Abstract
Aim:
The aim of this objective is to evaluate the antinociceptive and anti-inflammatory potential of sulfated polysaccharide purified fractions isolated from brown seaweed Sargassum wightii and seagrass Halophila ovalis in male Wistar rats.
Subjects and Methods:
Crude sulfated polysaccharide from S. wightii and H. ovalis was subjected to anion exchange chromatography, and the chemical composition was investigated. The antinociceptive activity of purified fractions was investigated using formalin and hot plate test. Carrageenan-induced paw edema, peritonitis model, and Freund's Complete Adjuvant-induced arthritis model were employed to determine the anti-inflammatory activity.
Results:
In the formalin test, there was a significant reduction in licking time in both phases of the test at a dose of 10 mg/kg. In the hot plate test, the antinociceptive effect was observed only in animals treated with 5, 10 mg/kg suggesting that the analgesic effect occurs through a central action mechanism. Sw FrIV and Ho FrIV significantly inhibited paw edema induced by carrageenan, especially at 3 h after treatment and potentially decreased neutrophil migration at 10 mg/kg, respectively. In Freund's adjuvant-induced arthritic rats, a significant reduction in paw volume was observed in Sw FrIV and Ho FrIV-treated groups (10 mg/kg).
Conclusion:
Purified components from S. wightii and H. ovalis have strong antinociceptive and anti-inflammatory effect on animal model. However, to determine the molecular mechanism, it is necessary to investigate the effect of purified fractions on inhibition of nitric oxide synthase expression mediated by inhibiting the phosphorylation of various signal proteins in lipopolysaccharide-stimulated RAW264.7 cells.
Keywords: Anti-inflammatory properties, antinociception, Halophila ovalis, polysaccharides fractions, Sargassum wightii
Inflammation has been linked with physiological and pathological processes by the activation of immune system, local vascular system, and various cells within the damaged tissue.[1] Prolonged inflammation leads to various diseases and disorders such as pain, swelling, edema, redness, and heat. High levels of inflammatory cytokines and reactive oxygen species are proposed contributors to the pathophysiological mechanisms associated with various inflammatory disorders.
Formalin test is the most predictive model of tonic and localized inflammatory pain since it encompasses inflammatory, neurogenic, and central mechanisms of nociception.[2] Injection of 1% formalin into the paw exhibits two distinct phases in the licking response. The first phase (acute neurogenic pain) caused by the direct chemical stimulation of nociceptors and the second phase (inflammatory pain) is triggered by a combination of stimuli, including inflammation of the peripheral tissues and mechanism of central sensitization.
Carrageenan has been used for decades to induce inflammation and to study the mediators of inflammation and the effectiveness of anti-inflammatory mediators. The edema induced by carrageenan is temporal and multimediated, with release of various mediators and is characterized by an intense neutrophil infiltrate.[3] Carrageenan-induced neutrophil migration in the inflamed tissue increases the vascular permeability with protein exudation and vice versa.[4] The selectin-adhesion molecules have been shown to be responsible for mediating leukocyte rolling along the endothelial cells of blood vessels at the sites of inflammation, and the neutrophil participates in the cascade of events leading to mechanical hypernociception.[5] Rheumatoid arthritis (RA), a destructive inflammatory polyarticular joint disease, leads to the formation of a very aggressive tissue, which invades and destroys adjacent cartilage and bone.[6] The suppression of inflammatory response through blockade of the activation, proliferation of inflammatory cells, and production of inflammatory cytokines is one such anti-inflammatory goal.
In recent times, there is a strong interest in searching for new anti-inflammatory agent from natural products due to indiscriminate use of commercially available analgesics and nonsteroidal anti-inflammatory drugs. Currently, sulfated polysaccharides of seaweeds and seagrasses have gathered unprecedented attention in recent times being a prolific source of natural analgesic and anti-inflammatory agents.[7,8,9] The sulfated polysaccharides from Sargassum species have been explored for possible anti-inflammatory effects.[10,11] In general, chemical characteristics such as molecular size, sugar composition, sulfate content, type of linkage, and molecular geometry have a great influence on their biological activities.[12] Seagrasses are marine vascular plants, which grow in seawater and survive the tides unscathed. The presence of sulfated polysaccharide was determined in three species, of which the Ruppia maritima sulfated polysaccharide was purified and its structure was characterized. To date, sulfated polysaccharide of Halodule wrightii has been characterized for its antioxidant and anticoagulant activities.[13] We reported the existence of antinociceptive and anti-inflammatory activity in crude sulfated polysaccharides of brown seaweed Sargassum wightii and seagrass Halophila ovalis[9] might be a synergistic effect of the fractions. Therefore, the present study is designed to investigate the antinociception and anti-inflammatory effects of sulfate polysaccharide purified fractions in animal models and delineate the chemical motifs of active fractions such as monosaccharide composition, molecular mass, sugar, and sulfate content.
Subjects and Methods
Experimental Animals
Male Wistar rats (160–200 g) were maintained in polypropylene cages under temperature controlled rooms with a 12 h light/dark cycle and had free access to food and water ad libitum. The Institutional Animal Ethics Committee of Committee for the Purpose of Control and Supervision of Experiments on Animals approved the study.
Purification of Biopolymer by Anion Exchange Chromatography
Crude sulfated polysaccharide was extracted from brown seaweed S. wightii and seagrass H. ovalis as described previously.[9] Purification was achieved on successive solubilization and precipitation of the biopolymer in aqueous medium. Water and enzyme extracted polysaccharide was applied to a diethylaminoethyl (DEAE)–cellulose column (20 cm × 2 cm) equilibrated with 50 mM sodium chloride solution. Thereafter, the column was then eluted successively with 50 mM (FrI), 0.2 M (FrII), 0.6 M (FrIII), and 2 M (FrIV) sodium chloride in a stepwise manner. The flow rate of the column was 0.2 ml/min, and the fractions were collected through auto collector. The collected fractions were dialyzed extensively against deionized water for 48 h and then lyophilized. The lyophilized samples were stored at 4°C for further characterization.
Estimation of Total Sugar and Sulfate Content
Total sugar was estimated by the phenol-sulfuric acid method using fucose as standard. Briefly, 200 μl of sample was mixed with same amount of 5% phenol solution. Then, 1 ml of concentrated H2SO4 acid was added to the sample mixture and kept for 30 min after vortexing for 2 min. Absorbance was measured at 490 nm using spectrophotometer (Hitachi, U-2000, Japan).
Sulfate content in purified fractions was determined by the barium chloride-gelatin method. A known amount of purified fractions was dissolved in deionized water and 2 ml of the sample was transferred into glass ampoules and dried under flow of nitrogen gas. To this ampoule, 1 ml of 1 N HCl was added, and the sample was flushed with nitrogen. Thereafter, the ampoule was sealed, and the sample was hydrolyzed at 105°C for 17 h. After hydrolyses, the sample was cooled, and the content was transferred to test tube containing 3.8 ml of 3% trichloroacetic acid solution. To this mixture, 1 ml of BaCl2-gelatin reagent was added and mixed thoroughly. The solution was kept at room temperature for 15–20 min and the turbidity formed was measured at 360 nm. Potassium sulfate dissolved in 1 N HCl was used as a standard for calibration. To estimate blanks in all the above estimations, deionized water was used in place of the samples and analyzed.
Molecular Mass Determination
The average molecular weight of purified fractions of S. wightii, and H. ovalis was determined by size exclusion chromatography using sephadex G-75 as a matrix. The column was packed with pre-swollen sephadex G-75 beads and connected to AKTA prime purification system (GE, Health Care, Germany). The column was stabilized with 50 mM NaCl solution at the flow rate of 1 ml/min. To determine the molecular weight of the samples, standard dextrans of different molecular weight 10 kDa, 15 kDa, and 20 kDa were chromatographed initially using 0.6 M sodium chloride solution as eluant, and the retention time was determined. The flow rate of the column was maintained at 1 ml/min. Purified fractions (50 mg) of S. wightii and H. ovalis were chromatographed using the same parameters, and the molecular mass was calculated according to the retention time of standard dextrans.
Antinociceptive Activity of Sulfated Polysaccharides
Formalin test
This method was used as a model for tonic pain and localized inflammatory pain. Male Wistar rats were injected with Sw-SP fractions Sw FrIII, IV and Ho-SP fractions Ho Fr III, IV (2.5, 5 or 10 mg/kg; intravenous [i.v.]) or sterile saline (0.9%, w/v, NaCl). After 30 min of administration, 1% aqueous formalin (20 μl) was injected ipl into the right hind paw. The amount of time that the animal spent licking was then recorded from 0 to 5 min (Phase 1, corresponding to the direct stimulation of nociceptors) and 20–25 min after formalin injection (Phase 2, inflammatory). Indomethacin (5 mg/kg, s.c.) was also administered 30 min before formalin injection and used as reference.
Hot plate test
The analgesic activity was measured using hot plate test. Each rat was placed twice onto a heated plate (51°C ± 1°C) separated by a 30 min intertrial interval. The reaction latency of thermal stimuli was recorded. Animals showing a response (licking the paw or jumping) greater than 10s were discarded. After the second trial, group of animals (n = 6) received sterile saline (0.9%, w/v, NaCl), Sw-SP fractions Sw FrIII, IV and Ho-SP fractions Ho FrIII, IV (2.5, 5 or 10 mg/kg; i.v.), morphine or indomethacin (both 5 mg/kg, s.c.). The reaction times were measured at time zero (0 time) and 30, 60, and 90 min after the fractions were administered, with a cutoff time of 40 s to avoid paw lesions.
Anti-inflammatory Activity of Sulfated Polysaccharides
Carrageenan-induced rat paw edema
The rats (n = 6 per group) were pretreated with Sw-SP and Ho-SP fractions at doses of 2.5, 5, or 10 mg/kg (0.1 ml/100 g body weight; s.c.) or sterile saline (0.9%, w/v, s.c.) 1 h before carrageenan injection. Carrageenan (prepared in 0.9% NaCl) was injected into the subplantar region of the right hind paw (500 μg/paw, 0.1 ml, ipl). Diclofenac (1 mg/kg; s.c.) was also administered 1 h before carrageenan as a reference compound. Paw volume was measured immediately before (0 time) the carrageenan injection and at selected time intervals (1, 2, 3, and 4 h) with a plethysmometer (Panlab, Spain). The results were expressed as a variation in paw volume (ml) calculated as the difference from the basal volume (0 time) and the volume at selected time interval.
Peritonitis model in rats
The peritoneal inflammation was induced in rats as described earlier.[14] Briefly, the rats (n = 6 per group) received sterile saline (0.9%, w/v, s.c.) or dexamethasone (1 mg/kg, s.c.), Sw FrIII, IV (10 mg/kg, s.c.), and Ho FrIII, IV (10 mg/kg, s.c.) 1 h before carrageenan (500 μg/cav, 100 μl, ipl). After 4 h, the animals were sacrificed, and the peritoneal cavity was washed with 10 ml saline contains heparin (5 IU/ml). The peritoneal fluid was collected, and the total leukocyte count was performed using Neubauer chamber. To differentiate the leukocyte count, samples were stained by hematoxylin and eosin method, and the cells were counted by microscopy. The data were expressed as the means ± standard error of mean of the number of cells × 103 ml of peritoneal fluid.
Freund's adjuvant-induced arthritis
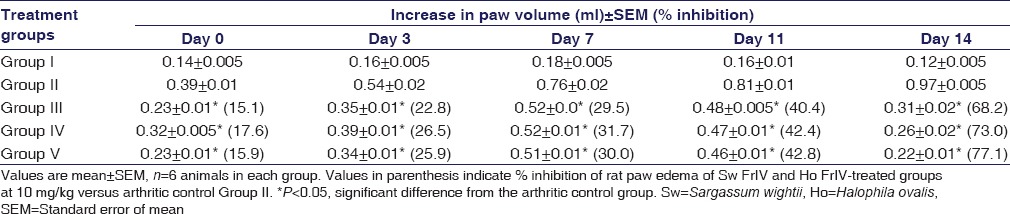
Male Wistar albino rats weighing 180 ± 20 g were used for the chronic inflammation study. Animals were divided into five groups of six animals each. Group I served as control (without treatment), Group II served as arthritic control (negative control), Group III was treated with diclofenac sodium at 5 mg/kg (positive control), Group IV received Sw FrIV (10 mg/kg), and Group V received Ho FrIV (10 mg/kg). After 30 min of drug treatment, arthritis was induced by injecting a 0.1 ml (0.1% w/v) of Freund's Complete Adjuvant (FCA) into the left hind paw. The drug treatment was continued from the initial day, i.e., from the day of adjuvant injection (0 day) and continued till 14th day. The paw volume of all the groups was measured on 0, 3, 7, and 14 days of drug treatment using a plethysmometer. The arthritic index was determined on respective days and % inhibition was calculated.
Statistical Analysis
All data were expressed as mean ± standard deviation. Statistical differences between the experimental groups were determined by one-way ANOVA, and differences were considered to be statistically significant if P < 0.05. Tukey's post hoc test was performed for multiple group comparison between groups. All computations were done by employing statistical software (SPSS Version 7.5, SPSS Inc.).
Results
Purification of Biopolymer
The crude extract was purified initially by anion exchange chromatography (DEAE–cellulose) separated the crude polysaccharide into four fractions (Sw FrI, Sw FrII, Sw FrIII, and Sw FrIV) eluted with increasing concentration of sodium chloride. Similarly, crude sulfated polysaccharide of seagrass H. ovalis was separated into four fractions (Ho FrI, Ho Fr II, Ho FrIII, and Ho Fr IV) using step-wise gradient method [Figure 8].
Figure 8.
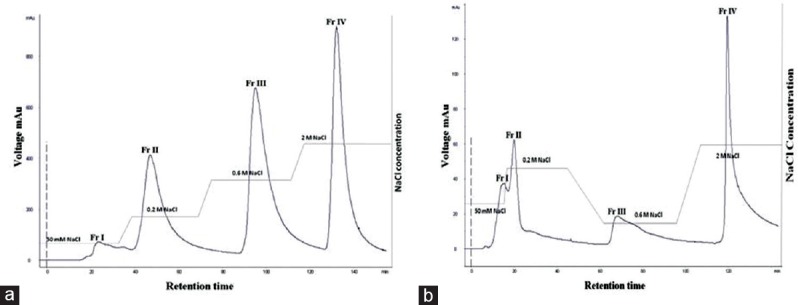
Ion exchange chromatography purification of crude polysaccharides
Chemical Composition Analysis
The chemical motif such as monosaccharide composition, molecular weight, total sugar, and sulfate content of purified fractions was determined. Purified fraction Sw FrIV contained relatively high amount of total sugars (74.5%) and sulfate content (21.2%) based on dry weight when compared with other fractions. Similarly, the purified fraction Ho FrIV contained 75.2% of neutral sugar and 21.3% sulfate based on dry weight. The average molecular weight of Sw-SP and Ho-SP purified fractions was determined by size exclusion chromatography using different molecular weight standard dextrans. The results suggested that the average molecular weight of Sw FrIII, Ho FrIII (>10 kDa), and Sw FrIV, Ho FrIV (10 kDa) was observed.
Formalin Test
The antinociceptive effect of Sw-SP and Ho-SP fractions was characterized by decrease in licking time in 1% formalin administered rats. There was no significant reduction of licking time observed during the first phase following 1% formalin administration (neurogenic) with 2.5 or 5 mg/kg of Sw-SP and Ho-SP fractions, respectively. Whereas there was a considerable reduction of licking time by 51.8 (Sw FrIII), 59.9 (Sw FrIV), 54.3 (Ho FrIII), and 61.9% (Ho FrIV) with 10 mg/kg [Figures 1a and 2a]. In addition, the Sw-SP and Ho-SP fractions (10 mg/kg; i.v.) injected 30 min before the formalin injection elicited a dose-dependent inhibition of the formalin response during the second phase (inflammatory) by 73.5 (Sw FrIII), 79.3 (Sw FrIV), 74.7 (Ho FrIII), and 82.7% (Ho FrIV), respectively [Figures 1b and 2b].
Figure 1.
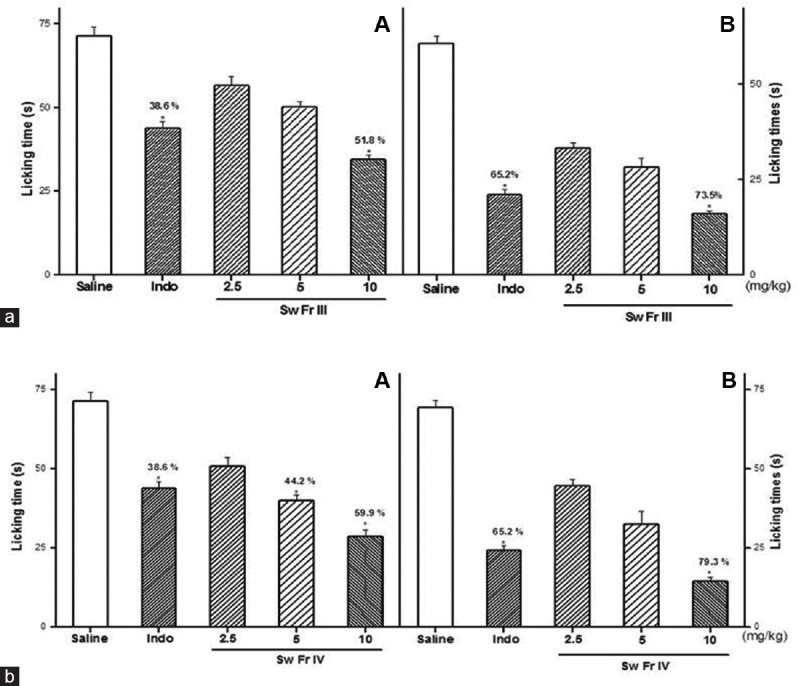
Effect of Sw FrIII (A) and Sw FrIV (B) on the chemical stimuli (formalin test). Sw FrIII, Sw FrIV (2.5, 5 or 10 mg/kg) or saline were given intravenous 30 min before the formalin and the licking time was determined during the first 5 min (Phase I, [a]) and during 20–25 min (Phase II, [b]) after a 1% formalin injection in rats. Data are expressed as the mean ± standard error of the mean of six animals per group. *P < 0.05 indicates significant difference from the saline group. *,&Indicates significant difference from indomethacin-treated group (ANOVA, Tukey's test)
Figure 2.
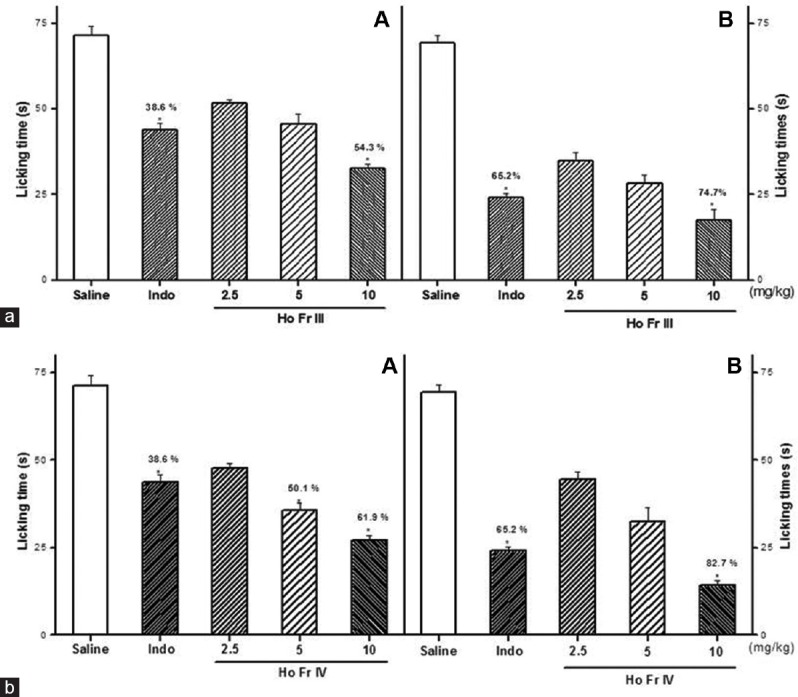
Effect of Ho FrIII (A) and Ho FrIV (B) on the chemical stimuli (formalin test). Ho FrIII and Ho FrIV (2.5, 5 or 10 mg/kg) or saline were given intravenous 30 min before the formalin, and the licking time was determined during the first 5 min (Phase I, [a]) and 20–25 min (Phase II, [b]) after a 1% formalin injection in rats. Data are expressed as the mean ± standard error of mean of six animals per group. *P < 0.05 indicates significant difference from the saline group. *,& Indicates significant difference from indomethacin-treated group (ANOVA, Tukey's test)
Hot Plate Test
The treatment of rats with Sw-SP and Ho-SP fractions (5 or 10 mg/kg, i.v.) after the second hot-plate trial produced significant antinociceptive effects, as measured by the reaction time during, the 90 min observation [Figure 3a and b]. The systemic administration of Sw FrIII and Sw FrIV (5 or 10 mg/kg) significantly increased latency in the hot-plate test at 30, 60, and 90 min. At 5 or 10 mg/kg, the maximum latency to thermal stimuli was observed at 60 min after administration when compared to indomethacin-treated control. Similarly, Ho FrIII and Ho FrIV (5 or 10 mg/kg) significantly increased latency in hot-plate test at all time intervals compared to the indomethacin-treated group [Figure 4a and b]. The minimal doses of Sw-SP and Ho-SP fractions (2.5 mg/kg) were ineffective at inducing antinociception at all time intervals.
Figure 3.
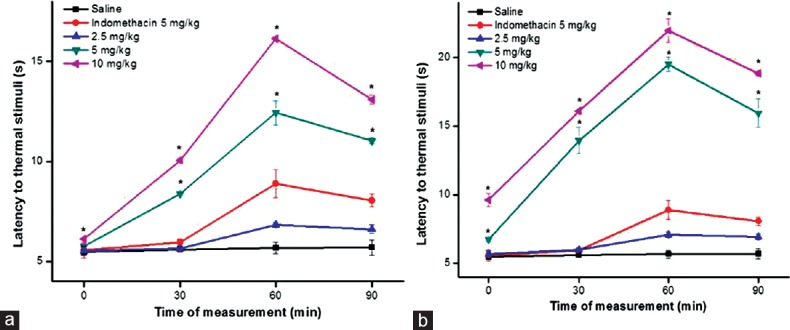
Effect of Sw FrIII (a) and Sw FrIV (b), morphine or indomethacin on the latency period to thermal stimuli (hot-plate) induced in male Wistar rats. Animal received morphine or indomethacin (both 5 mg/kg, s.c.). Saline, Sw FrIII or Sw FrIV (2.5, 5 or 10 mg/kg) was injected intravenous. Data are expressed as mean ± standard error of mean of six rats per group. *P < 0.05 indicates significant difference from the saline group (ANOVA, Tukey's test)
Figure 4.
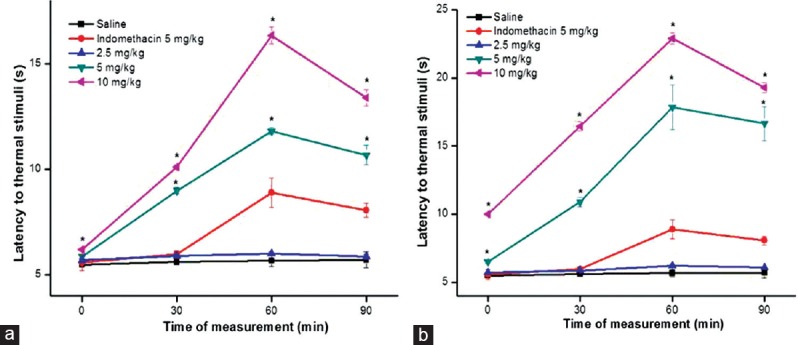
Effect of Ho FrIII (a) and Ho FrIV (b), indomethacin on the latency period to thermal stimuli (hot-plate) induced in male Wistar rats. Animal received indomethacin (5 mg/kg, s.c.). Saline, Ho FrIII or Ho FrIV (2.5, 5 or 10 mg/kg) was injected intravenous. Data are expressed as mean ± standard error of mean of six rats per group. *P < 0.05 indicates significant difference from the saline group (ANOVA, Tukey's test)
Carrageenan-induced Paw Edema
In this study, acute anti-inflammatory activity of Sw-SP and Ho-SP fractions (2.5, 5, or 10 mg/kg) was evaluated by carrageenan-induced paw edema in rats [Figures 5 and 6]. The intense paw edema reached a maximum level (0.59 ± 0.03 ml) at 3 h after carrageenan (500 μg, ipl) administration, and then decreased over the subsequent hour. Sw FrIV (10 mg/kg) showed significantly inhibited carrageenan-induced acute paw edema after 1 h administration at all time intervals, 1st h (0.16 ± 0.02), 2nd h (0.18 ± 0.02), 3rd h (0.23 ± 0.02), and 4th h (0.14 ± 0.01) when compared to diclofenac-treated groups [Figure 5a and b]. Similarly, Ho FrIV (10 mg/kg) reduced the paw edema at all time intervals, 1st h (0.18 ± 0.02), 2nd h (0.19 ± 0.01), 3rd h (0.22 ± 0.01), 4th h (0.13 ± 0.02) than diclofenac-treated groups [Figure 6a and b]. However, there was no significant inhibition in the carrageenan-induced paw edema in Sw FrIII (2.5, 5 or 10 mg/kg) and Sw FrIV (2.5 or 5 mg/kg) treated groups at any interval. Similarly, Ho FrIII (10 mg/kg, but not 2.5 or 5 mg/kg) and Ho FrIV (5 mg/kg, but not 2.5 mg/kg) significantly inhibited the carrageenan-induced acute paw edema during 4th h.
Figure 5.
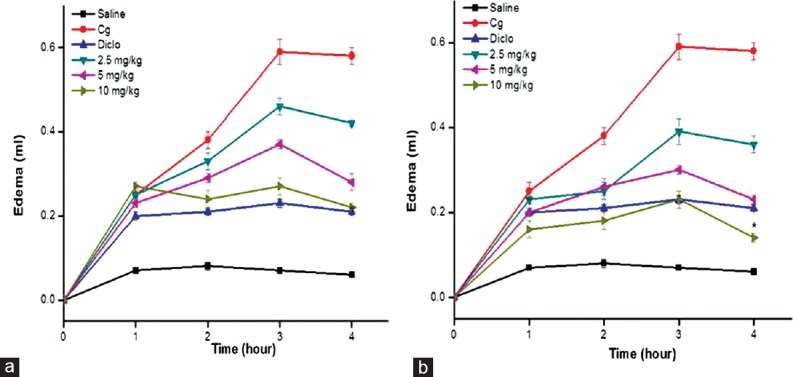
Effect of Sw FrIII (a) and Sw FrIV (b) on paw edema induced by carrageenan in rats. Before receiving a 0.1 ml injection of carrageenan (Cg, 500 μg/paw, ipl), rats received saline or Sw FrIII or Sw FrIV (2.5, 5 or 10 mg/kg). Diclofenac sodium (5 mg/kg) was injected s.c. Another group received only saline (s.c.) without Cg. Data are expressed as mean ± standard error of the mean of six rats per group. *P < 0.05 indicates significant difference from the diclofenac-treated group (ANOVA, Tukey's test)
Figure 6.
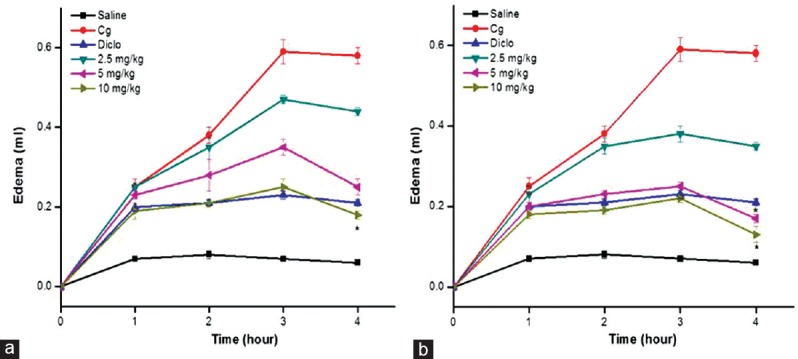
Effect of Ho FrIII (a) and Ho FrIV (b) on paw edema induced by carrageenan in rats. Before receiving a 0.1 ml injection of carrageenan (Cg, 500 μg/paw, ipl), rats received saline or Ho FrIII or Ho FrIV (2.5, 5 or 10 mg/kg). Diclofenac sodium (5 mg/kg) was injected s.c. Another group received only saline (s.c.) without Cg. Data are expressed as mean ± standard error of mean of six rats per group. *P < 0.05 indicates significant difference from the diclofenac-treated group (ANOVA, Tukey's test)
Peritonitis Model
The anti-inflammatory activity of Sw FrIV and Ho FrIV (10 mg/kg) was evaluated in a carrageenan-induced peritonitis model. As expected, carrageenan (500 μg/cav, ipl)-induced significant leukocyte and neutrophil migrations when injected into the peritoneal cavity of rats. On the other hand, Sw FrIV and Ho FrIV (10 mg/kg) showed anti-inflammatory effects by decreasing leukocyte migration into the peritoneal cavity of these rats by 72 and 75%, respectively [Figure 7a]. The reference drug, dexamethasone (1 mg/kg, s.c.) significantly inhibited the leukocyte migration by 69% into peritoneum of animals treated with carrageenan. Similarly, at the test dose of 10 mg/kg, Sw FrIV and Ho FrIV inhibited neutrophil migration by 64% and 70% [Figure 7b]. The reference drug dexamethasone (1 mg/kg, s.c.) decreased the carrageenan-induced neutrophil migration by 81%. The inhibitory action of Sw FrIV and Ho FrIV on neutrophil migration is perhaps due to its interaction with P-selectin as supported by the inhibition of the chemotaxis of leukocytes into peritoneal cavity of rats suffering from experimentally induced peritonitis.
Figure 7.
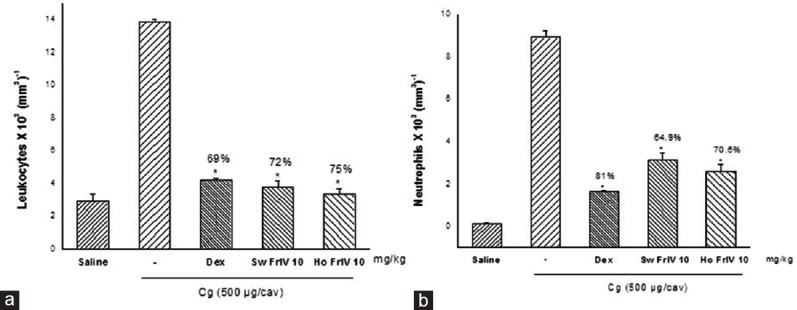
Effect of Sw FrIV and Ho FrIV on leukocyte (a) and neutrophil (b) migrations induced in rats. Rats received Sw FrIV and Ho FrIV at 10 mg/kg s.c. before an injection of carrageenan (Cg, 500 μg/cav, ipl). Dexamethasone (1 mg/kg) was injected s.c. Another group received saline without carrageenan. Data are expressed as mean ± standard error of mean of six rats per group. *P < 0.05 indicates significant difference from the carrageenan-treated group (ANOVA, Tukey's test)
Freund's Complete Adjuvant-Induced Arthritis
The chronic anti-inflammatory activity of Sw FrIII, Sw FrIV, Ho FrIII, and Ho FrIV (10 mg/kg) was evaluated by FCA-induced arthritis model in male Wistar albino rats during 0, 3, 7, 11, and 14 days intervals [Table 1]. FCA-treated rats showed significant increase in paw volume over saline-treated rats at all intervals. Whereas Sw-SP and Ho-SP fractions (10 mg/kg) showed significant reductions in rat paw volume when compared with the arthritic group. On the 14th day, the percentage inhibition of FCA induced paw edema exhibited by Sw FrIV and Ho FrIV (10 mg/kg) were 73.0% and 77.1%, respectively. However, the inhibition was not significant when compared to diclofenac (5 mg/kg; 68.2% inhibition)-treated groups.
Table 1.
Effect of Sargassum wightii FrIV and Halophila ovalis FrIV fractions on rat paw edema in Freund's adjuvant arthritic rats and degree of weight loss
Discussion
Marine natural products have become more sophisticated nowadays as it contains wide biological active components with different structures and interesting functional properties. Of such active components, polysaccharides of seaweeds expanded the global industries. Over the last decade, sulfated polysaccharides from seaweeds and seagrasses exhibited several health benefit effects and are used in food, pharmaceutical, and other products for human consumption. It has been reported that, sulfated polysaccharides from Sargassum siliquosum were non-cytotoxic and significantly induced peripheral blood mononuclear cells (PBMC's) proliferation. Similarly, preliminary non-cytotoxic effect of S. wightii and H. ovalis sulfated polysaccharide fractions were carried out in vitro in PBMC's before attempting biological screening. In our previous study, crude polysaccharide of S. wightii and H. ovalis was characterized for aninociceptive and anti-inflammatory activities.[9] The present was designed to reveal the antinociceptive and anti-inflammatory effect from purified fractions of S. wightii and H. ovalis polysaccharide.
The average molecular weight of purified fractions of sulfated polysaccharides was determined using standard dextrans. Similar kind of low-molecular-weight polysaccharides was reported from brown seaweeds Sargasssum fulvellum.[15] Notably, the molecular weight of biopolymer of the present study was lower than the other brown seaweed Sargassum tenerrimum.[16] However, the low-molecular weight biopolymer of S. wightii well reflected the already well-known low molecular weight polymers reported in Sargassacea family. In the present study, a low-molecular-weight polysaccharide was determined from H. ovalis purified fractions. Similarly, a 11 kDa sulfated polysaccharide-rich fraction was obtained from seagrass H. wrightii Asch., Cymodoceaceae.[13]
Administration of substances by the same route may result in adverse effects on experimental animals and confounded results. Earlier reports showed that highest bioavailability of substances achieved by parenteral administration methods and avoid the first-pass effect of hepatic metabolism, which occurs commonly with orally administered chemicals. Therefore, the present study was carried out with different administration routes in determining the antinociceptive, and anti-inflammatory activity of S. wightii and H. ovalis sulfated polysaccharide purified fractions.
Formalin test is the most predictive model for tonic and localized inflammatory pain. In the present study, fractions of S. wightii and H. ovalis showed more than 50% inhibition with low dose (10 mg/kg) during the first phase. Similarly, green seaweed Caulerpa cupressoides fraction 2 (Cc-sp2) showed 52.1% inhibition with 27 mg/kg during the first phase.[7] Whereas fraction I of red seaweed Solieria filiformis (SP-Sf) did not reduce the licking time during the first phase. However, it showed 86.4% inhibition during the second phase at the test dose of 9 mg/kg.[17] Similarly, Cc-SP2 at 9 mg/kg showed 82.34% inhibition during the second phase. Substances that inhibit both phases are central analgesics while those that inhibit only the second phase are peripheral analgesics.[18] Our results showed that fractions of Sw-SP and Ho-SP act as central analgesics by inhibiting both phases.
Hot plate test is known to evaluate the possible specific central action in which analgesic effects are exerted via supra spinal and spinal receptors. The present study showed that fractions of S. wightii and H. ovalis increased the latency to thermal stimuli in a dose-dependent manner. Similarly, Cc-SP2 of green seaweed C. cupressoides (9 mg/kg) showed maximum latency at 60 min.[7] Therefore, the present study revealed that the antinociceptive action of Sw FrIV and Ho FrIV occur via a central acting mechanism similar to green seaweeds C. cupressoides.[7]
Carrageenan-induced paw edema was adopted for decades as the acute inflammatory model and to study the mediators of inflammation and the efficiency of anti-inflammatory mediators. To evaluate this, purified fractions of S. wightii and H. ovalis were investigated in the paw edema model. In this test, edema evoked by carrageenan was significantly inhibited by purified fractions of S. wightii and H. ovalis in a dose-dependent manner. Similarly, sulfated polysaccharide fractions of Padina tetrastromatica significantly reduced the paw edema in a dose-dependent manner.[19] Whereas sulfated galactan of Champia feldmannii and SP-Sf of red seaweed S. filiformis at 9 mg/kg demonstrated edematogenic activity.[17,20]
Neutrophil recruitment into the systemic inflammatory site of animals is P-selectin-dependent. Inhibition of neutrophil migration to the inflammation site is one way in which the intensity of the inflammation process is decreased by sulfated polysaccharides.[21] In our study, we found that purified fractions of S. wightii and H. ovalis inhibited neutrophil migration in a dose-dependent manner. Similar result was observed from fucoidan extracted from brown seaweed Laminaria saccharina and sulfated polysaccharides from Fucus vesiculosus.[22,23]
The antiarthritic activity of various drugs can be measured by paw swelling index. Adjuvant-induced arthritis in rats is a classical model to study the pathological and serological changes, including the inflammatory mediators in association with the destruction of joints resembles RA in humans.[24] In the present study, purified fractions Sw FrIV and Ho FrIV (10 mg/kg) exhibited significant paw edema inhibition in arthritis-induced rats on the day 14, respectively, which is higher than that of standard drug, diclofenac. Similarly, heterofucan and alginic acid at 10, 100 mg/kg extracted from S. wightii reduced paw edema in arthritic rats.[9,25] The ability of Sw FrIV and Ho FrIV to reduce edema formation in adjuvant-induced arthritis related to its inhibition of prostaglandin synthesis.
Conclusion
Our results show that the sulfated polysaccharide purified fractions of S. wightii and H. ovalis possesses antinociceptive and anti-inflammatory effect that can be a possible candidate for the development of new drugs to treat various inflammatory diseases such as osteoarthritis, gout, and RA. However, its molecular mechanism of the inflammatory effect associated with nociception remains unknown.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields SD, Cavanaugh DJ, Lee H, Anderson DJ, Basbaum AI. Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors. Pain. 2010;151:422–9. doi: 10.1016/j.pain.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris CJ, Ferreira SH, Vane JR. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Med. 2003;225:115–21. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- 4.Issekutz AC. Role of polymorphonuclear leukocytes in the vascular responses of acute inflammation. Lab Invest. 1984;50:605–7. [PubMed] [Google Scholar]
- 5.Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, et al. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83:824–32. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa T, Mishawaka F, Miyata S, Hirayama Y. Prevention of progressive joint destruction in adjuvant induced arthritis in rats by a novel matrix metalloproteinase inhibitor. Eur J Pharmacol. 2005;508:239–47. doi: 10.1016/j.ejphar.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues JA, Vanderlei ES, Silva LM, Araújo IW, Queiroz IN, Paula GA, et al. Antinociceptive and anti-inflammatory activities of a sulfated polysaccharide isolated from the green seaweed Caulerpa cupressoides. Pharmacol Rep. 2012;64:282–92. doi: 10.1016/s1734-1140(12)70766-1. [DOI] [PubMed] [Google Scholar]
- 8.Coura CO, de Araujo IW, Vanderlei ES, Rodrigues JA, Quindere AL, Fontes BP, et al. Antinociceptive and anti-inflammatory activities of sulfated polysaccharides from the red seaweed Gracilaria cornea. Basic Clin Pharm Toxicol. 2012;110:335–41. doi: 10.1111/j.1742-7843.2011.00811.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuvaraj N, Kanmani P, Satishkumar R, Paari A, Pattukumar V, Arul V. Antinociceptive and anti-inflammatory activities of Sargassum wightii and Halophila ovalis sulfated polysaccharides in experimental animal models. J Med Food. 2013;16:740–8. doi: 10.1089/jmf.2012.2719. [DOI] [PubMed] [Google Scholar]
- 10.Wen ZS, Xiang XW, Jin HX, Guo XY, Liu LJ, Huang YN, et al. Composition and anti-inflammatory effect of polysaccharides from Sargassum horneri in RAW264.7 macrophages. Int J Biol Macromol. 2016;88:403–13. doi: 10.1016/j.ijbiomac.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Wu GJ, Shiu SM, Hsieh MC, Tsai GJ. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll. 2016;53:16–23. [Google Scholar]
- 12.Vishchuk OS, Tarbeeva DV, Ermakova SP, Zvyagintseva TN. Structural characteristics and biological activity of fucoidans from the brown algae Alaria sp. and Saccharina japonica of different reproductive status. Chem Biodivers. 2012;9:817–28. doi: 10.1002/cbdv.201100266. [DOI] [PubMed] [Google Scholar]
- 13.Silva JM, Dantas-Santos N, Gomes DL, Costa LS, Cordeiro SL, Costa MS, et al. Biological activities of the sulfated polysaccharide from the vascular plant Halodule wrightii. Braz J Pharmacogn. 2012;22:94–101. [Google Scholar]
- 14.de Souza GE, Ferreira SH. Blockade by antimacrophage serum of the migration of PMN neutrophils into the inflamed peritoneal cavity. Agents Actions. 1985;17:97–103. doi: 10.1007/BF01966691. [DOI] [PubMed] [Google Scholar]
- 15.de Zoysa M, Nikapitiya C, Jeaon YJ, Jee Y, Lee J. Anticoagulant activity of sulfated polysaccharide isolated from fermented brown seaweed Sargassum fulvellum. J Appl Phycol. 2008;20:67–74. [Google Scholar]
- 16.Sinha S, Astani A, Ghosh T, Schnitzler P, Ray B. Polysaccharides from Sargassum tenerrimum: Structural features, chemical modification and anti-viral activity. Phytochemistry. 2010;71:235–42. doi: 10.1016/j.phytochem.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 17.de Araujo IW, Vanderlei ES, Rodrigues JA, Coura CO, Quindere AL, Fontes BP, et al. Effect of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydr Polym. 2011;86:1207–15. [Google Scholar]
- 18.Deraedt R, Jouquey S, Delevallée F, Flahaut M. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980;61:17–24. doi: 10.1016/0014-2999(80)90377-5. [DOI] [PubMed] [Google Scholar]
- 19.Sulaiman M, Muraleedhara Kurup G, Rauf AA. Anti-inflammatory and antioxidant effect of sulphated polysaccharide isolated from marine algae Padina tetrastromatica from Kerala coast. J Pharm Res. 2011;4:784–8. [Google Scholar]
- 20.Assreuy AM, Gomes DM, da Silva MS, Toress VM, Siqueira RC, Pires AF, et al. Biological effects of a sulfated polysaccharide isolated from the marine red algae Champia feldmannii. Biol Pharm Bull. 2008;31:691–5. doi: 10.1248/bpb.31.691. [DOI] [PubMed] [Google Scholar]
- 21.Besednova NN, Zaporozhets TS, Makarenkova ID, Kunetsova TA, Kryzhanoskli SP, Zvyagintseva TN, et al. Anti-inflammatory effects of sulfated polysaccharides extracted from brown marine algae. Biol Bull Rev. 2012;2:525–32. [Google Scholar]
- 22.Preobrazhenskaya ME, Berman AE, Mikhailov VL, Ushakova NA, Mazurov AV, Semenov AV, et al. Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem Mol Biol Int. 1997;43:443–51. doi: 10.1080/15216549700204231. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso ML, Xavier CA, Bezerra MB, Paiva AO, Carvalho MF, Benevides NM, et al. Assessment of zymosan-induced leukocyte influx in a rat model using sulfated polysaccharides. Planta Med. 2010;76:113–9. doi: 10.1055/s-0029-1186003. [DOI] [PubMed] [Google Scholar]
- 24.Astusi O, Kawahito Y, Prudovsky I, Tubouchi Y, Kimura M. Copper chelation with tetrathiomolybdate suppresses adjuvants induced arthritis and inflammation associated cachexia in rats. Arthritis Res Ther. 2005;7:1174–82. doi: 10.1186/ar1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarithakumari CH, Renju GL, Kurup GM. Anti-inflammatory and antioxidant potential of alginic acid isolated from the marine algae, Sargassum wightii on adjuvant-induced arthritic rats. Inflammopharmacology. 2013;21:261–8. doi: 10.1007/s10787-012-0159-z. [DOI] [PubMed] [Google Scholar]


