Abstract
Objectives:
Methotrexate (MTX) is the most commonly used cost-effective disease-modifying antirheumatoid drug (DMARD). Its main dose-limiting adverse effects are hepatic and hematopoietic. This cross-sectional, observational study evaluated the prevalence of hepatic and hematological adverse effects with long-term low-dose MTX therapy.
Materials and Methods:
Rheumatoid arthritis (RA) patients taking ≤15 mg/week MTX for at least 2 years were enrolled from the rheumatology outpatient department. Demographic, disease, drug treatment profiles, and hematological and hepatic enzyme levels were noted.
Results:
Of the 204 patients enrolled, the frequency of raised alanine transaminase level (≥3-fold rise above the upper limit of normal) was 6.37% (95% confidence interval of 3.76–10.59) including two biopsy-proven hepatic fibrosis cases. About 5.4% had severe anemia (<8 g/dl) and 4.4% had leukopenia.
Conclusion:
Long-term low-dose MTX is safe in RA patients in the Indian population. The patterns of adverse effects were similar to those documented in earlier studies. However, our study results suggest that disease duration, cumulative MTX dose, concomitant DMARD intake are not risk factors associated with hepatic or hematological adverse effects.
Keywords: Adverse effects, hematological, hepatotoxicity, methotrexate, rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune disease with a global prevalence of approximately 1%,[1] while Indian data suggest a prevalence of 0.5% in the rural and about 1% in the urban population.[2] Treatment with disease-modifying antirheumatoid drug (DMARD) plays a pivotal role in the management of RA.[3] MTX is most commonly prescribed first-line DMARD due to favorable cost-effectiveness profile. Published literature has documented various adverse effects of MTX, but there are limited data in the Indian population pertaining to long-term adverse effects of low-dose therapy.
Potential risk factors suggested for hepatic adverse effects are increased age, female gender, alcohol intake, smoking, disease duration, cumulative dose of MTX, concomitant medications mainly nonsteroidal anti-inflammatory drug (NSAID), and other DMARDs.[4,5,6] Genetic factors may also play a role in predicting such adverse effects; however, the data are as yet inconclusive.[7,8,9]
The suggested molecular mechanism for hepatotoxicity is not well delineated. However, it is proposed that MTX enters the cell utilizing the folate transporter 1 and is pumped out by ATP-binding cassette family of transporters. Impaired activity of these transporters results in excess accumulation of drug in liver cells. MTX is retained within the cells as polyglutamates which inhibit enzymes dihydrofolate reductase, thymidylate synthase, and 5-aminoimidazole-4-carboxamideribonucleotide transformylase, leading to impaired pyrimidine and purine synthesis.[10] This mechanism is implicated for hematological adverse effects. MTX affects methylenetetrahydrofolate reductase and hence the generation of methionine from homocysteine. Excess homocysteine so generated lead to oxidative stress and activated proinflammatory cytokines leading to fatty infiltration of the liver. As published data on the prevalence of hepatic and hematological adverse effects are lacking, this study was undertaken to estimate the frequency of hepatic and hematological adverse effects of long-term low-dose MTX therapy in RA patients. The secondary objectives were to characterize the extent of rise of liver enzymes and to identify potential risk factors, if any for these adverse effects.
Materials and Methods
The study was approved by the Institutional Ethics Committee and was registered with the CTRI (CTRI/2015/05/005773). Patients were enrolled from the rheumatology outpatient department of the hospital for a period of 18 months. Since this was a time bound observational study, no formal sample size calculation was done.
Diagnosed patients of RA as per the American College of Rheumatology 2010 criteria were screened for subject eligibility criteria. Patients ≥18 years of age of either gender taking low-dose (≤15 mg/weekly) MTX for ≥2 years either as monotherapy or in combination with other drugs were included in the study after taking written informed consent. As per the treatment guidelines adopted at the Rheumatology Department of this institute, RA patients who are on MTX therapy undergo routine hemogram (hemoglobin, total count and differential count) and liver enzymes, serum alanine transaminase, aspartate transaminase, and alkaline phosphatase (ALT and AST, respectively) every 6 months. Detailed clinical, disease, drug-related, and laboratory data of these patients were recorded.
Hepatic and hematological adverse reactions were coded as per the World Health Organization (WHO) Adverse Drug Reaction Terminology Criteria. Only those adverse drug reactions where the WHO-UMC and Naranjo's causality assessment was “probable” or “possible” association were recorded.
Statistical Analysis
Data were collected in a study-specific patient case record form and analyzed using the GraphPad Prism (Version 5) software (GraphPad Software Inc., CA, USA). The extent of rise of liver enzyme was computed in terms of the number of fold rise of the respective enzyme above the upper limit of normal (ULN). Summary statistics of the study population and inferential statistics was undertaken. Comparison of normally distributed numeric variables between groups was done using unpaired t-test. Univariate analysis for identification of risk factors was done using Mann–Whitney U-test or Fisher's exact test as applicable. Test statistics along with their 95% confidence interval were computed. P ≤ 0.05 was considered statistically significant.
Results
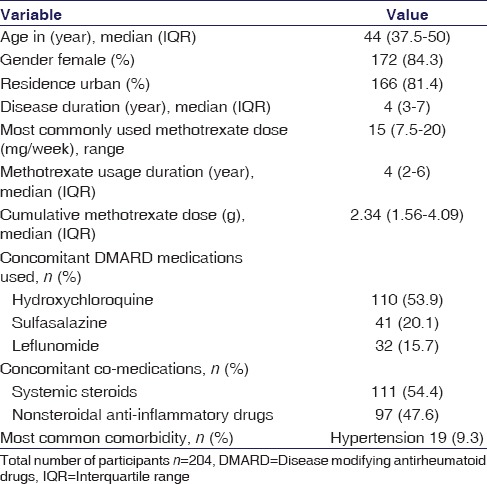
During the study period, 204 RA patients were recruited. The baseline characteristics of the study population are presented in Table 1. The median age of the study population was 44 years (interquartile range [IQR] 37.5–50), median disease duration 4 years (IQR 3–7), and MTX intake of 4 years (IQR 2–6). The most frequently used dose was 15 mg weekly and all received folic acid supplementation. Concomitant DMARD intake frequencies were hydroxychloroquine (53.9%), sulfasalazine (20.1%), and leflunomide (15.7%). Systemic steroids and NSAID were taken by 54.4% and 47.5%, respectively.
Table 1.
Demographic, disease, and drug intake profile of study participants
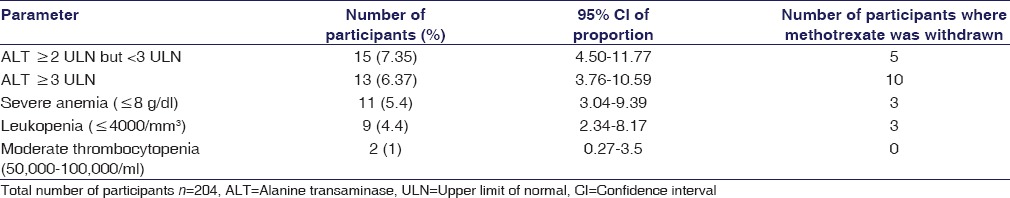
Patients having abnormal laboratory parameters in at least one assessment were considered evaluable for the respective adverse event. As per the laboratory standards, the ULN for serum ALT level estimation was 35 IU/L. Table 2 shows that the frequency of raised ALT levels in the study population. About 13.73% had ALT ≥2-fold rise among whom 7.35% (n = 15) had ALT levels ≥2 but <3-fold rise and 6.37% (n = 13) had ≥3-fold rise of ALT levels. Of those with raised ALT levels, only one case had raised serum bilirubin and AST levels, and all except one were seropositive for RA factor. Causality analysis was performed of the 28 cases using both the WHO and Naranjo's scale and 16 were “probable” and 12 “possible” association.
Table 2.
Frequency of raised serum alanine transaminase level and hematological adverse effect profile in the study population
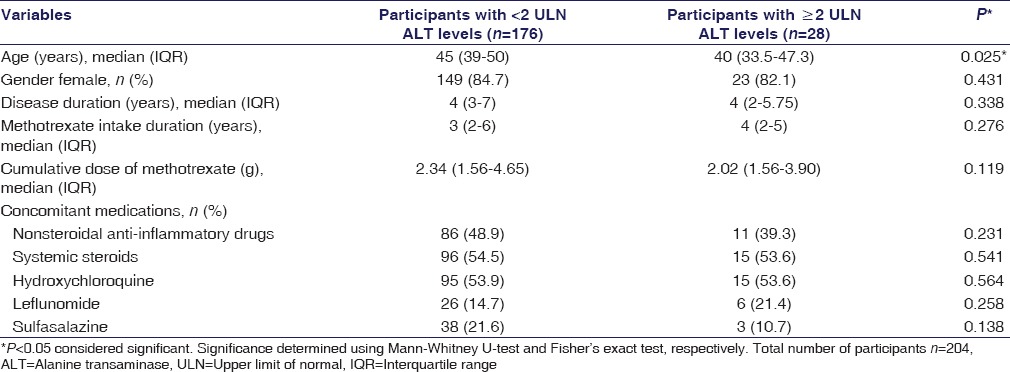
Univariate analysis to determine the association of raised ALT (levels ≥2 ULN) with age, gender, disease duration, duration of MTX intake, cumulative MTX dose, and concomitant DMARD or steroid, and NSAID intake was undertaken and enlisted in Table 3. Results reveal that there was no statistically significant difference (P > 0.05) in the two groups except for age (P = 0.025). MTX was withdrawn in all patients (n = 13) who had ≥3-fold rise of ALT and one case with ≥2 but <3-fold rise had associated raised alkaline phosphatase level with portal tract fibrosis, and two had associated leukopenia.
Table 3.
Comparison of demographic, disease, and drug intake profile of participants with or without alanine transaminase elevations
Fourteen patients experienced gastrointestinal adverse effects such as nausea, vomiting, and heartburn but had normal liver enzyme levels.
A 24-year-old male patient had biopsy-proven hepatic necrosis and fibrosis. He had been on oral MTX for nearly 10 years, cumulative dose of 7.8 g, raised ALT levels, hyperbilirubinemia (14.1 mg/dl) and >3-fold rise of AST level. In addition, a 51-year-old female patient who was on 7.5 mg weekly MTX for 6 years with a cumulative dose of 5 g had mild lobular lymphocytic infiltrates and portal tract fibrosis when liver histopathology was done. Serum alkaline phosphate level was 189 IU/L and ALT was >2 ULN. In both the cases, virological markers for hepatitis B, C, and HIV infection were negative.
The most commonly observed hematological adverse effect was anemia of varying severity. About 34.8% patients were moderately anemic (Hb 8–10.9 g/dl) while 5.4% (n = 11) had severe anemia (<8 g/dl). Leukopenia was the second most commonly observed hematological adverse effect which was noted in 4.4% (n = 9) of study participants. Severe thrombocytopenia (<50,000/μL) was not detected. MTX was withdrawn in four patients because of hematological adverse effects. All these patients were on folic acid supplementation. Univariate analysis conducted to detect the association between severe anemia and various study variables did not show statistical significance. Table 2 depicts the detailed profile of hematological adverse effects.
Discussion and Conclusion
The use of DMARD plays a pivotal role in the treatment of RA as it has been suggested to arrest disease progression. MTX, an immunomodulator drug, is the most commonly used DMARD due to its favorable cost-effectiveness profile. However, there are several issues concerning the safety profile of long-term MTX therapy. The commonly reported hematological adverse effects are leukopenia, pancytopenia, anemia, megaloblastic anemia, and thrombocytopenia. The hepatic adverse effects comprise asymptomatic rise of liver enzymes, hepatic fibrosis, portal hypertension, and fatty liver have been reported. Other systemic adverse effects include pulmonary fibrosis, nephrotoxicity, and muco-cutaneous reactions.[11]
However, there is a dearth of knowledge concerning the occurrence of such adverse effects in the Indian population. This study is the first to provide information regarding the same. RA patients tolerate it well when given in low doses even for several years. The results found in this study are in concordance with the two recently published studies published in Pakistan[12] and Saudi Arabia.[13] The incidence of MTX-induced adverse effects is similar in the developed countries even though the prevalence of malnutrition and anemia is more prevalent in our country. A study conducted in the UK reported drug withdrawal in RA patients due to MTX-induced liver injury in 5.5% cases which are slightly higher compared to this study.[14] However, the incidence of raised liver enzymes was 14.1% in a study conducted in the USA which is similar to that in our study.[15] A study conducted in Srinagar (India) in 2006 showed that the prevalence of ≥2 ULN ALT levels among RA patients on MTX therapy was 1.2%, the low prevalence could be due to inclusion of newly diagnosed cases of RA.[16]
Although the prevalence of hepatic enzyme rise is to the tune of 14% in this study, only two patients develop clinically relevant liver injury, which shows that liver enzyme rise if diagnosed at the right time may avert the development of frank liver injury. Patients with mild or moderate anemia did not require withdrawal of MTX.
The limitations of this observational study included those that are inherent of all observational studies. In addition, several patients did not have complete liver function test (LFT) reports but had only serum ALT levels. Serial estimations, and baseline LFT values were not available for majority of the patients. However, interventional studies cannot be done for addressing such study objective and a prospective cohort study though ideal could not be undertaken due to logistic and financial constraints.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Wong R, Davis A, Badley E, Grewal R, Mohammed M. Prevalence of arthritis and rheumatic diseases around the world: A growing burden and implications for health care needs. Model Care Arthritis Bone Joint Dis. 2010;7:6–7. [Google Scholar]
- 2.Chopra A, Patil J, Billempelly V, Relwani J, Tandle HS WHO-ILAR COPCORD Study. WHO International League of Associations from Rheumatology Community Oriented Program from Control of Rheumatic Diseases. Prevalence of rheumatic diseases in a rural population in western India: A WHO-ILAR COPCORD Study. J Assoc Physicians India. 2001;47:240–6. [PubMed] [Google Scholar]
- 3.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. Update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Am Coll Rheumatol. 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent PD, Luthra HS, Michet C., Jr Risk factors for methotrexate-induced abnormal laboratory monitoring results in patients with rheumatoid arthritis. J Rheumatol. 2004;31:1727–31. [PubMed] [Google Scholar]
- 5.Gupta R, Bhatia J, Gupta SK. Risk of hepatotoxicity with add-on leflunomide in rheumatoid arthritis patients. Arzneimittelforschung. 2011;61:312–6. doi: 10.1055/s-0031-1296204. [DOI] [PubMed] [Google Scholar]
- 6.Hoekstra M, van Ede AE, Haagsma CJ, van de Laar MA, Huizinga TW, Kruijsen MW, et al. Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:423–6. doi: 10.1136/ard.62.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslibekyan S, Brown EE, Reynolds RJ, Redden DT, Morgan S, Baggott JE, et al. Genetic variants associated with methotrexate efficacy and toxicity in early rheumatoid arthritis: Results from the treatment of early aggressive rheumatoid arthritis trial. Pharmacogenomics J. 2014;14:48–53. doi: 10.1038/tpj.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swierkot J, Slezak R, Karpinski P, Pawlowska J, Noga L, Szechinski J, et al. Associations between single-nucleotide polymorphisms of RFC-1, GGH, MTHFR, TYMS, and TCII genes and the efficacy and toxicity of methotrexate treatment in patients with rheumatoid arthritis. Pol Arch Med Wewn. 2015;125:152–61. doi: 10.20452/pamw.2707. [DOI] [PubMed] [Google Scholar]
- 9.Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: Basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014;7:53–64. doi: 10.2147/PGPM.S38295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan ES, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res. 2002;4:266–73. doi: 10.1186/ar419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilani ST, Khan DA, Khan FA, Ahmed M. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. J Coll Physicians Surg Pak. 2012;22:101–4. [PubMed] [Google Scholar]
- 12.Attar SM. Adverse effects of low dose methotrexate in rheumatoid arthritis patients. A hospital-based study. Saudi Med J. 2010;31:909–15. [PubMed] [Google Scholar]
- 13.Lampropoulos CE, Orfanos P, Bournia VK, Karatsourakis T, Mavragani C, Pikazis D, et al. Adverse events and infections in patients with rheumatoid arthritis treated with conventional drugs or biologic agents: A real world study. Clin Exp Rheumatol. 2015;33:216–24. [PubMed] [Google Scholar]
- 14.Kinder AJ, Hassell AB, Brand J, Brownfield A, Grove M, Shadforth MF. The treatment of inflammatory arthritis with methotrexate in clinical practice: Treatment duration and incidence of adverse drug reactions. Rheumatology (Oxford) 2005;44:61–6. doi: 10.1093/rheumatology/keh512. [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz RS, Goldstein DP, Bernstein MR. Ten year's experience with methotrexate and folinic acid as primary therapy for gestational trophoblastic disease. Gynecol Oncol. 1986;23:111–8. doi: 10.1016/0090-8258(86)90123-x. [DOI] [PubMed] [Google Scholar]
- 16.Buhroo AM, Ortho MS. Adverse effects of low-dose methotrexate in patients with rheumatoid arthritis. Indian J Phys Med Rehabil. 2006;17:21–5. [Google Scholar]


