Abstract
Objective:
The current study was designed to explore anxiolytic, antidepressant, and antistress actions of Cinnamomum tamala (CT) leaves (aqueous extract) in rats.
Materials and Methods:
Behavioral procedures of anxiety, depression, and stress were assessed in rats. CT (100, 200, and 400 mg/kg) was given once a daily for 7 days via oral route and the efficacy was matched by those elicited by lorazepam (1 mg/kg, p.o.), imipramine (10 mg/kg, p.o.), and Withania somnifera (100 mg/kg, p.o.) for anxiolytic, antidepressant, and antistress studies, respectively. Standard drugs were given 1 time, 30 min preceding the behavioral trials.
Results:
One-way analysis of variance followed by Newman–Keuls multiple comparison test was employed to analyze the results. P < 0.05 was considered statistically significant as compared to control. CT at 400 mg/kg produced an antianxiety effect equivalent to lorazepam, in the elevated plus maze, open field, and social interaction tests among selected doses of the CT. CT at 400 mg/kg also induced an antidepressant activity similar to imipramine, in the behavioral despair, learned helplessness test, and tail suspension among selected doses of the CT. Moreover, CT at 400 mg/kg produced a significant antistress effect comparable to W. somnifera in water immersion-restraint stress by decreasing ulcer index, adrenal gland weight, and by normalizing the plasma levels of corticosterone, glucose, cholesterol, and triglyceride levels when related to stress control.
Conclusion:
The study shows that among the different CT doses, CT at 400 mg/kg possesses significant anxiolytic, antidepressant, and anti-stress effects and has therapeutic beneficial for the management of psychological ailments.
Keywords: Antidepressant, antistress, anxiolytic, Cinnamomum tamala, ulcer index, Withania somnifera
Anxiety, depression, and stress are the most predominant psychological diseases globally. The estimated range of their occurrence among adolescents across the globe is from 5% to 70%.[1] Implausibly, women are more affected by this unwellness rather than men.[2] Currently, antianxiety, antidepressants, and antistress drugs are the only choice of treatment for these maladies. Regardless of therapeutic benefits, these drugs also cause unusual adverse effects which ensue patient's noncompliance. The drugs derived from natural origin barely have side effects and moreover, are economically cheaper.
Plants are considered as a wealthy source of safe and effective medicines for a long time. India has a great history of herbal medicine practice such as Ayurveda, Unani, Siddha, Homeopathy, and Naturopathy. Even today, many rural and tribal populations in India are relying upon the natural plant wealth for healing and mitigation of certain diseases. It was already evidenced that traditional herbal medicine could provide a novel treatment for affective and other central nervous system (CNS) disorders.[3]
Cinnamomum tamala (CT) Nees and Eberm or Indian Cassia, also known as tejpat (in Hindi), is an evergreen tree with a height of 8 m, moderate sized within the Lauraceae family. Natural habitat includes the tropical and the subtropical Himalayas and Khasi and Jaintia hills. The leaves are usually called “bay leaves,” often used as a traditional spice in cooking because of their aromatic, carminative, and antiflatulent properties. Till date, the CT leaves have been evidenced and reported to exert antidiabetic,[4] antioxidant,[4] antihyperlipidemic,[5] and gastroprotective[6] activities.
The literature survey suggests that the neuropharmacological effects of CT were not studied at the time of conceptualization of this investigation. Therefore, we proposed to explore the antidepressant, anxiolytic, and antistress activity of the CT leaves extract on rodent models and compared the efficacy to standard drugs.
Materials and Methods
Animals
Adult Wistar albino rats of either sex weighing 180 ± 20 g were housed in polypropylene cages in groups of five (evenly matched with male and female rats) and maintained under conditions of temperature (25°C ± 1°C) and relative humidity (50% ± 5%), under a standard light/dark cycle of 12 h. The animals were provided with a standard pellet diet (“Amrut” brand, M/s. Nav Maharashtra Chakan oil mills Ltd.; Pune, India) and water ad libitum. Experiments were performed between 09.00 A.M. and 02.00 P.M. Guidelines of laboratory animal care (NIH publication number # 85–23, revised in 1985) were exercised. All studies were carried out in accordance with the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments on Animals, India. The experimental protocol was approved by the Central Animal Ethical Committee (No. Dean/10-11/352).
Plant Material and Preparation of the Extract
Fresh leaves of CT Nees and Eberm (Family-Lauraceae) were gathered in August month from Lucknow region, India, and they were taxonomically identified and authenticated. The leaves were shade dried and powdered with a mechanical grinder and preserved in an airtight vessel. The dried powder material was defatted with petroleum ether and later extracted with distilled water by hot maceration method. The solvent was wholly evaporated, and 9% yield of aqueous extract of CT was acquired. The final yield was kept at 4°C in an airtight vessel till needed. Fresh solutions of CT were prepared in distilled water and used for the investigation.
Drugs
Imipramine (standard antidepressant drug) was a gift sample from Sun Pharmaceutical Industries Ltd., India. Lorazepam (reference standard for antianxiety effect) was provided by Intas Pharmaceutical Ltd., India, as a gift sample. All chemicals used in the present study were of analytical grade and were obtained from Sigma Chemical Co. and Merck. Withania somnifera, herbal adaptogen, was used as a standard for antistress activity[7] and was obtained from the Department of Dravyaguna, Faculty of Ayurveda, IMS, Banaras Hindu University, Varanasi, India.
Experimental Protocol
The animals were housed for 1 week in a laboratory room for acclimatization. They were grouped into five, containing six animals each group.
Group I: Control (10 mL/kg/p.o.)
Group II: CT 100 mg/kg/p.o.
Group III: CT 200 mg/kg/p.o.
Group IV: CT 400 mg/kg/p.o.
Group V: Standard drugs (lorazepam 1 mg/kg, imipramine 10 mg/kg, and W. somnifera 100 mg/kg in the case of anxiolytic, antidepressant, and antistress activities, respectively).
In addition, stress control group was included in the antistress activity study design to investigate the influence of stress on neurochemical and histopathological changes compared to normal rats.
CT dose was selected on the basis of randomization and was administered in distilled water to Groups (II, III, and IV) at 100, 200, and 400 mg/kg orally, once in a day for 7 days. The control group (Group I) received an equal volume of distilled water. Standard drugs were also employed to Group V accordingly in each set of protocol and were dispensed orally to animals 1 h before the experiments. On day 7, animals were subjected to behavioral studies.
Behavioral Evaluation
Anxiolytic activity
Elevated plus maze
apparatus for elevated plus-maze test contained 2 open arms (50 cm × 10 cm × 40 cm) and 2 closed arms (50 cm × 10 cm × 40 cm), for rats facing each other with an open roof that extends from a common central platform (10 cm × 10 cm). The maze was kept in a dimly lit room and elevated at a height of 50 cm above the ground level. The rats were positioned in the center of the maze independently, facing one of the closed arms. Thereafter, a number of entries and time spent on the open and closed arms were documented throughout the 5 min observation period. An arm entry was defined when four paws of the rats were inside the arm. A neutral “blind” observer made observation.[8]
Open field test
The open field test apparatus was built of plywood (60 cm × 60 cm ×60 cm) and was painted black. White marks of 6 mm wide divide the floor into sixteen squares (15 cm × 15 cm). The open field was lit with 16 W bulb focusing onto the arena from a height of 100 cm excluding the open field; the whole room was kept dark throughout the experiment. Animals were positioned individually at the center of the test apparatus for 5 min, and the following behavioral aspects were observed:[9]
Ambulation: Assessed in terms of the number of squares traversed by the rat
Rearings: Measured in terms of the number of times the rat raised on its rear limbs
Self-grooming: Measured in terms of the number of times the rat cleaned facial region and licked different body parts
Activity in center: Measured in terms of the number of central squares traversed by the rat
Fecal dropping: Measured in terms of the number of fecal droppings excreted during the observation.
Social interaction test
rodents were individually accommodated for 5 days prior investigation. The test device consists of a wooden box (60 cm × 60 cm × 35 cm) located in a faintly illuminated room. On the 6th day, the rodents were individually positioned in the wooden box and offered two 7.5 min familiarization sessions at 2 h time period. On the 7th day, based on sex and weight, rodents were paired and retained in the apparatus for 7.5 min. The total time devoted by the rodent pair in “social interaction,” included sniffing, biting, grooming, boxing, kicking, and crawling under or over the partner, was documented during 7.5 min time, by a neutral blind observer.[10]
Antidepressant activity
Behavioral despair test
Each animal was restrained in a cylinder (45 cm × 20 cm) filled with water at a height of 38 cm (25°C ± 2°C) so that it could not contact with the bottom of the cylinder with its rear paws or mount over the edge of the cylinder. Dual swim sessions were performed, an early 15 min pretest followed by a 5 min test after 24 h of drug administration. During the test session, the immobility period characterized by absolute cessation of swimming and performing necessary movement's essential to place its head beyond the aquatic level was observed.[11]
Tail suspension test
Each rat in the group was hanged by the tail (50 cm above the floor) with an adhesive tape to a cord in an upside down position so that its nostrils touch the water surface in a vessel. After the early escape-oriented actions, the rat rapidly turns out to be immobile, and the immobility period (the absence of initiating movements and includes passive swaying) was recorded during 5 min observation period.[12]
Learned helplessness test
Rats were exposed to a shock of 0.7 mA for 10 s every minute for 1 h. The gadget was a box with dimensions of 30 cm × 45 cm × 30 cm having grid floor. At an altitude of 20 cm above the base, a platform (7.5 cm × 7.5 cm) was introduced from one side wall to permit a jump-up escape reaction. The platform was not provided for the whole period of training. After the proper treatment, the rats were allowed for acquisition of a jump-up response. At the beginning of a trial, the platform was provided in the box, and a current of 0.7 mA was introduced. The shock was ceased in 10 s if the rat could not escape onto the platform within the specified period. If an escape retort ensued, the rat was permitted to stand on the platform for the length of 10 s, and then reverted to the base.[13]
Antistress activity
Water immersion-restraint stress
The rats were deprived of food for 24 h before the stress application. Rats were restrained in iron net and submerged upright to the level of the xiphoid in a water bath maintained at 20°C, for 3.5 h.[14] At the end of the experiment, 5 mL of blood was collected into centrifuge tubes containing heparin (10 μL, 1000 IU/mL) by cardiac puncture after anesthetizing animals with diethyl ether. All animals were sacrificed by cervical dislocation. Then, their stomachs were isolated and expanded slightly 10 min after injecting 15% formalin. Later, the stomachs were incised alongside the superior curvature and ulcer scoring was done using dissecting microscope bearing square grid-eye piece. The isolated stomachs were held in 15% formalin solution and then forwarded for histopathological scrutiny. Adrenal glands were collected and their weights were recorded as well.
Biochemical Parameters
Collection of samples
For biochemical estimation, blood was collected in heparin spilt Eppendorf tubes by cardiac puncture before sacrificing the rats. The collected blood was centrifuged at 4°C and 4000 rpm, for 15 min, to separate plasma. Thus, separated plasma was stored in −80°C till the day of biochemical estimation. Animals were sacrificed and adrenal glands were gathered, weighed, and preserved at −80°C.
Estimation of plasma corticosterone
Rat plasma corticosterone levels were measured by high-performance liquid chromatography (HPLC)/ultraviolet (Waters, USA) according to Woodward and Emery[15] with slight changes. Dexamethasone was used as an internal standard. Five hundred microliters of plasma comprising a 50 μL of dexamethasone was extracted with 5 mL of dichloromethane. The dichloromethane extract was allowed to dry and dissolved in 100 μL of the mobile phase. Twenty microliters of the sample was inserted into the HPLC for quantification purpose. Mobile phase contained methanol and water in the ratio of 70:30. The flow rate was 1.0 mL/min and analytical column used was Waters Spherisorb® C 18 (250 mm × 4.6 mm, 5 μm). Plasma corticosterone was identified at 250 nm wavelength by photodiode array detector (Model 2998, Waters, USA). The chromatogram was documented and evaluated by “Empower” software.
Estimation of plasma glucose
Plasma glucose was estimated by autoanalyzer (BioMasteR touch screen biochemistry analyzer, Qualisystems, Fisher Scientific, Mumbai) employing commercially available diagnostic kit (Span Diagnostics Ltd., Surat, India) by glucose oxidase–peroxidase method.
Estimation of plasma cholesterol
Plasma cholesterol was estimated by autoanalyzer (BioMasteR touch screen biochemistry analyzer, Qualisystems, Fisher Scientific, Mumbai) using the commercially available diagnostic kit (Span Diagnostics Ltd., Surat, India).
Estimation of plasma triglycerides
Plasma triglyceride level was estimated by autoanalyzer (BioMasteR touch screen biochemistry analyzer, Qualisystems, Fisher Scientific, Mumbai) using the commercially available diagnostic kit (Span Diagnostics Ltd., Surat, India).
Statistical Analysis
Results of this study were statistically analyzed by GraphPad Prism 5 software. The data were expressed as a mean ± standard error of mean. The data from various groups were statistically analyzed using one-way analysis of variance, followed by post hoc Tukey's multiple comparisons test. P < 0.05 was considered statistically significant.
Results
Effect of the Cinnamomum tamala Extract on Elevated Plus Maze
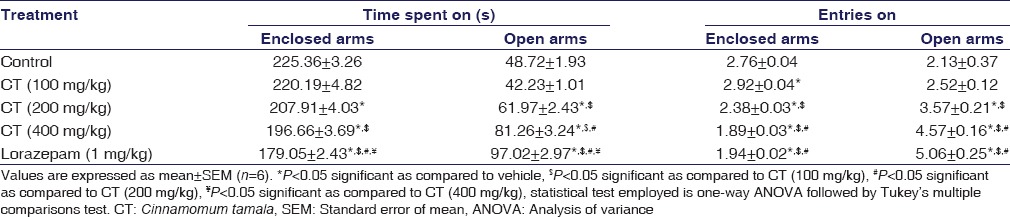
CT (200 and 400 mg/kg) treated rats exhibited a significant increase in entries made (P = 0.0021, P < 0.001) and time spent in open arms (P = 0.0063, P < 0.001) except at 100 mg/kg (P > 0.05). CT-treated rats exhibited a significant decrease in entries made (P < 0.001, P < 0.001) and time spent (P = 0.0218, P < 0.001) in enclosed arms at 200 and 400 mg/kg, respectively. Lorazepam (P < 0.001) also produced significant anxiolysis, and its effect was qualitatively comparable to that of CT. The results were summarized in Table 1.
Table 1.
Effect of the Cinnamomum tamala extract on elevated plus-maze test in rats
Effect of the Cinnamomum tamala Extract on Open Field Test
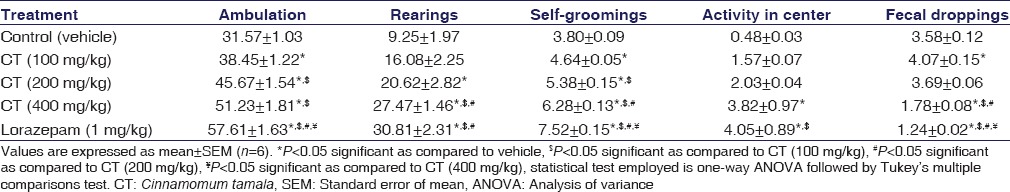
Rats treated with doses (100, 200, and 400 mg/kg) of CT showed a significant increase in open field ambulation (P = 0.0221, P < 0.001, and P < 0.001), rearing (P > 0.05, P < 0.001, and P < 0.001), activity in center (P > 0.05, P > 0.05, and P = 0.0041), and self-grooming (P < 0.001, P < 0.001, and P < 0.001) when compared to vehicle-treated rats and produced comparable activity with lorazepam; indicating anxiolytic activity of CT. Simultaneously, a significant decrease in fecal droppings was observed at all doses of CT, except at 200 mg/kg (P > 0.05). Lorazepam (P < 0.001) also induced significant anxiolytic activity and its effect was also found to be qualitatively comparable to that of CT. The results were summarized in Table 2.
Table 2.
Effect of the Cinnamomum tamala extract on open field test in rats
Effect of the Cinnamomum tamala Extract on Social Interaction Test
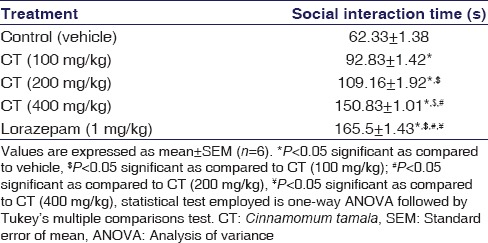
Rats treated with CT spent significantly more time in social interaction as compared to control rats in a dose-dependent wise (P < 0.001). Similarly, lorazepam-treated group exhibited a significant increase in social interaction (P < 0.001) in rats and its effect was comparable to CT at all doses. The results were shown in Table 3.
Table 3.
Effect of the Cinnamomum tamala extract on social interaction test in rats
Effect of the Cinnamomum tamala Extract on Behavioral Despair Test
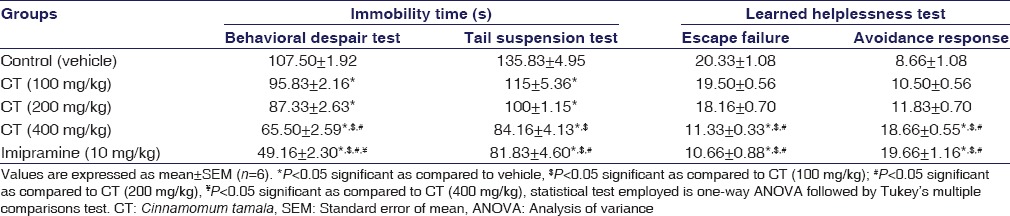
CT at 100 (P = 0.013), 200 (P < 0.001), and 400 mg/kg (P < 0.001) treated rats caused a significant dose-dependent decrease in immobility time. Imipramine also showed similar activity and effects were qualitatively comparable to that of CT (P < 0.001). The results were summarized in Table 4.
Table 4.
Effect of the Cinnamomum tamala extract on behavioral despair test, tail suspension test, and learned helplessness test in rats
Effect of the Cinnamomum tamala Extract on Tail Suspension Test in Rats
CT at 100 (P = 0.017), 200 (P < 0.001), and 400 mg/kg (P < 0.001) treated rats significantly reduced the immobility time dose-dependently, and CT at 400 mg/kg (P > 0.05) produced the equivalent effect as that of imipramine, a well-established antidepressant drug, which showed a significant reduction in immobility time. The results were summarized in Table 4.
Effect of the Cinnamomum tamala Extract on Learned Helplessness Test
The escape failure significantly decreased in rats treated with the dose of CT 400 mg/kg (P < 0.001). In addition, the number of avoidance response also significantly increased, respectively, with the same dose of CT (P < 0.001). Imipramine (P < 0.001) also showed significant reduction of learned helplessness and its effect was qualitatively comparable to that of CT at 400 mg/kg. The results were summarized in Table 4.
Effect of the Cinnamomum tamala Extract on the Ulcer Index in Water-immersion Restraint Stress Test
Stress increases the ulcer index (P < 0.001) and treatment with CT at 200 mg/kg (P < 0.001) and 400 mg/kg (P < 0.001) significantly decreases the ulcer index through its antistress effect. W. somnifera at 100 mg/kg significantly decreased the ulcer index (P < 0.001). The results were shown in Table 5.
Table 5.
Effect of the Cinnamomum tamala extract on the ulcer index, adrenal gland weight, plasma corticosterone, glucose, cholesterol, and triglycerides in rats
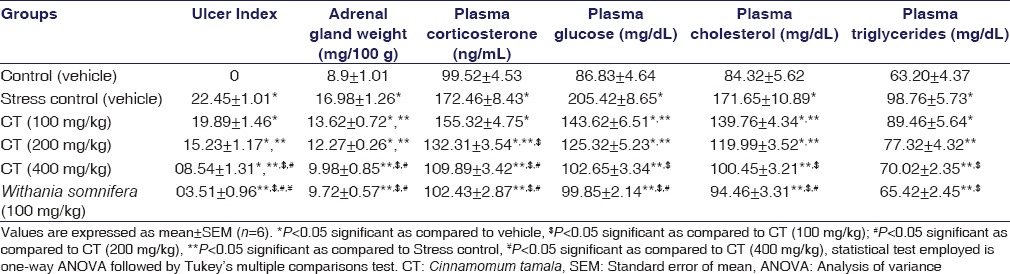
Effect of the Cinnamomum tamala on the Weight of the Adrenal Gland and Plasma Corticosterone Levels
Stress increased the weight of the adrenal gland (P < 0.001) and plasma corticosterone levels (P < 0.001) and treatment with CT significantly decreased both weight of the adrenal gland (P < 0.001) at all doses, but reduced plasma corticosterone levels at 200 and 400 mg/kg (P < 0.001), comparable to the W. somnifera significant reduction. The results were shown in Table 5.
Effect of the Cinnamomum tamala Extract on Plasma Glucose
Stress increased the plasma glucose levels (P < 0.001), whereas CT-treated rats showed the significant decrease in plasma glucose levels at all doses (P < 0.001), comparable to the effect of W. somnifera (P < 0.001). The results were shown in Table 5.
Effect of the Cinnamomum tamala Extract on Plasma Cholesterol
Stress increased the plasma cholesterol levels (P < 0.001), whereas CT significantly decreased plasma cholesterol levels at 100 (P = 0.013), 200 (P < 0.001), and 400 mg/kg (P < 0.001), comparable to the effect of W. somnifera (P < 0.001). The results were shown in Table 5.
Effect of the Cinnamomum tamala Extract on Plasma Triglycerides
Stress increased the plasma triglyceride levels (P < 0.001), whereas CT-treated rats showed a significant decrease in plasma triglyceride levels at 200 (P = 0.018) and 400 mg/kg (P < 0.001), comparable to the effect of W. somnifera (P < 0.001). The results were shown in Table 5.
Discussion
The present results of these experiments show for the first time that aqueous extract of CT leaves possesses anxiolytic, antidepressant, and antistress effects following long-term administration to rats.
Anxiolytic Activity
Both the open field test and elevated plus-maze tests are used to evaluate neurobehavioral profiles of rodents under conditions of anxiety.[16] In the open field test, rodents are exposed to an unusual background, where they express fear and anxiety in terms of increased defecation and decreased activities such as rearing, ambulation, and self-grooming along with less activity in the center. Similarly, in elevated plus-maze test, animals upon exposure to an open arm causes an approach conflict that is significantly robust than the response elicited by exposure to an enclosed arm of the maze. Thus, open/enclosed arms entries and time spent in respective arms provide a measure of fear provoked suppression of exploratory action. Generally, classical anxiolytics antagonize these behavioral changes,[16] and benzodiazepines are used to validate the anxiolytic activity.[17]
The results obtained from elevated plus-maze test demonstrate that CT at 200 mg/kg and 400 mg/kg significantly increased the open arm entries and time spent in open arm. Similarly, in the open field test, CT at 400 mg/kg significantly increased ambulation, self-grooming, rearing, and increased activity at center as well as significantly decreased fecal droppings compared to lorazepam at 1 mg/kg. Furthermore, antianxiety drugs increase the social interaction time in a new environment.[16] CT at 400 mg/kg significantly prolonged the social interaction time and an equal effect was recorded with lorazepam at 1 mg/kg. These behavioral changes are indicative of diminished anxiety and confirm the anxiolytic activities of CT.
Antidepressant Activity
Learned helplessness[13] and behavioral despair test[11] are the two most persuasive and commonly used in vivo animal models of depression. In the learned helplessness test, rodents are exposed to inescapable and unavoidable electric shocks in one circumstance and later fail to escape a shock in another circumstance when escape is feasible. If the number of failures to escape is lessened and learned helplessness is decreased, then the drug is assumed to be effective in depression. Likewise, in behavioral despair test, rodents are obliged to swim in a confined area from which they unable to escape and exhibit a distinctive rigidity.[11] In general, antidepressants can abolish or reduce this state of despair.
In the learned helplessness test, CT at 400 mg/kg found to produce a significant decline in a number of escape failures and a significant rise in avoidance response, which is comparable to imipramine antidepressant activity. Similarly, CT produced a dose-dependent reduction in time of immobility in despair test. However, the results obtained in the tail suspension test provide an additional measure for assessing antidepressant activity and CT at 400 mg/kg significantly decreased immobility time in this test also. The results presented here show that CT given orally is effective in producing significant antidepressant-like effects.
Antistress Activity
Stress is an adaptive physiological response to dysregulation of homeostasis. Stress consists of diverse, intricate neurological, biochemical, and immunological pathways and has been involved in the etiopathogenesis of numerous disease conditions such as anxiety, depression, and cognitive dysfunction.[18] Stress-induced gastric ulcer is a notable example of stress-associated visceral injuries and considered as an indication of stress syndrome.[19]
Water-immersion restraint stress (WIRS) simulates the clinical condition of acute gastric ulcerations, and our results showed that WIRS caused significant gastric ulceration in rats. Furthermore, CT significantly mitigated WIRS-induced ulcer index [Figure 1]. The observed gastroprotective effect of CT extract might be due to its antioxidant activity.[6]
Figure 1.
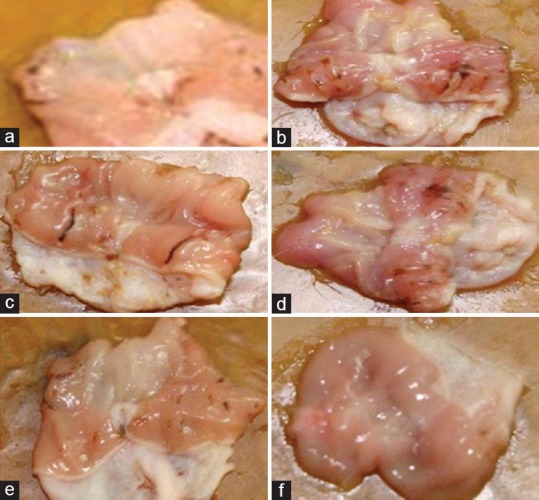
Images of stomach of rats after water-immersion restraint stress. (a) Control rats showing normal mucosa. (b) Water immersion restraint rats showing mucosal ulceration. (c) Cinnamomum tamala 100 mg/kg treated rats showing mucosal ulceration. (d) Cinnamomum tamala 200 mg/kg treated rats showing focal mucosal ulceration. (e) Cinnamomum tamala 400 mg/kg treated rats showing intact mucosa with mild ulceration. (f) Withania somnifera 100 mg/kg treated rats showing normal mucosa
It was reported that stress increases adrenal gland weight and plasma corticosterone levels owing to the stimulation of adrenomedullary response[20] and hypothalamic–pituitary–adrenocortical axis,[18] respectively. Furthermore, elevated corticosterone has a deleterious effect on lipid and glucose metabolism. In our study, stress treatment increased the adrenal gland weight, plasma corticosterone, glucose, cholesterol, and triglyceride levels, whereas prior administration of CT treatment significantly (P < 0.05) normalized those parameters. These results indicate that CT effectively suppressed the stress-induced hormonal and metabolic alterations and preserved the homeostatic mechanism.
The present experimental data could not reveal the mode of action through CT-induced anxiolytic, antidepressant, and antistress actions. The pharmacological effects of CT noticed in this investigation might be credited to plant's phytoconstituents such as linalool, p-cymene, eugenol, cinnamic aldehyde, alpha- and beta-pinene, 3,3,4,5,7-pentahydroxyflavone, kapeferol-3-o-glucopyranoside, kaempferol-3-o-saphoroside, and quercetin-3-o-rutenoside[21] have been reported previously from this plant. Singh et al. have isolated the flavonoids such as kaempferol, myricetin, quercetin, kaempferol-3-O-rhamnoside, and quercitrin from the leaves of CT.[22] It has been demonstrated that linalool,[23,24] kaempferol,[25] and quercetin[25] exert significant anxiolytic actions in animals, as well as in human beings. It is uncertain whether the reported CNS beneficial actions occur as a result of individual stimulation of neuronal regions either by a single constituent or by the synergistic effect of different phytoconstituents of this plant. Therefore, further neurochemical and pharmacological studies are necessary to establish the mechanism of action of CT.
Conclusion
The investigation demonstrated that CT at 400 mg/kg possesses significant anxiolytic, antidepressant, and antistress effects and is therapeutically beneficial for the management of psychological ailments.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
Authors are grateful to the University Grants Commission, New Delhi, India, for providing financial support in the form of junior research fellowship to one of the co-authors Mr. Gaya Prasad.
References
- 1.Sahoo S, Khess CR. Prevalence of depression, anxiety, and stress among young male adults in India: A dimensional and categorical diagnoses-based study. J Nerv Ment Dis. 2010;198:901–4. doi: 10.1097/NMD.0b013e3181fe75dc. [DOI] [PubMed] [Google Scholar]
- 2.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cott J. NCDEU update. Natural product formulations available in europe for psychotropic indications. Psychopharmacol Bull. 1995;31:745–51. [PubMed] [Google Scholar]
- 4.Chakraborty U, Das H. Antidiabetic and antioxidant activities of Cinnamomum tamala leaf extracts in Stz-treated diabetic rats. Glob J Biotechnol Biochem. 2010;5:12–8. [Google Scholar]
- 5.Dhulasavant V, Shinde S, Pawar M, Naikwade NS. Antihyperlipidemic activity of Cinnamomum tamala Nees, on high cholesterol diet induced hyperlipidemia. Int J PharmTech Res. 2010;2:2517–21. [Google Scholar]
- 6.Eswaran MB, Surendran S, Vijayakumar M, Ojha SK, Rawat AK, Rao ChV. Gastroprotective activity of Cinnamomum tamala leaves on experimental gastric ulcers in rats. J Ethnopharmacol. 2010;128:537–40. doi: 10.1016/j.jep.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 7.Singh B, Chandan BK, Sharma N, Singh S, Khajuria A, Gupta DK. Adaptogenic activity of glyco-peptido-lipid fraction from the alcoholic extract of Trichopus zeylanicus Gaerten (part II) Phytomedicine. 2005;12:468–81. doi: 10.1016/j.phymed.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Vijayalakshmi, Adiga S, Bhat P, Chaturvedi A, Bairy KL, Kamath S. Evaluation of the effect of Ferula asafoetida Linn. gum extract on learning and memory in Wistar rats. Indian J Pharmacol. 2012;44:82–7. doi: 10.4103/0253-7613.91873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav AV, Kawale LA, Nade VS. Effect of Morus alba L. (mulberry) leaves on anxiety in mice. Indian J Pharmacol. 2008;40:32–6. doi: 10.4103/0253-7613.40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 12.Bourin M, Chenu F, Ripoll N, David DJ. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav Brain Res. 2005;164:266–9. doi: 10.1016/j.bbr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Seligman ME, Beagley G. Learned helplessness in the rat. J Comp Physiol Psychol. 1975;88:534–41. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- 14.Nishida K, Ohta Y, Kobayashi T, Ishiguro I. Involvement of the xanthine-xanthine oxidase system and neutrophils in the development of acute gastric mucosal lesions in rats with water immersion restraint stress. Digestion. 1997;58:340–51. doi: 10.1159/000201464. [DOI] [PubMed] [Google Scholar]
- 15.Woodward CJ, Emery PW. Determination of plasma corticosterone using high-performance liquid chromatography. J Chromatogr. 1987;419:280–4. doi: 10.1016/0378-4347(87)80287-6. [DOI] [PubMed] [Google Scholar]
- 16.Cryan JF, Sweeney FF. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br J Pharmacol. 2011;164:1129–61. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada T, Nakajima R, Kurihara E, Narumi S, Masuoka Y, Goto G, et al. Pharmacologic characterization of a novel non-benzodiazepine selective anxiolytic, DN-2327. Jpn J Pharmacol. 1989;49:337–49. doi: 10.1254/jjp.49.337. [DOI] [PubMed] [Google Scholar]
- 18.Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, Herman JP. Stress risk factors and stress-related pathology: Neuroplasticity, epigenetics and endophenotypes. Stress. 2011;14:481–97. doi: 10.3109/10253890.2011.604751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selye H. A syndrome produced by diverse nocuous agents 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230–1. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 20.Sardessai SR, Abraham ME, Mascarenhas JF. Effect of stress on organ weight in rats. Indian J Physiol Pharmacol. 1993;37:104–8. [PubMed] [Google Scholar]
- 21.Bharadwaj DK, Chand G, Gupta AK, Jain RK. Polyphenolic component of C. tamala. Proc Indian Natl Sci Acad. 1983;49:413–7. [Google Scholar]
- 22.Singh VP, Pandey R, Yadav B, Pandey VB. Flavonoids of Cinnamomum tamala. Nat Prod Sci. 2002;8:16–7. [Google Scholar]
- 23.Höferl M, Krist S, Buchbauer G. Chirality influences the effects of linalool on physiological parameters of stress. Planta Med. 2006;72:1188–92. doi: 10.1055/s-2006-947202. [DOI] [PubMed] [Google Scholar]
- 24.Linck VM, da Silva AL, Figueiró M, Piato AL, Herrmann AP, Dupont Birck F, et al. Inhaled linalool-induced sedation in mice. Phytomedicine. 2009;16:303–7. doi: 10.1016/j.phymed.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Vissiennon C, Nieber K, Kelber O, Butterweck V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin – Are they prodrugs? J Nutr Biochem. 2012;23:733–40. doi: 10.1016/j.jnutbio.2011.03.017. [DOI] [PubMed] [Google Scholar]


