Abstract
Background:
Diabetes-induced oxidative stress and hypertension play a major role in the development of nephropathy. Hence, the present study was undertaken to evaluate the protective effects of molsidomine, a nitric oxide donor in streptozotocin (STZ)-induced diabetic nephropathy (DN) in rats.
Materials and Methods:
Type 1 diabetes was induced through a single dose of STZ (52 mg/kg, i.p.) in male Wistar rats and then treated with molsidomine (5 and 10 mg/kg; p.o.) for 8 weeks. Physical parameters, vital and renal function test including blood glucose, albuminuria, blood urine nitrogen, serum creatinine, and kidney index were determined. Oxidative stress and lipid peroxidation were assessed in the kidney homogenate by means of antioxidant enzymes and malondialdehyde levels.
Results:
DN rats exhibited a significant renal dysfunction with a reduction in body weight, excessive oxidative stress, and pathological changes. Molsidomine treatment significantly improved vital sign, renal functions, and oxidative stress in DN rats in a dose-dependent manner. The protective effect of molsidomine was also substantiated by pathological changes in the architect of the kidney.
Conclusion:
Molsidomine shows a significant beneficial effect in Type 1 DN in rats.
Keywords: Albuminuria, molsidomine, nitric oxide, oxidative stress
Diabetes-related morbidity and mortality are accounted for its complications such as nephropathy, neuropathy, and retinopathy. Chronic hyperglycemia is the root cause for unique changes in renal structure. It is one of the leading causes of end-stage renal disease. Approximately, 40% of diabetic patients diagnosed with nephropathy either require kidney dialysis or transplantation. Diabetic nephropathy (DN) is characterized by microalbuminuria, renal hypertrophy, and mesangial expansion with glomerular basement membrane thickening.[1] Hypertension, smoking, and dyslipidemia are the major contributors for the progression of nephropathy. As per the UK Prospective Diabetes Study, tight control on systolic blood pressure (SBP) lessened the risk for the development of microalbuminuria.[2]
Streptozotocin (STZ) destroys the beta cells of the pancreas which leads to hyperglycemia. The mechanism for its cell injury is associated with the generation of reactive oxygen species (ROS) and oxidative stress.[3] An STZ-induced rodent model of Type 1 diabetes develops renal injury which resembles to human DN.[4] It is a valuable and broadly accepted model for interpretation of therapeutic interventions in preclinical diabetic renal injury.
Nitric oxide (NO), a potent endogenous vasodilator, has a role in maintaining systemic blood pressure and renal functions. Basal production of NO is crucial for the routine glomerular functions.[5] Its bioactivity is reduced by superoxide, a major ROS.[6] Reduction of antioxidant enzymes in diabetes results in overproduction of ROS which converts NO into peroxynitrite and produces nitrosative stress. Iqbal et al. have shown that exogenous NO donor administration, prevents the oxidative stress, and restores the renal functions.[7]
Molsidomine, an NO donor, has antioxidant and vasodilator effects. It is used for the treatment of stable angina. Molsidomine relaxes vascular smooth muscle by stimulating guanylate cyclase.[8] The purpose of the present study was to investigate whether NO donor can attenuate DN in STZ-induced diabetic rats.
Materials and Methods
Chemicals and Reagents
Molsidomine was procured as a gift sample from Taj Pharmaceutical, Mumbai, with quality control report. STZ was purchased from the MP Biomedical India Pvt. Ltd. The kits used for biochemical analysis were procured from the Autospan diagnostic, Surat, India. Other chemicals and reagents used were of analytical grade.
Animals
Healthy weight-matched (250–300 g) male Wistar rats were selected and housed in polypropylene cages layered with husk and kept for 12 h light/12 h in dark cycle with free access to water and standard pellet diet. The use of experimental animals was approved by the Institutional Animal Ethical Committee of Al-Ameen College of Pharmacy (Approval No: AACP/IAEC/Aug 2014/01). The experiment was conducted according to the CPCSEA guidelines.
Induction of Diabetes and Experimental Design
Type 1 diabetes was induced by a single intraperitoneal injection of STZ (52 mg/kg) in freshly prepared citrate buffer (0.1 M, pH 4.5).[9] Rats were given 5% w/v glucose solution for 48 h to prevent hypoglycemia-induced mortality. A week later, the rats with fasting blood glucose (FBG) values ≥230 mg/dL were considered to be diabetic and used in the study. Total 24 Wistar rats were randomly divided into four groups of six animals each.
Group I served as normal control.
Group II served as DN control.
Group III and IV served as a test group, (diabetic rats were administered molsidomine [5 and 10 mg/kg, p.o.] for 8 weeks).
Doses of molsidomine were chosen based on the previous study in which it has shown the nephroprotective effect and did not produce any side effects.[10]
Measurement of Physical Parameter
The animals were monitored for physical parameters such as body weight (BW), weakness, and mortality throughout the study. BW was measured before induction of diabetes and at the 8th week using digital balance (Essae® DS-252). Percentage change was calculated from the BW of beginning and at the end of the study.
Measurement of Vital Signs
Progression of DN is strongly associated with hypertension. Hence, SBP was measured at the 8th week of the study by tail cuff method.[11,12] Each measurement was repeated 5–6 times and values, not different by more than 3 mmHg, were considered valid. The mean of the three measurements was then recorded and reported as the value of SBP for each animal.
Measurement of Renal Function
On the 8th week, an individual rat from each group was placed in metabolic cages for 24 h to collect urine with free access to food and water; urine volume (ml/24 h) was measured. Urinary albumin levels were detected using assay kits by autoanalyzer according to the manual provided by the manufacturer. Urinary albumin excretion rate (UAER), an indication of albuminuria, was calculated by the formula, UAER (mg/24 h) = 24 h urine volume × urinary albumin (mg/dl). Overnight-fasted animals were anesthetized; blood was withdrawn through retro-orbital and collected in clot activator Vacutainer then centrifuged at 3000 RPM for 10 min to separate the serum. FBG, blood urine nitrogen, and serum creatinine (Scr) were determined by autoanalyzer. All kits were used in accordance with manufacturer instructions. Then, animals were sacrificed, and their kidneys were isolated and weighed. The kidney index was calculated as 100 × kidney weight/BW. Right kidneys were used for homogenate preparation and left kidneys were stored in formalin for histopathology study.
Measurement of Oxidative Stress
Kidneys were washed with saline, chopped on ice, and homogenate (10%, w/v) was prepared with 0.1 M phosphate buffer and centrifuged at 3000 g for 10 min at 4°C using Sorvall refrigerated centrifuge and supernatant was used for biochemical estimations.
Lipid peroxidation or malondialdehyde (MDA) formation was determined by the method of Slater and Sawyer.[13] Catalase (CAT) activity was detected by the method of Aebi's description[14] and glutathione (GSH) was determined by the method of Moron et al.[15] superoxide dismutase (SOD) was determined by the method of Misra and Fridovich.[16]
Histopathology
Paraffin-embedded specimens were cut into 5 μm thick sections and stained with hematoxylin and eosin. The sections were examined under a light microscope for the presence of pathological changes and photomicrographs were taken. The pathologist was blinded to the animal treatment group.
Statistical Analysis
Values are expressed as mean ± standard error of mean (SEM). Statistical significance with respect to DN control was evaluated using one-way ANOVA followed by Tukey's t-test using Graph Pad Prism software (Version 5.0, Graph Pad Prism Software Inc., San Diego, CA).
Results
Effect of Molsidomine on Physical Parameter
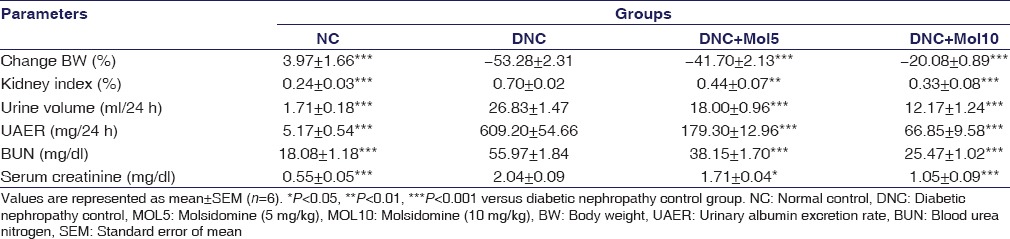
In the present study, there was a continuous reduction in BW of DN rats throughout the study, while there was a gain in the BW of normal rats. At the 8th week, the percentage change in BW of diabetic and normal rats was −53.28 and 3.97, respectively. Treatment with molsidomine significantly (P < 0.001) prevented the percentage change in BW dose dependently compared to diabetic rats. Molsidomine prevented the diabetic rats from vast BW loss [Table 1].
Table 1.
Effect of molsidomine on in body weight, kidney index, and renal function-related parameters in rats
Effect of Molsidomine on Vital Function
At the 8th week, SBP of DN rats was 30 mmHg higher than that of the normal control rats. Molsidomine-treated rats significantly (P < 0.001) reduced the elevated blood pressure level dose dependently compared to diabetic rats [Figure 1].
Figure 1.
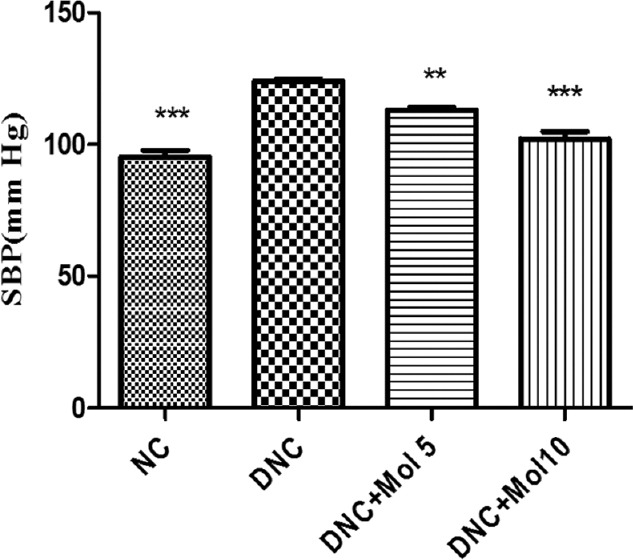
Effect of molsidomine on systolic blood pressure (mmHg) in rats. NC - Normal control, DNC = Diabetic nephropathy control, MOL5 = Molsidomine (5 mg/kg), MOL10 = Molsidomine (10 mg/kg). Values are represented as mean ± standard error of the mean (n = 6). ***P < 0.001 versus diabetic nephropathy control group
Effect of Molsidomine on Renal Functions
The FBG level in the DN control rats was 330 mg/dL, nearly 3 times higher compared to normal control rats. By contrast, molsidomine-treated rats significantly decreased the elevated fasting serum glucose level (P < 0.001) compared to diabetic rats [Figure 2].
Figure 2.
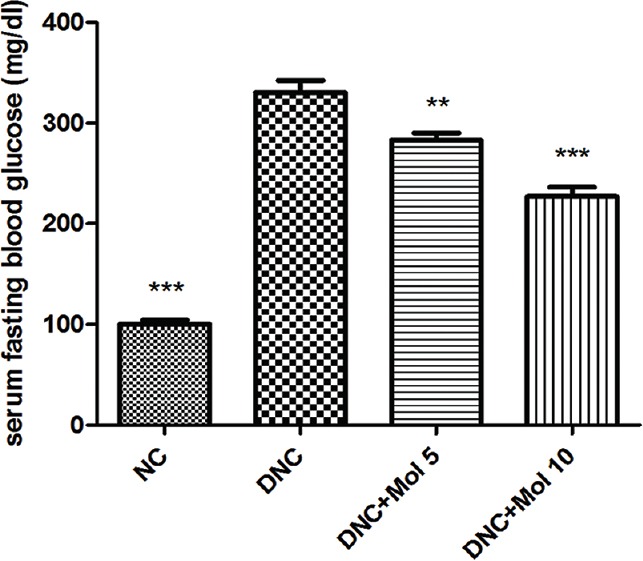
Effect of molsidomine (5 and 10 mg/kg, p.o.) on fasting blood glucose level in rats. (mg/dl). NC = Normal control, DNC = Diabetic nephropathy control, MOL5 = Molsidomine (5 mg/kg), MOL10 = Molsidomine (10 mg/kg). Values are represented as mean ± standard error of the mean (n = 6). **P < 0.01, ***P < 0.001 versus diabetic nephropathy control group
In DN rats, all mentioned renal parameters were significantly (P < 0.001) increased compared to normal control rats, showing that diabetic rats were progressing to nephropathy. As depicted in Table 1, treatment with molsidomine prevented diabetes-induced renal abnormality compared to diabetic control rats in dose-dependent manner by preserving its normal functions.
Effect of Molsidomine on Oxidative Stress and Lipid Peroxidation
DN control rats exhibited a significant (P < 0.001) decrease in SOD, reduced glutathione and CAT activity [Figure 3] compared to normal control rats.
Figure 3.
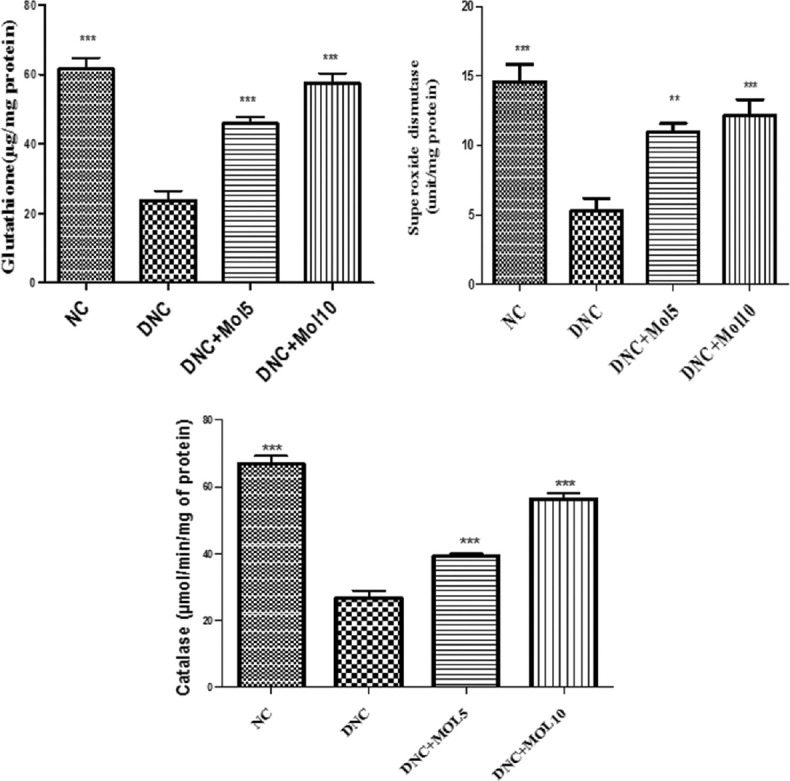
Effect of molsidomine on renal antioxidant enzymes in rats. NC = Normal control, DNC = Diabetic nephropathy control, MOL5 = Molsidomine (5 mg/kg), MOL10 = Molsidomine (10 mg/kg). Values are represented as mean ± standard error of the mean (n = 6). ***P < 0.001 versus diabetic nephropathy control group
The MDA level which is the end product of lipid peroxidation was increased significantly in kidney homogenate of diabetic rats (P < 0.001) when compared to normal control rats. Molsidomine-treated rats significantly reduced MDA level in dose-dependent manner compared to DN rats [Figure 4].
Figure 4.
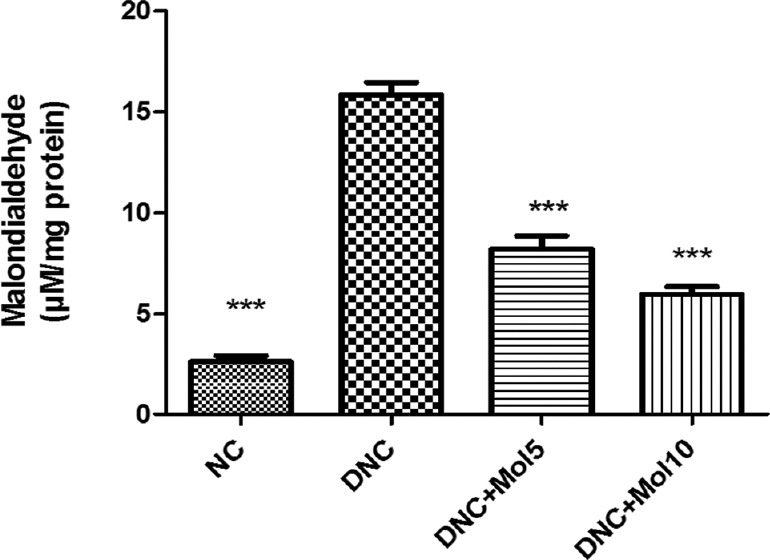
Effect of molsidomine on renal malondialdehyde (μM/mg protein). NC = Normal control, DNC = Diabetic nephropathy control, MOL5 = Molsidomine (5 mg/kg), MOL10 = Molsidomine (10 mg/kg). Values are represented as mean ± standard error of the mean (n = 6). ***P < 0.001 versus diabetic nephropathy control group
Effect of Molsidomine on Renal Pathological Changes
The histopathological examination of kidney tissue of normal rats showed the normal appearance. Renal tissue section of DN control rats showed glomerulosclerosis, tubular degeneration, mononuclear cell (MNC) infiltration, and thickening of glomerular basement membrane. The treatment with Mol 5 showed a moderate glomerular necrosis and MNC infiltration. However, the treatment with Mol 10 showed no abnormality and the architect of kidney tissue appeared normal [Figure 5a–d].
Figure 5.
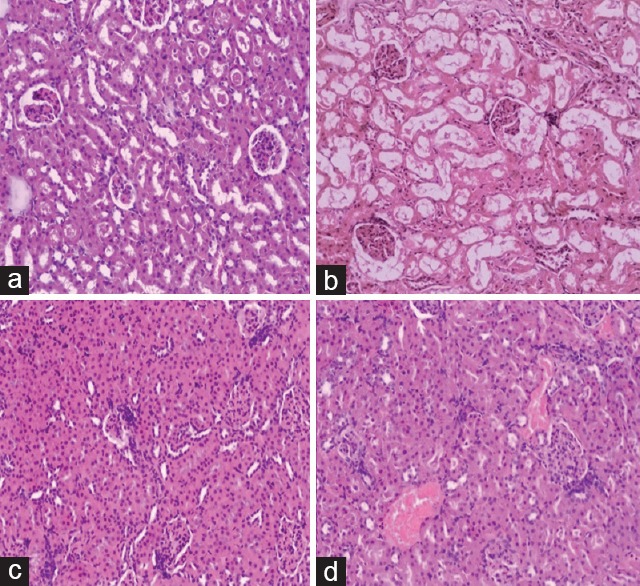
Histopathology of kidney tissue of normal control, diabetic nephropathy control, and treated groups rats. (a) Section of normal rat kidney tissue showing normal appearance of glomerulus and tubules. (b) Section of diabetic rat kidney tissue showing glomerulosclerosis, tubular degeneration, and mononuclear cell infiltration. (c) Section of diabetic rat kidney tissue treated with mol 5 mg/kg showing moderate glomerular necrosis and mononuclear cell infiltration. (d) Section of diabetic rat kidney tissue treated with mol 10 mg/kg showing no abnormality (×10)
Discussion
DN is one of the leading causes of morbidity and mortality in diabetic patients globally. Preventing the progression of DN has been a challenge in biomedical research. Hypertension is a risk factor for progression of DN. Reduction of blood pressure is beneficial in preventing progression of DN. On other hand, many studies have shown that oxidative stress plays a major role in the development diabetic renal injuries. Molsidomine (NO donor) has antioxidative and vasodilator effects. Hence, the present study was undertaken to evaluate the effects of molsidomine in DN.
STZ-induced diabetic rats are more liable to develop hypertension.[17] Elevated blood pressure has a damaging effect on kidney. In the present study, the SBP was significantly higher (P < 0.001) in DN group compared to the normal group, and this rise was prevented by molsidomine treatment. The antihypertensive effect of molsidomine is the major intervention in the prevention of albuminuria which is due to its vasodilatation effect.
Microalbuminuria (defined as UAER 30–300 mg/day) is considered a predictor of worse outcomes for both kidney and heart patients.[18] High blood pressure may cause microalbuminuria by increasing glomerular filtration pressure and subsequent renal damage. The findings of our study revealed that treatment with molsidomine has significantly prevented microalbuminuria in the diabetic group.
Insulin deficiency in Type 1 diabetes prevents the entry of glucose into the cells. As a result, cells start starvation and loss of BW occurs. BW was observed in concurrence with the continuation of diabetes in the study. Molsidomine treatment prevented massive loss of BW.
Several studies have shown that multiple factors caused by hyperglycemia play a role in the development of diabetic kidney disease. The hyperglycemic condition results in irreversible tissue damage through the protein glycation reaction that leads to the formation of glycosylated protein and advanced glycation end product.[19,20] In the present study, molsidomine has prevented the hyperglycemia of diabetic rats which shows its role in nephroprotection.
In our study, STZ-induced diabetic rats successfully developed DN which was evidenced by polyurea, albuminuria, and increased in metabolic wastes in the blood. Scr is considered as a marker of altered glomerular filtration rate (GFR) in DN.[21] However, molsidomine treatment showed a significant decrease in urine volume, albuminuria, and also improved the GFR. These results are in accordance with the recent study in which it had shown that molsidomine has a beneficial effect in cisplatin-induced nephrotoxicity.[22] On the other hand, it has been reported that protective effect of molsidomine in iron-induced nephrotoxicity is due to its NO-donating property,[10] as NO play an important role in maintaining normal physiology of kidney.
Oxidative stress has a link in the pathogenesis of diabetic complications, including DN. Hyperglycemia-induced activation of several pathways results in the excessive formation ROS that is toxic to the cell. They also interact with the lipid bilayer and produce lipid peroxidation product like MDA which further damages the cells. SOD, CAT, and GSH are responsible for the detoxification of the ROS.[23] Dugan et al.[24] revealed that mitochondrial-derived superoxide anion production is reduced in diabetes and plays a pivotal role in preserving renal function during hyperglycemia. In our study, the ability of molsidomine to increase renal antioxidant enzyme level is in line with the finding of Chander and Chopra[8] who found that molsidomine restored the depleted renal antioxidant enzyme level in ischemia-reperfusion renal injury. They also suggested that the beneficial antioxidant effect of molsidomine is due to the decreasing neutrophil infiltration and reducing plasma levels of pro-inflammatory mediators, which increase oxidative stress and the severity of inflammation process. Attia et al.[25] found that NO deficiency resulted in podocyte stress in hypercholesterolemic rats, while molsidomine prevented podocyte injury suggesting its additional role in our study for its nephroprotective action. The histopathology results obtained from the study further confirmed our biochemical findings. Along with improving renal function, molsidomine prevented diabetes-induced tubular degeneration, and glomerulosclerosis. However, further research is required to investigate its molecular mechanism and its comparative efficacy to the available treatment of DN.
Conclusion
Treatment with molsidomine prevented renal dysfunction dose dependently in diabetic rats. Nephroprotective effect of molsidomine is due to its antihypertensive, antihyperglycemic, and antioxidant effect. Molsidomine may be promising as a drug for delaying and preventing Type 1 DN in rats.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Parving HH, Osterby R, Ritz E. Diabetic nephropathy. In: Brenner BM, editor. The Kidney. 6th ed. Philadelphia: WB Saunders; 2000. pp. 1731–73. [Google Scholar]
- 2.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 3.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 4.Tesch GH, Allen TJ. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology (Carlton) 2007;12:261–6. doi: 10.1111/j.1440-1797.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 5.Waz WR, Van Liew JB, Feld LG. Nitric oxide metabolism following unilateral renal ischemia/reperfusion injury in rats. Pediatr Nephrol. 1998;12:26–9. doi: 10.1007/s004670050397. [DOI] [PubMed] [Google Scholar]
- 6.Araujo M, Welch WJ. Oxidative stress and nitric oxide in kidney function. Curr Opin Nephrol Hypertens. 2006;15:72–7. doi: 10.1097/01.mnh.0000191912.65281.e9. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal M, Okazaki Y, Sharma SD, Okada S. Nitroglycerin, a nitric oxide generator attenuates ferric nitrilotriacetate-induced renal oxidative stress, hyperproliferative response and necrosis in ddY mice. Biochim Biophys Acta. 2003;1623:98–108. doi: 10.1016/j.bbagen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Chander V, Chopra K. Renal protective effect of molsidomine and L-arginine in ischemia-reperfusion induced injury in rats. J Surg Res. 2005;128:132–9. doi: 10.1016/j.jss.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Kiran G, Nandini CD, Ramesh HP, Salimath PV. Progression of early phase diabetic nephropathy in streptozotocin-induced diabetic rats: Evaluation of various kidney-related parameters. Indian J Exp Biol. 2012;50:133–40. [PubMed] [Google Scholar]
- 10.Gupta A, Sharma S, Chopra K. Reversal of iron-induced nephrotoxicity in rats by molsidomine, a nitric oxide donor. Food Chem Toxicol. 2008;46:537–43. doi: 10.1016/j.fct.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 11.NIBP Controller- owner's guide: AD Instruments, Australia. www.adinstruments.com/products/manuals/NIBP_Controller_OG.pdf .
- 12.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall J. Recommendations for blood pressure measurement in experimental animals. A statement for professionals from the subcommittee of professional & public education of the American heart association council on blood pressure research. Circulation. 2005;111:697–716. [Google Scholar]
- 13.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro.General features of the systems used. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 15.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 16.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 17.Browne D, Meeking D, Shaw K, Cummings M. Endothelial dysfunction and pre-symptomatic atherosclerosis in type 1 diabetes – Pathogenesis and identification. Br J Diabetes Vasc Dis. 2003;3:27–34. [Google Scholar]
- 18.Guerrero-Analco JA, Hersch-Martínez P, Pedraza-Chaverri J, Navarrete A, Mata R. Antihyperglycemic effect of constituents from Hintonia standleyana in streptozotocin-induced diabetic rats. Planta Med. 2005;71:1099–105. doi: 10.1055/s-2005-873137. [DOI] [PubMed] [Google Scholar]
- 19.Cooper ME, Gilbert RE, Epstein M. Pathophysiology of diabetic nephropathy. Metabolism. 1998;47(12 Suppl 1):3–6. doi: 10.1016/s0026-0495(98)90362-6. [DOI] [PubMed] [Google Scholar]
- 20.Yabe-Nishimura C. Aldose reductase in glucose toxicity: A potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–33. [PubMed] [Google Scholar]
- 21.Falk RJ, Scheinman JI, Mauer SM, Michael AF. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983;32(Suppl 2):34–9. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- 22.Karakoc HT, Altintas R, Parlakpinar H, Polat A, Samdanci E, Sagir M, et al. Protective effects of molsidomine against cisplatin-induced nephrotoxicity. Adv Clin Exp Med. 2015;24:585–93. doi: 10.17219/acem/47742. [DOI] [PubMed] [Google Scholar]
- 23.Ugochukwu NH, Cobourne MK. Modification of renal oxidative stress and lipid peroxidation in streptozotocin-induced diabetic rats treated with extracts from Gongronema latifolium leaves. Clin Chim Acta. 2003;336:73–81. doi: 10.1016/s0009-8981(03)00325-5. [DOI] [PubMed] [Google Scholar]
- 24.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, DeCleves AE, et al. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–99. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attia DM, Feron O, Goldschmeding R, Radermakers LH, Vaziri ND, Boer P, et al. Hypercholesterolemia in rats induces podocyte stress and decreases renal cortical nitric oxide synthesis via an angiotensin II type 1 receptor-sensitive mechanism. J Am Soc Nephrol. 2004;15:949–57. doi: 10.1097/01.asn.0000118528.00817.8e. [DOI] [PubMed] [Google Scholar]


