.1200/JCO.2014.58.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamo CE, Bloom MW, Cardinale D, Ky B, Nohria A, Baer L, et al. Cancer therapy-related cardiac dysfunction and heart failure: Part 2: Prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9:e002843. doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch X, Rovira M, Sitges M, Domènech A, Ortiz-Pérez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies) J Am Coll Cardiol. 2013;61:2355–62. doi: 10.1016/j.jacc.2013.02.072. [DOI] [PubMed] [Google Scholar]
- 20.Ky B, Putt M, Sawaya H, French B, Januzzi JL, Jr, Sebag IA, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–16. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pavo N, Raderer M, Hülsmann M, Neuhold S, Adlbrecht C, Strunk G, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–80. doi: 10.1136/heartjnl-2015-307848. [DOI] [PubMed] [Google Scholar]
- 22.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokkel MP, de Wit-van der Veen LJ, Boekhout A. I-123-MIBG myocardial imaging in trastuzumab-based cardiotoxicity: The first experience. Nucl Med Commun. 2013;34:19–24. doi: 10.1097/MNM.0b013e32835ae523. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. 2012;60:2504–12. doi: 10.1016/j.jacc.2012.07.068. [DOI] [PubMed] [Google Scholar]
- 25.de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32:2159–65. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Xu T, Li Y, Liang C, Chen J, Lu Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: A meta-analysis. Cancer Treat Rev. 2011;37:312–20. doi: 10.1016/j.ctrv.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Bonifazi M, Franchi M, Rossi M, Moja L, Zambelli A, Zambon A, et al. Trastuzumab-related cardiotoxicity in early breast cancer: A cohort study. Oncologist. 2013;18:795–801. doi: 10.1634/theoncologist.2013-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano C, Cortés J, De Mattos-Arruda L, Bellet M, Gómez P, Saura C, et al. Trastuzumab-related cardiotoxicity in the elderly: A role for cardiovascular risk factors. Ann Oncol. 2012;23:897–902. doi: 10.1093/annonc/mdr348. [DOI] [PubMed] [Google Scholar]
- 29.Chavez-MacGregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russo G, Cioffi G, Gori S, Tuccia F, Boccardi L, Khoury G, et al. Role of hypertension on new onset congestive heart failure in patients receiving trastuzumab therapy for breast cancer. J Cardiovasc Med (Hagerstown) 2014;15:141–6. doi: 10.2459/JCM.0b013e328365afb5. [DOI] [PubMed] [Google Scholar]
- 31.Russo G, Cioffi G, Di Lenarda A, Tuccia F, Bovelli D, Di Tano G, et al. Role of renal function on the development of cardiotoxicity associated with trastuzumab-based adjuvant chemotherapy for early breast cancer. Intern Emerg Med. 2012;7:439–46. doi: 10.1007/s11739-012-0794-9. [DOI] [PubMed] [Google Scholar]
- 32.Cochet A, Quilichini G, Dygai-Cochet I, Touzery C, Toubeau M, Berriolo-Riedinger A, et al. Baseline diastolic dysfunction as a predictive factor of trastuzumab-mediated cardiotoxicity after adjuvant anthracycline therapy in breast cancer. Breast Cancer Res Treat. 2011;130:845–54. doi: 10.1007/s10549-011-1714-9. [DOI] [PubMed] [Google Scholar]
- 33.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 36.Marty M, Espié M, Llombart A, Monnier A, Rapoport BL, Stahalova V Dexrazoxane Study Group. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006;17:614–22. doi: 10.1093/annonc/mdj134. [DOI] [PubMed] [Google Scholar]
- 37.Vejpongsa P, Yeh ET. Topoisomerase 2ß: A promising molecular target for primary prevention of anthracycline-induced cardiotoxicity. Clin Pharmacol Ther. 2014;95:45–52. doi: 10.1038/clpt.2013.201. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: Cardio-oncology. Mayo Clin Proc. 2014;89:1287–306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 40.Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: Addressing the unresolved issues. Circulation. 2012;126:2749–63. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]


