Abstract
Objectives:
The defective apoptosis is believed to play a major role in the survival and proliferation of neoplastic cells. Hence, the induction of apoptosis in cancer cells is one of the targets for cancer treatment. Researchers are considering scorpion venom as a potent natural source for cancer treatment because it contains many bioactive compounds. The main objective of the current study is to evaluate the anticancer property of Androctonus bicolor scorpion venom on cancer cells.
Materials and Methods:
Scorpions were milked by electrical stimulation of telsons and lyophilized. The breast (MDA-MB-231) and colorectal (HCT-8) cancer cells were maintained in appropriate condition. The venom cytotoxicity was assessed by 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay, and the cellular and nuclear changes were studied with propidium iodide and 4’,6-diamidino-2-phenylindole stain, respectively. The cell cycle arrest was examined using muse cell analyzer.
Results:
The A. bicolor venom exerted cytotoxic effects on MDA-MB-231 and HCT-8 cells in a dose- and duration-dependent manner and induced apoptotic cell death. The treatment with this venom arrests the cancer cells in G0/G1 phase of cell cycle.
Conclusions:
The venom selectively induces the rate of apoptosis in MDA-MB-231 and HCT-8 cells as reflected by morphological and cell cycle studies. To the best of our knowledge, this is the first scientific evidence demonstrating the induction of apoptosis and cell cycle arrest by A. bicolor scorpion venom.
Keywords: 3-(4, 5-di-methylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide assay, Androctonus bicolor, G0/G1 phase
Cancer is a major public health problem in the world. Indeed, the struggle to find an effective therapeutic to cure the cancer is one of the greatest challenges of humanity. The WHO agency “The International Agency for Research on Cancer” is reporting that the rate of cancer is constantly increasing and 14 million new cases were registered in the year of 2012, and this figure may reach 22 million/year within the next two decades.[1] This increasing incidence indicates the inefficacy of currently available treatment methods such as chemotherapy, surgery and radiotherapy.[2] Nevertheless, these treatments lead to severe adverse effects.[3] These disadvantages are urged to find a new drug for the treatment of cancer from varied sources. Plants are the main source of anticancer drug, around 50% of the available anticancer drugs are plant products or its derivatives.[4] However, still search for anticancer molecules is not fulfilled; so, other natural sources such as animal is the optional source for finding a new anticancer agent. Among the animals, some venomous arthropods such as bees, wasps, scorpion, ants, spiders, and caterpillars are producing pharmacologically active molecules, and they are used for medicinal purpose.[5] Venom and its peptides is the new therapeutic target for the development of anticancer drugs.[6] Researchers are considering scorpion venom as a potent natural source for cancer treatment because it contains many bioactive compounds such as peptides, free amines, nucleotides, lipids, and biologically active molecules.[7]
Androctonus bicolor (AB) or black fat-tailed scorpion belongs to the family Buthidae, which is one among the largest family of scorpion. They are found in tropical, subtropical, and temperate habitats, and it is commonly found in the Middle East and North Africa. The biological activities of this venom species have been widely studied including our own study.[8,9] The chemical composition of AB venom is not fully known. However, androcin is one of the known peptides present in this venom. The chief function of this compound is to induce akinesia and anxiety-like symptoms in mice.[10,11] However, the anticancer property of AB has been not yet examined. The objective of the present study is to find the anticancer potential of AB venom.
Materials and Methods
Collection of Scorpions and their Venom
Scorpions were collected from different locations of Riyadh provinces in Saudi Arabia with the permission of Ethical Committee of the Prince Sultan Military Medical City, Riyadh. The animals were maintained individually in a glass hood with preferable condition[12] and fed with mealworms and water.[8] Scorpions were milked by electrical stimulation (10–20 V, 500 mV) of telsons, and the venom was dissolved in sterile water.[13] The lyophilized venom was stored at −80°C prior to use.
Cell Culture
Human breast cancer cell MDA-MB-231 (obtained from breast mammary glands) and colorectal cancer cell HCT-8 (obtained from the ileocecal adenocarcinoma of a 67-year-old male) were supplied by the cancer research facilities, King Saud Bin Abdulaziz Medical City, Riyadh, Kingdom of Saudi Arabia, and had been originally procured from the American Type Culture Collection, USA. The culture media were supplemented with 10% fetal bovine serum (Gibco, Life Technologies, UK), and 100 U/mL penicillin and 100 μg/mL streptomycin as antibiotics (Sigma-Aldrich, St. Louis, Mo, USA). The cells were maintained in a humidified atmosphere of 5% CO2 and 95% air in a CO2 incubator (New Branswick, Eppendorf, Hamburg, Germany). For cell viability, 100 μl of cell suspension was stained with trypan blue (0.2%) and cells were counted using an hemocytometer. All the experiments were performed using cells from passage 15 or less.
Determination of the Cytotoxic Property of AB Venom Adopting 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium Bromide Colorimetric Assay
The cytotoxic property of AB venom was assessed adopting 3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide assay (MTT) colorimetric assay.[14] The MDA-MB-231 and HCT-8 cells were seeded in 96-well plate at a density of 5 × 104 cells/well and treated with venom at concentrations ranging from 100 to 1000 μg/ml, at appropriate cell culture condition, for 24 h and 48 h. At the end of the respective exposure duration, 20 μl (5 mg/ml) of MTT in phosphate-buffered saline (PBS) was added to each well of the culture plates containing the venom-treated cells and incubated for 4 h at dark culture condition. The end product of MTT assay, purple formazan product, was dissolved by adding 100 μl of dimethyl sulphoxide. The absorbance was measured at 570 nm (measurement) and 630 nm (reference) using a multi-well plate reader (Hidex, Turku, Finland). Data were collected for four replicates each and used to calculate the respective means and the standard deviations (SDs). The percentage of viability was calculated from this data, using the following formula: Percentage of cell viability = optical density (OD) of test/OD of control × 100.
Morphological Assessment of Cell Death
Observation of cellular morphology by phase contrast microscope
Apoptotic cells were observed based on the morphological feature of the cell by phase contrast microscopic method.[15] In brief, the MDA-MB-231 and HCT-8 cells were seeded in 6-well plate at a density of 3 × 105 cells/well and treated with IC50 concentration of AB venom and incubated for 24 h and 48 h. After the treatment period, cells were harvested and suspended with PBS after a wash with PBS. The morphological changes of cells were assessed using phase contrast microscope (Olympus, Tokyo, Japan), and the images were captured using a scientific camera (Olympus D73, Tokyo, Japan) at ×200 magnification.
Propidium iodide staining
Cellular changes of venom-treated cells were observed using propidium iodide (PI) staining as described by Sarker et al.[16] For PI staining, 3 × 105 cells/wells were seeded in 6-well plate, and the adherent cells were treated with IC50 concentration of AB venom for 24 h and 48 h. After the incubation, cells were harvested and suspended with PBS after a wash. The 100 μl of cell suspension was mixed with an equal volume of PI (100 μ/ml) and incubated at 37°C for 30 min in dark condition. The stained cells were observed through a fluorescent microscope using green filter (Olympus, Tokyo, Japan), and the images were taken using the scientific camera (Olympus D73, Tokyo, Japan).
4’,6-diamidino-2-phenylindole staining
Cells were grown in 6-well plate at a seeding density of 3 × 105 cells/well, and adherent cells were treated with PI staining method. After harvesting, the cells were fixed with 3.7% formaldehyde prior to washing with PBS, and a drop of fixed cells was stained with 1 μg/ml 4’,6-diamidino-2-phenylindole (DAPI) for 15 min in a glass slide at dark condition. The stained cells were viewed with a fluorescent microscope at 340–380 nm and ×400 magnification (Olympus, Tokyo, Japan), and pictures were taken using the scientific camera (Olympus D73, Tokyo, Japan). Three hundred cells were counted per slide randomly and the cells were classified based on morphological features. Cells with smooth nuclear and cellular membranes are considered normal, and on the other hand, specialized features such as cell shrinkage, blebbing, chromatin condensation, and nuclear fragmentation are considered apoptotic. The bulged irregular swelled cells are considered necrotic. Thus, the percentage of normal, apoptotic, and necrotic cells was obtained.
Cell Cycle Analysis
AB venom-treated and untreated cells were harvested by trypsinization and counted using hemocytometer. Around 1 × 106 cells were transferred to a 2 ml tube. The cells were centrifuged at 300 ×g for 5 min. The cell pellets were washed twice with PBS. The washed cells were fixed with 70% ethanol. For fixation, cells were incubated for 3 h at −20°C. About 200 μl of fixed cells and an equal volume of Muse cell cycle reagent were mixed and incubated for 30 min at room temperature in dark. Cell cycle was analyzed using Muse cell analyzer (Millipore, Billerica, USA), and this experimental protocol was followed by the kit description.
Statistical Analysis
Student's t-test was performed using Graph Pad Prism software, version 4.0 (Graph Pad Software, Inc., San Diego, CA, USA). The mean was reported with SD (±SD). Differences were considered to be statistically significant when P ≤ 0.05.
Results
Cytotoxicity
The prime objective of this study was to check the cytotoxicity brought about by the venom and to find the toxicity levels in terms of IC50. When live and dead cell percentages are equal, this is considered the optimum dose for the various assays. MTT assay was conducted as an indirect measure to determine the viability of cells treated with AB venom. The cytotoxic activity was determined according to the dose values of the exposure of the venom required to reduce the survival of cells to 50% (IC50). The AB venom exerted cytotoxic effects on MDA-MB-231 and HCT-8 cells in a dose- and duration-dependent manner. The IC50 values of MDA-MB-231 were 839 ± 8 μg/ml and 735 ± 6 μg/ml for 24 h and 48 h, respectively [Figure 1]. The IC50 values for HCT-8 were 730 ± 6 μg/ml and 630 ± 7 μg/ml for 24 h and 48 h, respectively. The results are graphically shown in Figure 2.
Figure 1.
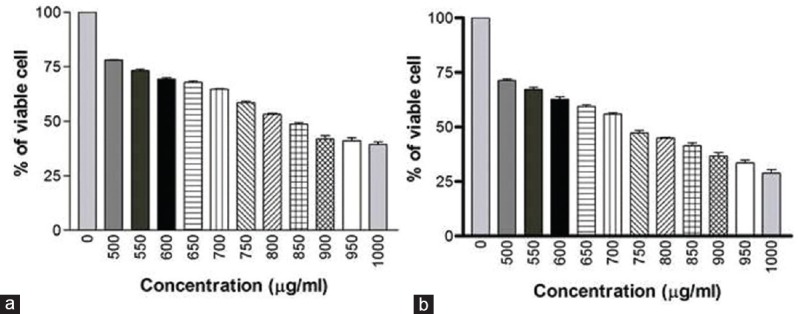
(a) Cytotoxic effect of AB venom on MDA-MB-231 cells at 24 h treatment (b) Cytotoxic effect of AB venom on MDA-MB-231 cells at 48 h treatment
Figure 2.
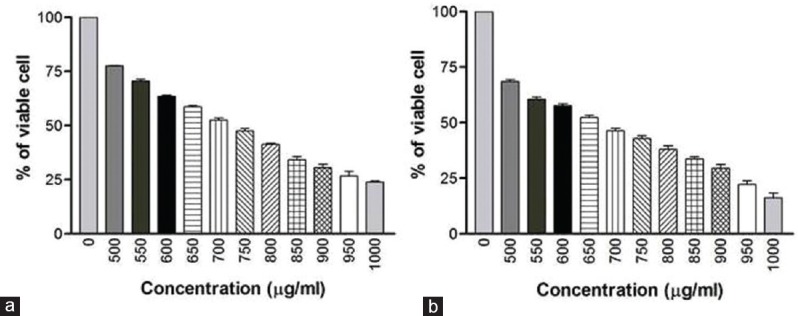
(a) Cytotoxic effect of AB venom on HCT-9 cells at 24 h treatment (b) Cytotoxic effect of AB venom on HCT-8 cells at 48 h treatment
Morphological Assessment of Cell Death
The cancer cells were treated with venom, at IC50 concentration for 24 h and 48 h, MDA-MB-231 and HCT-8 responded with apoptosis and necrosis. At 24 h treatment, in the MDA-MB-231 cell line, apoptosis is more than two times (36.0%) of the necrosis (14.7%). In the case of 48 h treatment, the rate of apoptosis further increased to 47% [Figure 3].
Figure 3.
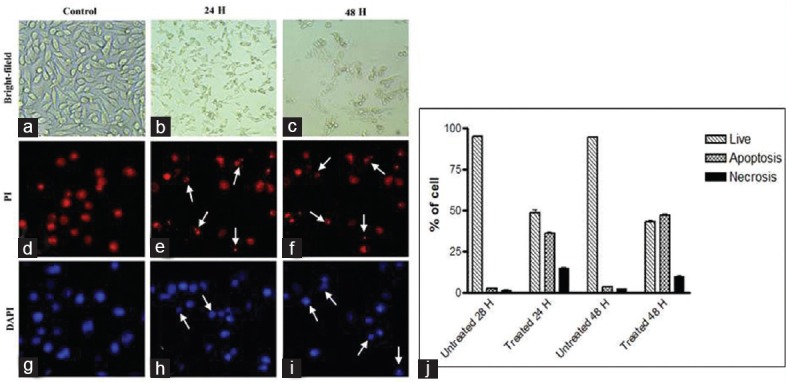
MDA-MB-231 breast cancer cells treated with AB venom for 24 h and 48 h (a) Bright field untreated. (b) Bright field 24 h treated. (c) Bright field 48 h treated. (d) 4’,6-diamidino-2-phenylindole stained untreated. (e) 4’,6-diamidino-2-phenylindole stained 24 h treated (f). 4’,6-diamidino-2-phenylindole stained 48 h treated. (g) Propidium iodide stained untreated. (h) Propidium iodide stained 24 h treated. (i) Propidium iodide stained 48 h treated. (j). Percentage of normal, apoptotic, and necrotic cells at 24 h and 48 h in control and AB venom-treated MDA-MB-231 cells. Arrows are indicating the apoptotic cells. Statistically significant (P ≤ 0.05)
Whereas in the case of HCT-8 cell line, at 24 h, the rate of apoptosis (25.66%) and necrosis (25.0%) were almost equal. In the case of 48 h treatment, the rate of apoptosis is significantly higher (33.66%) than the necrosis (22.66%) [Figure 4]. Untreated cells show <3% of apoptotic and necrotic cell death.
Figure 4.
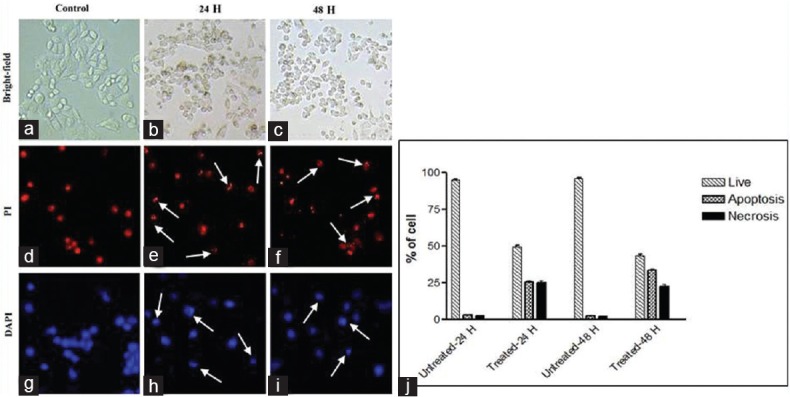
HCT-8 colorectal cancer cells treated with AB venom for 24 h and 48 h (a) Bright field untreated. (b) Bright field 24 h treated. (c) Bright field 48 h treated. (d) 4’,6-diamidino-2-phenylindole stained untreated. (e) 4’,6-diamidino-2-phenylindole stained 24 h treated (f). 4’,6-diamidino-2-phenylindole stained 48 h treated. (g) Propidium iodide stained untreated. (h) Propidium iodide stained 24 h treated. (i) Propidium iodide stained 48 h treated. (j) Percentage of normal, apoptotic, and necrotic cells at 24 h and 48 h in control and AB venom-treated HCT-8 cells. Arrows are indicating the apoptotic cells. Statistically significant (P ≤ 0.05)
Cell Cycle Analysis
Cell cycle stages can be recognized by Muse cell analyzer through their diminished stability with the DNA-specific fluorochrome, in which the hypodiploid population can be quantified by DNA content frequency histograms. When MDA-MB-231 and HCT-8 cells were treated with AB venom, there was a significant time-dependent increase of cells in the G0/G1 population, due to the arrest of cells in G0/G1 after 24 h and 48 h treatment. At 24 h treatment, MDA-MB-231 cells showed 55.35% of G0/G1 phase accumulation compared to 27.6% of untreated cells, and at 48 h treatment, 56.3% G0/G1 phase accumulation compared to 22.2% of untreated cells [Figure 5]. In the case of HCT-8 cells also, the trend was same. The percentage of cells in G0/G1 phase at 24 h was 53.3% and 36.6% in treated and untreated cells, respectively, and at 48 h, treated and untreated cells showed 54.8% and 29.1%, respectively [Figure 6]. Alltogether, the results show that AB venom is capable of inducing G0/G1 cell cycle arrest in both cell types and more prominently in MDA-MB-231 cells.
Figure 5.
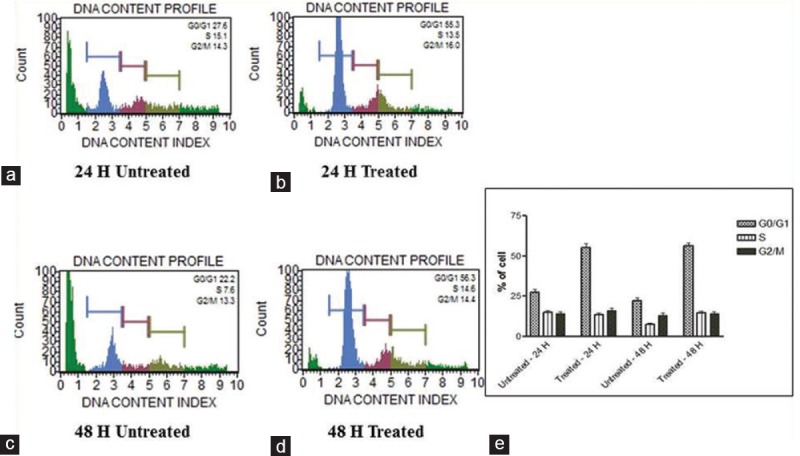
Cell cycle assay. Analysis of cell cycle arrest in AB scorpion venom-treated and untreated MDA-MB-231 cells. (a) 24 h untreated. (b) 24 h treated. (c) 48 untreated. (d) Treated. (e) Statistical analysis of cell cycle arrest on AB scorpion venom-treated MDA-MB231 cell. A significant G0/G1 enrichment was observed in venom-treated cell. Statistically significant (P ≤ 0.05)
Figure 6.
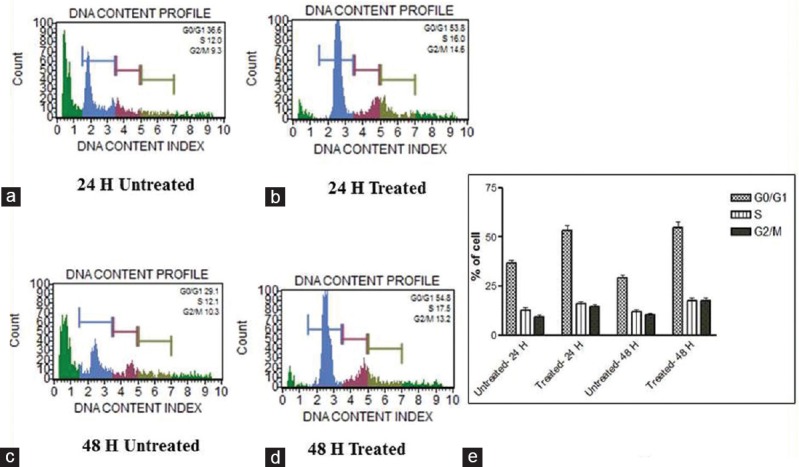
Cell cycle assay. Analysis of cell cycle arrest in AB scorpion venom-treated and AB scorpion venom-untreated HCT-8 cells. (a) 24 h untreated. (b) 24 h treated. (c) 48 untreated. (d) Treated. (e) Statistical analysis of cell cycle arrest on AB scorpion venom-treated HCT-8 cells. A significant G0/G1 enrichment was observed in venom-treated cells. Statistically significant (P ≤ 0.05)
Discussion
De-regulation of apoptosis and cell division are directly linked to the development of many types of cancers. Therefore, the induction of apoptosis has been recognized as one of the major targets for the development of new anticancer agents.[17] Scorpion venom is a potent natural source for cancer treatment,[18] and it can induce apoptosis and cell cycle arrest in cancer cells.[19] This study was conducted to examine the cytotoxicity, apoptosis inducing, and cell cycle arresting capability of AB scorpion venom on MDA-MB-231 and HCT-8 cancer cells. The exposure of scorpion venom to the cancer cells proved the cytotoxicity on the MDA-MB-231 and HCT-8 cells. Among the two cancer cell lines, the scorpion venom is more toxic on the HCT-8 cells than the MDA-MB-231. According to Díaz-García et al.,[20] the Cuban scorpion Rhopalurus junceus venoms’ IC50 value in colorectal cancer is 890 μg/ml and breast cancer is 700 μg/ml. Our results are also confirming this observation, but with slightly different IC50 values. This slight difference may be either due to the difference in the cell lines tested or species difference or both.
Apoptosis is a genetically controlled cell death process, which is characterized by chromatin condensation, DNA fragmentation to nucleosome-sized particles, membrane blebbing, cell shrinkage, and formation of apoptotic bodies.[21] Experiments were conducted to screen the apoptotic features in terms of cellular morphological changes which were assessed by PI staining and nuclear changes, adopting DAPI staining, and the result of this investigation showed that the AB venom induced a wide range of early apoptotic changes such as marginalization and/or fragmentation of chromatin, bi-nucleation, nuclear swelling, cytoplasmic blebbing, and late apoptotic indication of dot-like chromatin in a significant percentage of venom-treated MDA-MB-231 and HCT-8 cells. In the case of HCT-8 cells, the rate of necrosis was higher compared to apoptosis, whereas in the case of MDA-MB-231 cells, the rate of apoptotic was high after the treatment. Hence, AB venom may be more effective against MDA-MB-231 cells. It has already been reported that other scorpion venoms such as Centruroides limpidus, Odontobuthus doriae, R. junceus, Heterometrus bengalensis, and Scorpio maurus palamatus are capable of inducing apoptotic cell death in different cancer cells.[19,20,22,23] However, AB venom has not yet been studied for its anticancer potential. The limitation of this study is not to have the primary cells to use in parallel to the cancerous cells to evaluate the specificity of the venoms. However, based on the previous published work, it has been reported that R. junceus scorpion venom kills the cancer cells and no effect on normal cell line such as MRC-5, MDCK, and Vero had been reported.[20] Therefore, it can be postulated that in our study too, scorpion venom is selectively targeting the cancer cells and will not harm the normal cells. This study adds AB venom to the already reported list of anticancer potential of scorpion venom. Apoptosis and cell proliferation are linked by cell cycle regulators and apoptotic stimuli that affect both processes.[24] The well-known cancer drugs such as erloinib and cisplatin are arresting cancerous cells in G0-G1 phase.[25,26] The same phenomenon is observed in the present study. Most of the cancer types known to date have defects in one or more cell cycle checkpoints. For example, a number of studies[17,27] have shown an implication of p53-mediated induction of WAF1/Cip1/p21, resulting in a G1 cell cycle arrest. The loss of cell cycle checkpoints results in the selection of cells that have a growth advantage and a predisposition for acquiring more chromosome aberrations. This may also result in drug resistance, invasion, and metastasis. The G0-G1 arrest shown by scorpion venom, therefore, suggests that this agent may slow down the growth of cancer cells by artificially imposing the cell cycle checkpoint. Using crude venoms as the therapeutic agent for cancer patients is dangerous. The identified active principle should be tagged with the target specific nanoparticle to deliver the active component directly to the area of cancerous cells.[28]
Conclusion
AB venom is found to inhibit the growth of HCT-8 human colorectal cancer and MDA-MB-231 human breast cancer cells. The venom selectively induces the rate of apoptosis in MDA-MB-231 and HCT-8 cells as reflected by morphological and cell cycle studies. To the best of our knowledge, this is the first scientific evidence demonstrating the induction of apoptosis and cell cycle arrest by AB scorpion venom. The study is in progress to identify the active peptide of this scorpion venom and to decipher its molecular mechanism of induction of cell death.
Financial Support and Sponsorship
Research Center of Prince Sultan Military Medical City Hospital and King Abdulaziz City for Science and Technology (KACST).
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Stewart BW, Wild CP, editors. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 2.Fadeyi SA, Fadeyi OO, Adejumo AA, Okoro C, Myles EL. In vitro anticancer screening of 24 locally used Nigerian medicinal plants. BMC Complement Altern Med. 2013;13:79. doi: 10.1186/1472-6882-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meiyanto E, Hermawan A, Anindyajati Natural products for cancer-targeted therapy: Citrus flavonoids as potent chemopreventive agents. Asian Pac J Cancer Prev. 2012;13:427–36. doi: 10.7314/apjcp.2012.13.2.427. [DOI] [PubMed] [Google Scholar]
- 4.Amin A, Gali-Muhtasib H, Ocker M, Schneider-Stock R. Overview of major classes of plant-derived anticancer drugs. Int J Biomed Sci. 2009;5:1–11. [PMC free article] [PubMed] [Google Scholar]
- 5.Heinen TE, da Veiga AB. Arthropod venoms and cancer. Toxicon. 2011;57:497–511. doi: 10.1016/j.toxicon.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Jain D, Kumar S. Snake venom: A potent anticancer agent. Asian Pac J Cancer Prev. 2012;13:4855–60. doi: 10.7314/apjcp.2012.13.10.4855. [DOI] [PubMed] [Google Scholar]
- 7.Petricevich VL, Navarro LB, Possani LD. Therapeutic use of scorpion venom. Mol Asp Inflamm. 2013;9:209–31. [Google Scholar]
- 8.Al-Asmari A, Khan HA, Manthiri RA. Effect of Androctonus bicolor scorpion venom on serum electrolytes in rats: A 24-h time-course study. Hum Exp Toxicol. 2016;35:293–6. doi: 10.1177/0960327115584688. [DOI] [PubMed] [Google Scholar]
- 9.El-Bitar AM, Sarhan MM, Aoki C, Takahara Y, Komoto M, Deng L, et al. Virocidal activity of Egyptian scorpion venoms against hepatitis C virus. Virol J. 2015;12:47. doi: 10.1186/s12985-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Shi W, Zeng XC, Ge F, Yang M, Nie Y, et al. Unique diversity of the venom peptides from the scorpion Androctonus bicolor revealed by transcriptomic and proteomic analysis. J Proteomics. 2015;128:231–50. doi: 10.1016/j.jprot.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Zeng XC, Zhang L, Nie Y, Shi W, Liu Y. Androcin, a novel type of cysteine-rich venom peptide from Androctonus bicolor, induces akinesia and anxiety-like symptoms in mice. IUBMB Life. 2014;66:277–85. doi: 10.1002/iub.1261. [DOI] [PubMed] [Google Scholar]
- 12.Zargan J, Sajad M, Umar S, Naime M, Ali S, Khan HA. Scorpion (Odontobuthus doriae) venom induces apoptosis and inhibits DNA synthesis in human neuroblastoma cells. Mol Cell Biochem. 2011;348:173–81. doi: 10.1007/s11010-010-0652-x. [DOI] [PubMed] [Google Scholar]
- 13.Borges A, Silva S, Op den Camp HJ, Velasco E, Alvarez M, Alfonzo MJ, et al. In vitro leishmanicidal activity of Tityus discrepans scorpion venom. Parasitol Res. 2006;99:167–73. doi: 10.1007/s00436-006-0133-z. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 15.Syed Abdul Rahman SN, Abdul Wahab N, Abd Malek SN. In vitro morphological assessment of apoptosis induced by antiproliferative constituents from the Rhizomes of Curcuma zedoaria. Evid Based Complement Alternat Med 2013. 2013:257108. doi: 10.1155/2013/257108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarker KP, Obara S, Nakata M, Kitajima I, Maruyama I. Anandamide induces apoptosis of PC-12 cells: Involvement of superoxide and caspase-3. FEBS Lett. 2000;472:39–44. doi: 10.1016/s0014-5793(00)01425-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Q, Huo XC, Sun FD, Dong RQ. Polyphenol-rich extract of Salvia chinensis exhibits anticancer activity in different cancer cell lines, and induces cell cycle arrest at the G0/G1-phase, apoptosis and loss of mitochondrial membrane potential in pancreatic cancer cells. Mol Med Rep. 2015;12:4843–50. doi: 10.3892/mmr.2015.4074. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Gao R, Zhang Y, Gopalakrishnakone P. Purification and N-terminal sequence of a serine proteinase-like protein (BMK-CBP) from the venom of the Chinese scorpion (Buthus martensii Karsch) Toxicon. 2008;52:348–53. doi: 10.1016/j.toxicon.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Zargan J, Sajad M, Umar S, Naime M, Ali S, Khan HA. Scorpion (Androctonus crassicauda) venom limits growth of transformed cells (SH-SY5Y and MCF-7) by cytotoxicity and cell cycle arrest. Exp Mol Pathol. 2011;91:447–54. doi: 10.1016/j.yexmp.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Díaz-García A, Morier-Díaz L, Frión-Herrera Y, Rodríguez-Sánchez H, Caballero-Lorenzo Y, Mendoza-Llanes D, et al. In vitro anticancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013;4:5–12. [PMC free article] [PubMed] [Google Scholar]
- 21.Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis) Cytometry. 1997;27:1–20. [PubMed] [Google Scholar]
- 22.Das Gupta S, Debnath A, Saha A, Giri B, Tripathi G, Vedasiromoni JR, et al. Indian black scorpion (Heterometrus bengalensis Koch) venom induced antiproliferative and apoptogenic activity against human leukemic cell lines U937 and K562. Leuk Res. 2007;31:817–25. doi: 10.1016/j.leukres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Rahman MA, Omran MA, Abdel-Nabi IM, Nassier OA, Schemerhorn BJ. Neurotoxic and cytotoxic effects of venom from different populations of the Egyptian Scorpio maurus palmatus. Toxicon. 2010;55:298–306. doi: 10.1016/j.toxicon.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Alenzi FQ. Links between apoptosis, proliferation and the cell cycle. Br J Biomed Sci. 2004;61:99–102. doi: 10.1080/09674845.2004.11732652. [DOI] [PubMed] [Google Scholar]
- 25.Sutter AP, Höpfner M, Huether A, Maaser K, Scherübl H. Targeting the epidermal growth factor receptor by erlotinib (Tarceva) for the treatment of esophageal cancer. Int J Cancer. 2006;118:1814–22. doi: 10.1002/ijc.21512. [DOI] [PubMed] [Google Scholar]
- 26.Tao C, Lin H, Chen S. The regulation of ERK and p-ERK expression by cisplatin and sorafenib in gastric cancer cells. Gene. 2014;552:106–15. doi: 10.1016/j.gene.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya K, Bag AK, Tripathi R, Samanta SK, Pal BC, Shaha C, et al. Mahanine, a novel mitochondrial complex-III inhibitor induces G0/G1 arrest through redox alteration-mediated DNA damage response and regresses glioblastoma multiforme. Am J Cancer Res. 2014;4:629–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Misra SK, Ye M, Kim S, Pan D. Highly efficient anti-cancer therapy using scorpion ‘NanoVenin’. Chem Commun (Camb) 2014;50:13220–3. doi: 10.1039/c4cc04748f. [DOI] [PubMed] [Google Scholar]


