Abstract
Objectives:
The aim of this study was to investigate the antipsoriatic activity of ethanolic extract of Woodfordia fruticosa flowers (EEWF) using a novel in vivo screening model.
Materials and Methods:
For induction of psoriasis, 0.1 ml of prepared complete Freund's adjuvant (CFA) and formaldehyde mixture (1:10 ratio) was topically applied for 7 days on the dorsum surface of the skin of Swiss albino mice. Psoriasis severity index (PSI) was evaluated by phenotypic (redness, erythema, and scales) and histological features (epidermal thickness). Therapeutic effect of 0.05% and 0.1% (w/w) ointments of EEWF was evaluated after the induction of psoriasis. Ointments of EEWF flowers were applied once daily for 3 weeks, and antipsoriatic activity was evaluated by scoring the PSI and histological examination.
Results:
We observed the phenotypic and histological features and found a progressive reduction (P < 0.05) in the severity of psoriatic lesions (redness, erythema, and scales) from day 7 to 21st day and decreased epidermal thickness in animals treated with 0.05% and 0.1% (w/w) ointments of EEWF.
Conclusions:
The results showed that 0.05% and 0.1% (w/w) ointments of EEWF have dose-dependent beneficial effects in CFA and formaldehyde-induced psoriasis. The present investigation revealed that W. fruticosa flowers possess potent antipsoriatic activity and can be used for psoriasis treatment.
Keywords: Complete Freund's adjuvant, flavonoids, formaldehyde, inflammation, psoriasis, Woodfordia fruticosa (L.) Kurz
Psoriasis is an immune-mediated, inflammatory skin disorder characterized by red, thickened plaques with overlying silvery-white scaly patches mainly distributed into extensor surfaces and may also involve palms and scalp.[1] Psoriasis is a lifelong disorder with unpredictable remissions and relapse affecting the psychosocial life of the patients.[2] Although genetic, immunological, and environmental factors seem to be implicated, the exact cause is not yet known, and even today, psoriasis is a serious global problem.[3] Several treatment strategies are available, but affordability, availability, and side effects of prolonged use of synthetic drugs for psoriasis still remain a challenge.[4] In general, herbal formulations are less expensive and are known to minimize the risk of side effects, and therefore provide a viable alternative for psoriasis treatment.[5]
Woodfordia fruticosa (L.) Kurz is a straggling leafy shrub of family Lythraceae, frequently used English name is Fire flame bush, whereas locally in Hindi and Sanskrit known as Dhawai and Dhataki, respectively. In Ayurvedic and Unani systems of medicines, it is an extensively used medicinal plant. Flowers were traditionally used by the tribal peoples for the treatment of skin diseases, inflammation, and wound healing.[6] Phytochemical studies of W. fruticosa flowers can be ascribed for its important bioactive phytoconstituents such as flavonoids, glycoside, phenolics, and tannins, which may be valuable for the treatment of psoriasis.[7] However, till date, there are no validated scientific reports for its antipsoriatic activity.
Therefore, in light of ethnopharmacological facts of the plant, the aim of the present study was to investigate the antipsoriatic potential of ethanolic extract of W. fruticosa (L.) (EEWF) Kurz flowers in complete Freund's adjuvant (CFA) and formaldehyde-induced psoriasis.
Materials and Methods
Plant Material
W. fruticosa (L.) Kurz flowers for the present study were collected in the month of January from Swarn Jayanti Park, Bhopal, Madhya Pradesh, India. It was identified and authenticated by Dr. Zia Ul Hasan, Head of Department, Department of Botany, Saifia Science College, Bhopal, Madhya Pradesh, and a specimen voucher (444/Bot/Safia/13) deposited in the Department of Pharmacognosy, Truba Institute of Pharmacy, Bhopal, Madhya Pradesh, for future reference.
Extraction
The flowers of W. fruticosa were shade dried for 2 weeks, then pulverized to a coarse powder, passed through sieve no. 20 to maintain uniformity. Coarsely dried powder of flowers was first defatted with petroleum ether (60–80°C) for 72 h to remove fatty materials and then extracted with ethanol (95%) using Soxhlet apparatus for 36 h, the extract was collected, filtered through Whatman filter paper, concentrated in vacuum under reduced pressure using a rotary flash evaporator, and the dried extract was stored in airtight container at 4°C for further study. The percentage yield of the extract was calculated.
Preliminary Phytochemical Screening
EEWF flowers was subjected to various phytochemical screening tests for the identification of the phytoconstituents present in W. fruticosa flowers using standard procedures.[8]
Determination of Total Polyphenolic and Flavonoid Contents
The total polyphenols content of the EEWF was measured ultraviolet spectrophotometrically according to the Folin–Ciocalteu method.[9] A volume of 0.1 ml of the extract solution was mixed with 0.5 ml of Folin–Ciocalteu reagent in a test tube, and volume was made up to 3 ml of distilled water. After 3 min of incubation, 2 ml of 20% sodium carbonate solution was added and mixed thoroughly. The resulting mixture was incubated for 5 min at 50°C and cooled at room temperature. The absorbance of the mixture was measured at 650 nm against the reagent blank. All measurements were carried out in triplicate. The content of phenolic compounds was expressed as mg of gallic acid equivalents (GAEs)/g of dry extract using the linear equation obtained from the calibration curve of the standard gallic acid graph. The coefficient of determination (R2) was 0.9971.
The total flavonoid content of the EEWF was determined according to aluminum chloride (AlCl3) method.[10] A volume of 0.5 ml of AlCl3 ethanol solution (2%) was added to 0.5 ml of the sample solution. Extract sample was evaluated at a final concentration of 0.1 mg/ml. After 1 h of incubation at room temperature, the absorbance was measured at 420 nm. All measurements were carried out in triplicate. The total flavonoid content was calculated as mg of quercetin equivalents (QEs)/g of dry extract using the linear equation obtained from the calibration curve of the standard quercetin graph. The coefficient of determination (R2) was 0.9964.
Chemicals and Reagents
Retino-A 0.05% cream (Tretinoin Cream, USP) was purchased from Janssen-Cilag Pharmaceuticals (Trademark of Johnson & Johnson, USA). CFA, gallic acid, quercetin, and Folin–Ciocalteu reagent were purchased from Sigma-Aldrich-, USA. All other chemicals including solvent were of analytical grade and purchased from HiMedia Pvt. Ltd., Mumbai, India.
Animals
The experiment was carried out on healthy Swiss albino mice of 4 months of age and either gender weighing between 25 and 30 g. Animals were procured from the authorized animal house of Truba Institute of Pharmacy, Bhopal, Madhya Pradesh. The animals were acclimatized to the standard laboratory conditions in the cross-ventilated animal house at 25°C ± 2°C, relative humidity 44–56%, and light and dark cycles of 12:12 h, fed with standard diet and water ad libitum during the study. The study protocol was approved by the Institutional Animal Ethics Committee (Approval no. 1196/a/08/CPCSEA) as per the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines, India.
Induction of Psoriasis
The mouse tail model for psoriasis has been in use for the evaluation of antipsoriatic drugs.[11] We have developed a modified in vivo screening model in which combination of CFA and formaldehyde was used for the induction of psoriasis. The model is based on the potency of CFA to produce immune system stimulation and inflammation along with formaldehyde was used as a phlogistic agent, which could potentiate the inflammatory action of CFA.[12,13] A stable mixture of CFA and formaldehyde (1:10 ratio) was prepared. Hairs on the dorsum portion (nearly 2 cm × 2 cm) of each mouse were removed using depilatory cream (Reckitt Benckiser, Inc., UK). A volume of 0.1 ml of the prepared mixture was applied topically on the shaved area of all test animals (n = 10), at day 1, 2, and 3.
Animals were observed for psoriatic lesions daily for 7 days. An objective scoring system was developed based on the clinical psoriasis area and severity index.[14] Redness, erythema, and scales were scored independently on a scale from 0 to 4: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked. The cumulative score (sum of redness, erythema, and scaling) served as a measure of the psoriasis severity index (PSI) (scale 0–12). At the end of the study, animals were anesthetized using ketamine, and specimen of skin was collected and preserved in glass vials containing 10% formalin solution for histological examination. Longitudinal sections of mice skin specimen (about 5 μm thickness) were prepared by microtomy and stained with hematoxylin and eosin (H and E) dye for histological examination.
Preparation of Test Formulations
Simple ointments of EEWF, 0.05% and 0.1% (w/w) were formulated using stearyl alcohol, white petrolatum, and liquid paraffin as oleaginous phase and sodium lauryl sulfate, ethylparaben, glycerin, and water as the aqueous phase.
Acute Dermal Toxicity Study
The acute dermal toxicity of prepared ointments of EEWF was evaluated according to the Organization for Economic Cooperation and Development guidelines no. 402.[15] Swiss albino mice were divided into two groups, each group consisting of 6 animals. Twenty-four hour prior to the test, hairs were removed from the 10% of the body surface area on dorsum portion. Starting dose 2000 mg/kg, body weight, of prepared 0.05% and 0.1% (w/w) ointments was applied topically on the shaved area. The treated animals of both groups were monitored for 14 days for redness, erythema, and changes in fur, sleep pattern, behavior pattern, and mortality.
Antipsoriatic Activity of Test Formulations
Psoriasis was induced in the animals by topically applying the mixture of CFA and formaldehyde as mentioned in “Induction of psoriasis.” After the induction, all the animals were re-randomized before treatment to reduce the error in mean PSI between the groups. Disease-induced animals were divided into four groups of 6 each (n = 6). Group I (Untreated), Group II (Positive control) treated with Retino-A cream (0.05%), Group III and IV were treated with 0.05% and 0.1% (w/w) ointments of EEWF, respectively. Animals were treated after induction of psoriatic lesions once daily for 3 weeks. Reduction in the symptoms of psoriasis was evaluated by scoring the severity of psoriatic lesions every week. Redness, erythema, and scales were scored independently on a scale from 0 to 4: 0, none; 1, slight; 2, moderate; 3, marked; and 4, very marked. The cumulative score (sum of redness, erythema, and scaling) served as a PSI (scale 0–12). At the end of the study, animals were anesthetized using ketamine, and specimen of skin was collected and preserved in glass vials containing 10% formalin solution. Longitudinal sections of mice skin specimen (about 5 μm thickness) were prepared by microtomy and stained with H and E dye for histological examination.
Statistical Analysis
All the experimental results were represented as mean ± standard error of mean and analyzed using one-way analysis of variance by Tukey–Kramer multiple comparisons test. Statistical calculations were performed using GraphPad Software Inc, San Diego, CA, USA. P < 0.05 was considered statistically significant in all the cases.
Results
Acute Dermal Toxicity Study
Dermal toxicity study revealed the nontoxic nature of the ointments. There was no mortality or any sign of toxic reactions found at the maximum tested dose levels of 2000 mg/kg, body weight, throughout the study period.
Preliminary Phytochemical Screening
The percentage yield of EEWF was found to be 8.62% (w/w). The qualitative phytochemical analysis of the EEWF showed the presence of flavonoids, glycosides, alkaloids, tannins, triterpenoids, polyphenols, carbohydrates, and proteins.
Total Phenolic and Flavonoid Contents
The total phenolic and flavonoid content in the EEWF was found to be 276.13 ± 1.48 mg of GAEs/g of the dry extract and 31.74 ± 0.24 mg of QEs/g of the dry extract, respectively.
Induction of Psoriasis
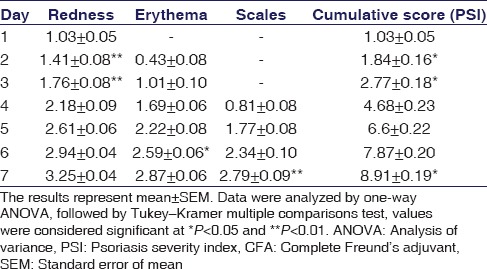
Topical application of 0.1 ml of CFA and formaldehyde for 7 days resulted in the development of induced psoriasis on the dorsum portion of the mice. Several phenotypic changes such as redness, erythema, and silvery scales on exposed area were marked visually and found an increase in severity progressively, whereas the cumulative score, PSI was significantly (P < 0.05) increased on the 7th day of induction as shown in Table 1.
Table 1.
Examination of redness, erythema, and scales in complete Freund's adjuvant- and formaldehyde-treated mice
Increased epidermal thickness, hyperproliferation of keratinocytes, granulocyte infiltration, the presence of Munro's microabscess, capillary loop dilatation, elongation of rete ridges, and absence of the granular cell layer (parakeratosis) were observed through histological examination in CFA- and formaldehyde-treated mouse skin as compared to normal mouse skin [Figure 1a and b]. All these phenotypic and histological features have a close resemblance with the human plaque psoriasis, for a valid and bona fide psoriasis mouse model.
Figure 1.
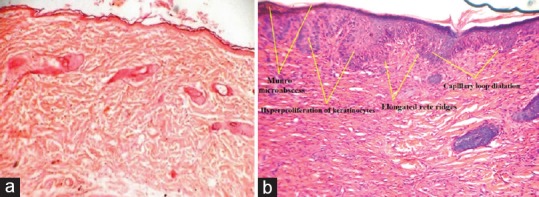
Longitudinal histological sections of mouse skin (H and E, ×40), (a) section of normal mouse skin and (b) section of complete Freund's adjuvant- and formaldehyde-treated mouse skin. (The arrows are pointing at Munro's microabscess, hyperproliferation of keratinocytes, elongated rete ridges, and capillary loop dilatation)
Effect of Test Formulations on Complete Freund's Adjuvant and Formaldehyde-induced Psoriasis
After induction of psoriasis, 0.05% and 0.1% (w/w) ointments of EEWF were applied once daily for 3 weeks, and the severity of psoriatic lesions was evaluated by visual and histological examinations. Results are shown in Figure 2. In visual examinations, the severity of psoriatic lesions (redness, erythema, and scales) was gradually increased in the untreated group (Group I) throughout the experimental period, which was reproducible in all animals [Figure 2a–c]. Cumulative score was significantly (P < 0.05) increased manifold in Group I [Figure 2d] on 21st day in comparison to other groups. In Group II, topical application of Retino-A cream (0.05%) progressively reduced (P < 0.05) the severity of redness, erythema, and scales from day 7 to 21st day. Cumulative score in Group II revealed the therapeutic effect of a standard drug on psoriatic lesions [Figure 2d]. In Group III, application of 0.05% (w/w) ointment showed a gradual decrease in redness, erythema, and scales [Figure 2a–c] along with a significant reduction (P < 0.01) in the cumulative score in comparison to the Group I [Figure 2d]. Application of 0.1% (w/w) ointment in Group IV animals was found to possess a significant therapeutic effect (P < 0.001) on psoriatic lesions. Redness, erythema, scales and cumulative score progressively reduced in animals [Figure 2d] showing the therapeutic efficacy of W. fruticosa flowers on induced psoriasis.
Figure 2.
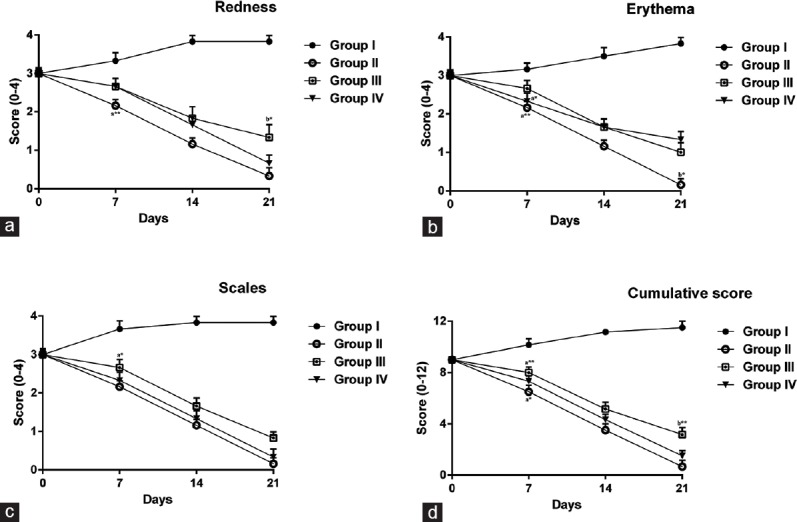
Changes in (a) redness, (b) erythema, (c) scales, and (d) cumulative score in the extract of Woodfordia fruticosa-treated mice. The results represent mean ± standard error of mean for 6 mice per group. Data were analyzed by one-way analysis of variance, followed by Tukey–Kramer multiple comparisons test, values were considered significant at *P < 0.05 and **P < 0.01, a-significant difference as compared to Group I, b-significant difference as compared to Group II
In histological examinations, increased epidermal thickness approximately 2-fold [Figure 3], hyperproliferation of keratinocyte cells and the presence of other features such as granulocyte infiltration and collection of neutrophils beneath the epidermis was an indication of psoriasis in untreated animals [Figure 4a] in comparison to standard [Figure 4b] and treated groups [Figure 4c and d]. EEWF-treated animals showed remarkable therapeutic effect based on histological examination and epidermal thickness. Epidermal thickness significantly reduced (P < 0.001) in terms of reduced parakeratosis [Figure 3] and granular layer retention, indicating the reduced hyperproliferation of keratinocytes and initiation of keratinization process [Figure 4c and d].
Figure 3.
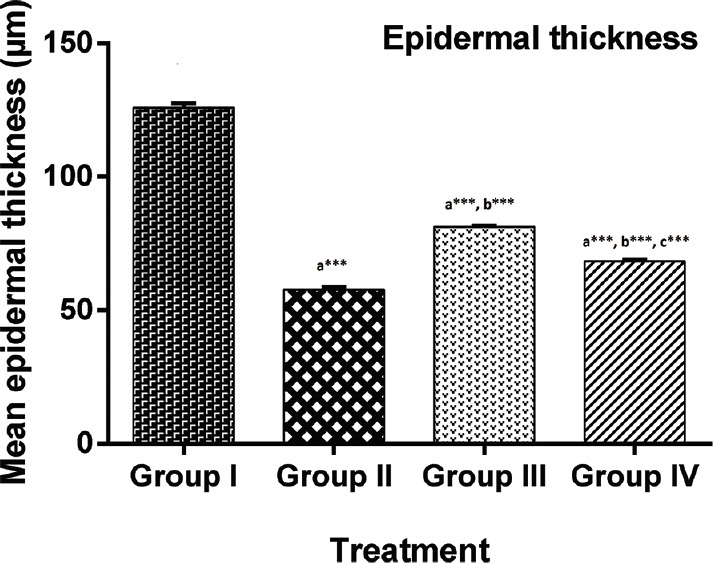
Changes in epidermal thickness of extract of Woodfordia fruticosa treated mice. Each bar represents mean ± standard error of mean for 6 mice per group. Data were analyzed by one-way analysis of variance, followed by Tukey–Kramer multiple comparisons test, values were significantly different from those of control group: *P < 0.05, **P < 0.01, and ***P < 0.001, a-significant difference as compared to Group I, b-significant difference as compared to Group II, c-significant difference as compared to Group III
Figure 4.
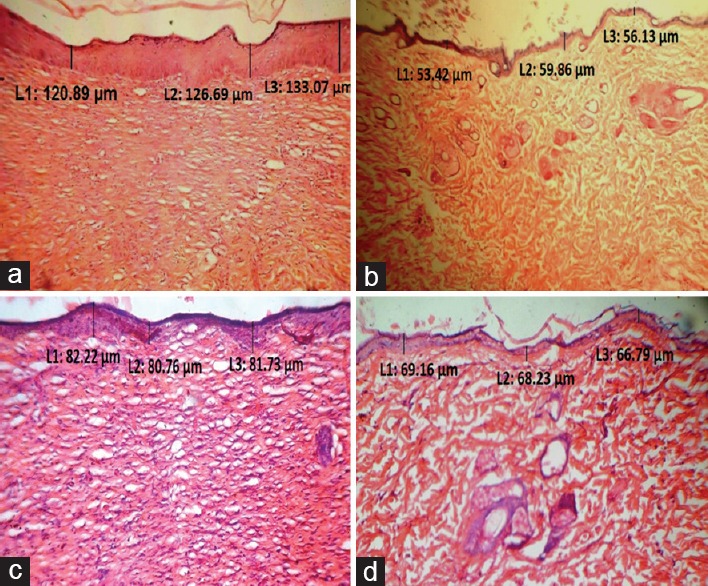
Longitudinal histological sections of mouse skin of different groups (H and E, ×40). (a) Group I (Untreated), (b) Group II (Positive control) treated with Retino-A cream (0.05%), (c) Group III treated with 0.05% (w/w) ointment of extract of Woodfordia fruticosa and (d) Group IV treated with 0.1% (w/w) ointment of extract of Woodfordia fruticosa. The section is marked with an arrow representing the epidermal thickness
Discussion
Since psoriasis is a disease of multiple etiologies, the exact cause is not yet known.[3] In the recent years, a lot of research work has been done to elucidate the exact pathogenesis of psoriasis along with the development of novel therapeutic strategies. However, the major dilemma in the area of new drug development for the treatment of psoriasis is the lack of the direct and efficient in vivo screening model. Existing animal models such as mouse tail model and xenograft model require extensive proficiency and technical expertise, and these models are less convenient in view of affordability and availability.[11]
The rationale behind the selection of CFA and formaldehyde to induce the psoriasis was the ability of CFA to provoke the precise immune response produced via influx of the different cells of the immune system.[16,17] Formaldehyde selected as a phlogistic agent, which could elicit the inflammatory response by enhancing the secretion of the different chemical mediators to potentiate the effect of CFA.[18]
Early and active psoriatic lesions are characterized by intraepidermal penetration of activated polymorphonuclear leukocytes, which causes the uncontrolled production of reactive oxygen species, leading to peroxidative damage to membranes of the skin and contributing to the exacerbation of lesions. Reactive oxygen species may also activate phospholipase A2 and thus increase the release of mediators of arachidonic acid. Prostaglandin E2 produced by the cyclooxygenase pathway also contributes to psoriasis by dilating capillaries in the dermis, increasing leukocyte infiltration, and stimulating keratinocyte cell growth.[19]
During induction of psoriasis, topical application of CFA and formaldehyde provoked several pro-inflammatory reactions (redness, erythema, and scales) in the mice skin. Fully developed psoriatic lesions were characterized by inflammatory erythematous papules covered with dry silvery and red scales; this condition shows a close resemblance with parakeratotic condition associated with an evident sign of plaque psoriasis that indicated precise immune response produced by the CFA.[16,17,20] Severity of psoriatic lesions indicates the role of formaldehyde in provoking the inflammation to potentiate the effect of CFA.[18]
Psoriasis is characterized by hyperproliferation and abnormal differentiation of epidermal keratinocytes, lymphocyte infiltration consisting principally of T-lymphocytes, and specific endothelial vascular changes within the dermal microvasculature including limited neoangiogenesis, capillary dilation, and high endothelial venules formation, histological examination of the CFA and formaldehyde-treated mice skin displayed all these characteristic features contributed to the visible redness of psoriatic lesions.[21]
In recent years, a lot of work has been carried out on herbal drugs to elucidate their potential effectiveness in psoriasis. At the present time, herbal medication is promising as an alternative treatment for psoriasis.[22] Previous studies have suggested that antioxidants could play an effective role in the management of psoriasis.[23] Flavonoids, triterpenoids, and polyphenolic compounds are well known as potent antioxidants and for their anti-inflammatory, antiproliferative, immunomodulatory, and free radical scavenging activities.[24,25] These characteristics of polyphenolic phytoconstituents may be beneficial for the treatment of diseases with multiple etiologies such as psoriasis. Phytochemical screening and the standardization of the EEWF revealed the presence of the rich amount of flavonoids and polyphenols in W. fruticosa flowers.
Although psoriasis is a recurrent chronic inflammatory skin disorder; EEWF exerted a protective effect by multiple mechanisms in combating psoriasis, which have a greater importance than other drugs which act by a single mechanism.
Conclusions
Developed in vivo screening model was characterized by phenotypic and histological examination as compared to normal. CFA- and formaldehyde-treated mice showed increased redness, erythema, and scales along with the elevated PSI. Histological features have also supported the resemblance of characteristic psoriatic lesions with human plaque psoriasis. Induced psoriasis by the topical application of the combination of CFA and formaldehyde may be considered as a bona fide screening model to evaluate the antipsoriatic activity of drugs.
Both the test formulations (0.05% and 0.1% ointment) of EEWF alleviated the sign of psoriasis along with mean PSI. Our results showed that the antipsoriatic activity of the W. fruticosa flowers was a result of the presence of the rich amount of bioactive phytoconstituents (flavonoids and polyphenols) in the ethanolic extract. These findings suggest the potential for use of W. fruticosa flowers in the treatment of psoriasis confirming their traditional use in skin disorders. Further studies are required to gain more insight to the isolated phytoconstituents.
Financial Support and Sponsorship
The study was supported by Truba Institute of Pharmacy, Karond Gandhi Nagar Bypass Road, Bhopal - 462 038, Madhya Pradesh, India.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
Authors are thankful to Truba Institute of Pharmacy, Bhopal, Madhya Pradesh, India, for providing experimental facilities for the successful completion of research work.
References
- 1.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–71. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 2.Nickoloff BJ, Nestle FO. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J Clin Invest. 2004;113:1664–75. doi: 10.1172/JCI22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberson ED, Bowcock AM. Psoriasis genetics: Breaking the barrier. Trends Genet. 2010;26:415–23. doi: 10.1016/j.tig.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim GK, Del Rosso JQ. Drug-provoked psoriasis: Is it drug induced or drug aggravated? understanding pathophysiology and clinical relevance. J Clin Aesthet Dermatol. 2010;3:32–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Deng S, May BH, Zhang AL, Lu C, Xue CC. Topical herbal formulae in the management of psoriasis: Systematic review with meta-analysis of clinical studies and investigation of the pharmacological actions of the main herbs. Phytother Res. 2014;28:480–97. doi: 10.1002/ptr.5028. [DOI] [PubMed] [Google Scholar]
- 6.Khare CP. Heidelberg: Springer Publishers; 2004. Encyclopedia of Indian Medicinal Plants: Rational Western Therapy, Ayurvedic and Other Traditional Usage, Botany. [Google Scholar]
- 7.Das PK, Goswami S, Chinniah A, Panda N, Banerjee S, Sahu NP, et al. Woodfordia fruticosa: Traditional uses and recent findings. J Ethnopharmacol. 2007;110:189–99. doi: 10.1016/j.jep.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Khandelwal KR. Pune: Nirali Prakashan; 2000. Practical Pharmacognosy Techniques and Experiments. [Google Scholar]
- 9.Sadasivam S, Manickam A. Biochemical Methods. New Delhi: New Age International (P) Limited; 1996. [Google Scholar]
- 10.Ordonez AA, Gomez JD, Vattuone MA, Isla MI. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006;97:452–8. [Google Scholar]
- 11.Vogel GH. Drug Discovery and Evaluation: Pharmacological Assays. New York: Springer; 2002. [Google Scholar]
- 12.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–60. [PubMed] [Google Scholar]
- 13.Xu B, Aoyama K, Takeuchi M, Matsushita T, Takeuchi T. Expression of cytokine mRNAs in mice cutaneously exposed to formaldehyde. Immunol Lett. 2002;84:49–55. doi: 10.1016/s0165-2478(02)00126-8. [DOI] [PubMed] [Google Scholar]
- 14.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl 2):ii65–8. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization Economic for Cooperation and Development (OECD). Guidelines for Testing of Chemicals. Acute Dermal Toxicity, Test No. 402. France: OECD; 2001. [Google Scholar]
- 16.Broderson JR. A retrospective review of lesions associated with the use of Freund's adjuvant. Lab Anim Sci. 1989;39:400–5. [PubMed] [Google Scholar]
- 17.Wanstrup J, Christensen HE. Granulomatous lesions in mice produced by Freund's adjuvant; morphogenesis and phasic development. Acta Pathol Microbiol Scand. 1965;63:340–54. doi: 10.1111/apm.1965.63.3.340. [DOI] [PubMed] [Google Scholar]
- 18.Saito A, Tanaka H, Usuda H, Shibata T, Higashi S, Yamashita H, et al. Characterization of skin inflammation induced by repeated exposure of toluene, xylene, and formaldehyde in mice. Environ Toxicol. 2011;26:224–32. doi: 10.1002/tox.20547. [DOI] [PubMed] [Google Scholar]
- 19.Amigó M, Payá M, De Rosa S, Terencio MC. Antipsoriatic effects of avarol-3’-thiosalicylate are mediated by inhibition of TNF-alpha generation and NF-kappaB activation in mouse skin. Br J Pharmacol. 2007;152:353–65. doi: 10.1038/sj.bjp.0707394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guenther LC, Ortonne JP. Pathophysiology of psoriasis: Science behind therapy. J Cutan Med Surg. 2002;6(3 Suppl):2–7. doi: 10.1177/12034754020060S302. [DOI] [PubMed] [Google Scholar]
- 21.Cameron AL, Kirby B, Fei W, Griffiths CE. Natural killer and natural killer-T cells in psoriasis. Arch Dermatol Res. 2002;294:363–9. doi: 10.1007/s00403-002-0349-4. [DOI] [PubMed] [Google Scholar]
- 22.Kaur A, Kumar S. Plants and plant products with potential antipsoriatic activity – A review. Pharm Biol. 2012;50:1573–91. doi: 10.3109/13880209.2012.690430. [DOI] [PubMed] [Google Scholar]
- 23.Young CN, Koepke JI, Terlecky LJ, Borkin MS, Boyd Savoy L, Terlecky SR. Reactive oxygen species in tumor necrosis factor-alpha-activated primary human keratinocytes: Implications for psoriasis and inflammatory skin disease. J Invest Dermatol. 2008;128:2606–14. doi: 10.1038/jid.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 25.González R, Ballester I, López-Posadas R, Suárez MD, Zarzuelo A, Martínez-Augustin O, et al. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51:331–62. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]


