Abstract
Purpose
Components of diet can modulate inflammation and therefore may have an important role in the development of nasopharyngeal carcinoma (NPC). Little is known about the inflammatory potential of diet in relation to nasopharyngeal carcinogenesis.
Methods
Data from an Italian multicentre case-control study conducted between 1992 and 2008 and including 198 cases with incident, histologically confirmed NPC, and 594 controls hospitalized for acute non-neoplastic diseases were used to estimate the relation between a dietary inflammatory index (DII) and the risk of NPC. The DII was computed based on the intake of selected dietary factors assessed by a validated 78-item food frequency questionnaire. Logistic regression models were used to estimate odds ratios (ORs) adjusted for study centre, place of living, sex, age, year of interview, education, tobacco smoking, alcohol drinking, and energy intake using the residual method.
Results
Subjects with higher DII scores had an increased risk of NPC, with each DII point increasing risk by nearly 20% (OR=1.19, 95 % confidence interval, CI, 1.05–1.36). Compared to subjects in the lowest DII tertile, those in the highest tertile had >60% higher risk of NPC (OR=1.64; CI, 1.06–2.55; ptrend=0.04).
Conclusion
These results indicate that inflammatory potential of diet plays a role in NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a rare malignancy, with significant worldwide variation in incidence [1]. Approximately 80,000 new cases reported per year, accounting 0.7% of all cancers [2]. The highest rates are observed in the southern part of China, other parts of South-Eastern Asia, Northern and Southern Africa, and in the Inuit population in Alaska. Populations living elsewhere in the United States of America and Europe have considerably lower NPC incidence rates [3,4]. Epstein-Barr virus (EBV) infection and genetic susceptibility appear to have a major impact in higher incidence populations; however, other environmental factors such as occupational exposure and tobacco smoke play a role [5,6].
The body mounts an acute inflammatory response to tissue injury or infection in the through the action of inflammatory stimulants such as cytokines [7,8]. Chronic inflammation is known to play a key role in the development of various cancers, including NPC [9,10]. Various dietary components, including fatty fish, whole grains, high energy diet and vitamins, have different effect on inflammation [11–13]. A prudent dietary pattern high in fish, yogurt, pulse, rice, fruit, vegetables, pasta and wine has been shown to be associated with lower concentrations of intermediary inflammatory markers [14].
A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual’s diet [13]. A pro-inflammatory diet is rich in consumption of food items rich in saturated fat, carbohydrate, and poor in the consumption of poly-unsaturated fatty acids, flavonoids, and other dietary components. The DII has been validated with various inflammatory markers, including C-reactive protein [15,16] and interleukin-6 [17–19] in several longitudinal and cross-sectional studies. Additionally, the DII has been associated with glucose intolerance and dyslipidemia components of the metabolic syndrome [20,21] in two cross-sectional studies, anthropometric measurements in a cohort study in Spain [22], asthma in a case-control study in Australia [17], bone mineral density among postmenopausal women in a cross-sectional study in Iran [23], two colorectal cancer case-control studies in Spain and Italy [24,25] and in cohort studies in the USA [26,27], and pancreatic and prostate cancers in case-control studies in Italy [28,29].
We tested the hypothesis that a higher DII score (indicating a pro-inflammatory diet) increases the risk of NPC. A multicentre case-control study conducted in Italy [30] provided original information on a Southern European population in which NPC rates are low; and dietary and life-style habits and awareness of diet-related health issues are different from those in countries where NPC is more common.
Methods
Recruitment and questionnaire
A case–control study on NPC was conducted between January 1992 and December 2008 within an established network of centres, including Aviano (Pordenone) and Milan in northern Italy, and Naples and Catania in southern Italy [31,30]. Cases were 198 European subjects (157 men, 41 women; median age 52 years, range 18–76 years) admitted to major teaching and general hospitals in the study areas with incident, histologically confirmed incident NPC diagnosed within one year before interview, and with no history of cancer at other sites. These included 137 (68.5%) undifferentiated NPCs (World Health Organization (WHO) type 3), 23 (11.5%) keratinising squamous cell carcinomas (WHO type 1; here referred to as differentiated NPCs), and 38 (20%) not otherwise specified NPCs [32]. EBV status was defined based on the detection of EBV nuclear antigen in tissue samples and was available for 61 cases only. Of these, 57 undifferentiated NPCs tested positive and to out of four differentiated NPCs were EBV positive.
The control group included 594 European patients (aged 19–76 years; median age: 52 years) admitted for a wide spectrum of acute conditions to the same hospitals as cases. Controls were admitted for trauma (34%), other orthopaedic disorders (32%), acute surgical conditions (22%), and miscellaneous other illnesses (12%). Controls admitted for malignant neoplasms, conditions related to tobacco smoking or alcohol consumption, or any other disorder associated to long-term modification of diet were not eligible for this study. All study participants signed an informed consent, according to the recommendations of the Internal Review Boards of each study hospital. Less than 10% of cases and controls refused to participate. Centrally trained interviewers administered a structured questionnaire to cases and controls during their hospital stay. The questionnaire included information on socio-demographic characteristics; anthropometric variables; life-style factors, including diet (described in more detail below), tobacco and alcohol; a problem-oriented personal medical history; family history of cancer; and, for women, menstrual and reproductive history. Controls were selected and interviewed within a year of case selection and interview.
Information on diet during the two years before cancer diagnosis (for cases) or hospital admission (for controls) was based on a food frequency questionnaire (FFQ) that was validated for nutrient intake and tested for reproducibility and validity for specific nutrients and food items [33,34]. Subjects were asked to indicate the average weekly consumption for the period under investigation. The FFQ consists of 78 foods and food groups, including some of the most common Italian recipes, and of 5 questions on various types of alcoholic beverages. Intakes reported at least once a month but less than once a week were coded as 0.5 per week. Dietary supplements were not considered, given their rare consumption in this population [35]. Other questions on fat-intake pattern, as well as portion size, were used to modulate the composition of recipes. In particular, a commonly used unit or serving size was pre-specified for 40 items; whereas for the remainder, portion was defined as small, average or large, and depicted in figures made available to participants. For a few vegetables and fruits, seasonal consumption and the corresponding duration were elicited. To compute energy and nutrient intakes, an Italian food composition database, as well as information from additional sources, were used [36]. Losses due to cooking were subtracted from the computation of the nutrients or food components, when appropriate.
Dietary Inflammatory Index (DII)
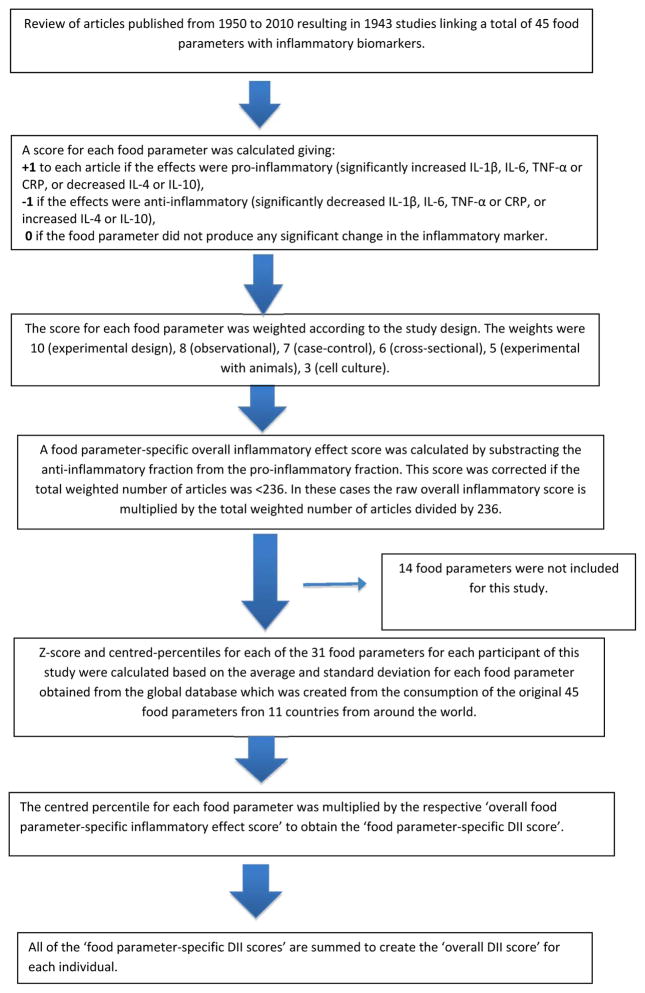
FFQ-derived dietary data were used to calculate the DII for each subject. A complete description of the DII is available elsewhere [13]. Briefly, dietary data were first linked to a regionally representative database that provided a robust estimate of the mean and the standard deviation for each food parameter included in the DII. These parameters then became the multipliers to express an individual’s exposure relative to the “standard global mean” as a z-score. This was achieved by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing,” this value was then converted to a centred percentile score by doubling and subtracting 1. The centred percentile score for each food parameter for each subject was then multiplied by the corresponding food parameter effect score in order to obtain a food parameter-specific DII score. All of the food parameter-specific DII scores were then summed to create the overall DII score for each subject. The DII computed on this study’s FFQ includes data on 31 of the possible 45 food parameters that comprise the DII: carbohydrate, protein, fat, alcohol, fiber, cholesterol, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamin, riboflavin, vitamin B6, iron, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic Acid, β-carotene, anthocyanidins, flavan-3-ol, flavonol, flavonones, flavones, isoflavones, caffeine, and tea. The DII has been previously used in this population in four case-control studies [37–40] and in one cohort study [41]. Steps involved in DII calculation are described in Figure 1.
Figure 1.
Sequence of steps in creating the dietary inflammatory index in the Italian Nasopharyngeal cancer case-control study
The DII was analyzed both as a continuous variable (i.e., a one-unit increment in the DII corresponds to one standard deviation (DII=±1.43) in the current study and by sex-specific tertiles of exposure, determined from controls in this study population. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) of NPC were estimated using logistic regression models, adjusting only for study centre, sex, age, and calendar year, and additionally adjusting for education, (<7, 7–11 and ≥12 years), tobacco smoking (never smokers, ex smokers, <15 and ≥15 cigarettes/day current smokers) and alcohol drinking (<14, 14–27 and ≥28 drinks/week). Energy adjustment was made using the residual method in both the models [42]. We also performed analyses across strata of sex, smoking, and alcohol. Sensitivity analyses were conducted by calculating DII scores without highly correlated micro and macronutrient. Tests for trend were performed using the median value within each tertile as an ordinal variable. Statistical analyses were performed using SAS® 9.3 (SAS Institute, Inc.).
Results
Table 1 shows the distribution of NPC cases and controls according to study centre, place of residence, sex, age, education, tobacco smoking and alcohol drinking habits. By design, cases and controls had similar distribution by study centre, sex and age. Cases tended to be more frequent alcohol drinkers and heavy smokers. The mean DII score among cases was 0.28 (SD=1.49) and among controls it was −0.09 (SD=1.40), indicating a slightly more pro-inflammatory diet for cases.
Table 1.
Distribution of 198 cases of nasopharyngeal cancer and 594 controls according to age, education and other selected covariates. Italy, 1992–2008.
| Cases | Controls | |
|---|---|---|
|
| ||
| N. (%) | N. (%) | |
| Study centrea | ||
| Aviano | 90 (45.5) | 270 (45.5) |
| Milan | 48 (24.2) | 144 (24.2) |
| Naples and Catania | 60 (30.3) | 180 (30.3) |
| Place of living | ||
| Northeast | 90 (45.5) | 270 (45.5) |
| Northwest | 48 (24.2) | 144 (24.2) |
| Centre, South, Islands | 60 (30.3) | 180 (30.3) |
| Sexa | ||
| Men | 157 (79.3) | 471 (79.3) |
| Women | 41 (20.7) | 123 (20.7) |
| Age (years)a | ||
| <45 | 52 (26.3) | 159 (26.7) |
| 45–54 | 64 (32.3) | 186 (31.3) |
| 55–64 | 47 (23.7) | 144 (24.2) |
| ≥65 | 35 (17.7) | 105 (17.7) |
| Education (years)b | ||
| <7 | 67 (33.8) | 221 (37.3) |
| 7–11 | 73 (36.9) | 222 (37.5) |
| ≥12 | 58 (29.3) | 149 (25.2) |
| Tobacco smoking statusb | ||
| Never smoker | 60 (30.6) | 196 (33.0) |
| Ex-smoker | 60 (30.6) | 181 (30.5) |
| Current smoker (cigarettes/day) | ||
| <15 | 21 (10.7) | 78 (13.1) |
| ≥15 | 55 (28.1) | 139 (23.4) |
| Alcohol drinking (drinks per week) | ||
| <14 | 72 (36.4) | 235 (40.3) |
| 14–20 | 39 (19.7) | 149 (25.1) |
| ≥21 | 87 (43.9) | 210 (35.4) |
Matching variable.
The sum does not add up to the total because of some missing values.
Control characteristics across tertiles of DII are provided in Table 2. There were some differences in socio-demographic, and lifestyle habits across DII tertiles. Compared to controls in the lowest tertile, subjects in the highest tertile were younger, more frequently males, current smokers and heavy alcohol drinkers.
Table 2.
Distribution of 594 controls by selected characteristics and tertiles of energy-adjusted dietary inflammatory index (DII). Italy, 1992–2008.
| Characteristics (%) | Energy-adjusted DII tertile | |||
|---|---|---|---|---|
| 1 Men ≤−0.64 Women ≤−1.06 |
2 Men−0.64 to 0.59 Women −1.05 to − 0 19 |
3 Men > 0.59 Women >−0.19 |
P-Valuea | |
| Sexa | 0.001 | |||
| Men | 71.8 | 79.7 | 87.4 | |
| Women | 28.2 | 20.3 | 12.6 | |
| Age (years)a | 0.01 | |||
| <45 | 16.3 | 30.2 | 31.7 | |
| 45–54 | 34.9 | 30.7 | 31.2 | |
| 55–64 | 29.2 | 22.3 | 20.8 | |
| ≥65 | 19.6 | 16.8 | 16.4 | |
| Education (years) | 0.41 | |||
| <7 | 42.1 | 36.1 | 32.8 | |
| 7–11 | 35.4 | 37.6 | 40.4 | |
| >11 | 22.5 | 26.2 | 26.8 | |
| Tobacco smoking | 0.003 | |||
| Never smoker | 40.2 | 32.2 | 25.7 | |
| Ex smoker | 30.1 | 31.7 | 29.5 | |
| Current smoker (cigarettes/day) | 29.6 | 36.2 | 44.8 | |
| <15 | 14.8 | 12.9 | 12.6 | |
| ≥15 | 14.8 | 23.3 | 32.2 | |
| Alcohol drinking (drinks | 0.01 | |||
| <14 | 25.8 | 31.2 | 35.5 | |
| 14–27 | 46.4 | 43.6 | 30.6 | |
| ≥28 | 27.8 | 25.2 | 33.9 | |
Estimated by Chi-square test.
ORs and 95% CIs of NPC according to tertiles of DII and continuous DII are shown in Table 3. When analyses were carried out using continuous DII, for an increase of 1 standard deviation DII (=1.43), a significant positive association was observed with NPC risk when adjusted only for energy and additionally adjusted for study centre, place of living, sex, age, and calendar year(OR=1.18; 95% CI 1.04–1.34, p-value=0.005). When also adjusted for education, smoking, alcohol drinking, there was minimal change in the risk estimate (OR=1.19; 95% CI 1.05–1.36, p-value=0.008). Subjects in the highest DII tertile had 58% higher risk of NPC compared to subjects in the lowest tertile (95% CI=1.04–2.40; p-trend=0.01) in the second model, and 64% (95% CI=1.06–2.55; p-trend=0.01) in the third model.
Table 3.
Odds ratios (ORs) of nasopharyngeal cancer and corresponding confidence intervals (CI) for energy-adjusted dietary inflammatory index (DII) both as continuous and expressed as tertiles among 198 cases and 594 controls. Italy, 1992–2008.
| Energy-adjusted DII tertile | p value | DII as Continuousa | |||
|---|---|---|---|---|---|
| 1b | 2 | 3 | |||
| Cases/controls | 53/198 | 55/198 | 90/199 | 198/594 | |
| Model 1c | 1.00 | 1.04 (0.68, 1.59) | 1.67 (1.13, 2.48) | 0.006 | 1.20 (1.07, 1.34) |
| Model 2d | 1.00 | 0.85 (0.54, 1.34) | 1.58 (1.04, 2.40) | 0.01 | 1.18 (1.04, 1.34) |
| Model 3e | 1.00 | 0.87 (0.55, 1.37) | 1.64 (1.06, 2.55) | 0.01 | 1.19 (1.05, 1.36) |
Referent.
One unit increase corresponding to 1SD (±1.43).
Adjusted for energy intake according to the residual method.
Adjusted for study centre, place of living, sex, age, year of interview, and energy intake according to the residual method.
Adjusted for study centre, place of living, sex, age, year of interview, education, smoking, alcohol drinking, and energy intake according to the residual method.
Table 4 shows the ORs among strata of selected covariates. Risk estimates were consistent across strata of sex, smoking and alcohol drinking (p for heterogeneity, 0.14, 0.33 and 0.81, respectively). Servings of fruit, vegetables, fish, soup and poultry significantly decreased across tertiles of DII, whereas servings of desserts significantly increased (data not shown).
Table 4.
Odds ratiosa (ORs) of nasopharyngeal cancer and corresponding confidence intervals (CI) for energy-adjusted dietary inflammatory index (DII) both as continuous and expressed as tertiles by sex and smoking status, among 198 cases and 594 controls. Italy, 1992–2008.
| Energy-adjusted DII tertile
|
p value | Continuousc | |||
|---|---|---|---|---|---|
| 1b | 2 | 3 | |||
|
|
|||||
| Sex | |||||
| Men | 1.00 | 0.78 (0.47, 1.31) | 1.43 (0.88, 2.32) | 0.05 | 1.14 (0.98, 1.32) |
| Women | 1.00 | 1.18 (0.38, 3.66) | 2.72 (0.91, 8.08) | 0.09 | 1.44 (1.05, 1.98) |
| Smoking status | |||||
| Never smokers | 1.00 | 0.74 (0.32, 1.71) | 2.00 (0.92, 4.35) | 0.06 | 1.28 (1.01, 1.64) |
| Ex-smokers | 1.00 | 0.55 (0.24, 1.27) | 1.36 (0.66, 2.82) | 0.28 | 1.05 (0.84, 1.31) |
| Current smokers | 1.00 | 1.56 (0.67, 3.65) | 1.96 (0.83, 4.62) | 0.25 | 1.31 (1.04, 1.65) |
| Alcohol drinking (drinks per week) | |||||
| <14 | 1.00 | 0.87 (0.39, 1.91) | 1.98 (0.94, 4.16) | 0.02 | 1.29 (1.05, 1.59) |
| 14–27 | 1.00 | 0.36 (0.13, 0.99) | 1.58 (0.63, 3.92) | 0.36 | 1.14 (0.86, 1.51) |
| ≥28 | 1.00 | 1.23 (0.56, 2.74) | 1.54 (0.71, 3.35) | 0.27 | 1.15 (0.92, 1.44) |
Adjusted for study centre, sex, age, year of interview, education, smoking, alcohol drinking, education, and energy intake according to the residual method.
Referent
One unit increase corresponding to 1SD (±1.43)
In the sensitivity analyses carried out after excluding strongly correlated micro- and macronutrients (total fat, total fibre, total protein, saturated fat, omega 3, omega 6, thiamin, vitamin b6, total folate and zinc) in the DII calculation, no significant change was observed in the results (Multivariate analyses with DII continuous: OR=1.22; 95% CI:1.03–1.45, p-value=0.02).
Discussion
The results of this study on the relationship between inflammatory potential of diet and NPC risk show that consuming a more pro-inflammatory diet, as reflected in higher DII values, was associated with an increased risk of NPC. Previous results based on these data indicated an increased consumption of carotenoids, including α-carotene and β-carotene, and yellow- or red-pigmented vegetables to be protective against NPC [30,43]. In addition, dietary intake of soluble and insoluble fibers was inversely related to NPC risk [31]. All of these nutrients and food items contribute to decreased DII scores [13]. Furthermore, diets rich in animal products, starch, and fats, which have been reported to increase DII scores [13], were positively associated with NPC risk [44].
Increased risks of NPC have been observed for high consumption of preserved meat, saturated fat, [45] and heavy alcohol drinking [46] in two case-control studies. A systematic review from 16 case-control studies showed inverse associations with non-preserved vegetable intake and a direct association with preserved vegetable [47]. Results from a case-control study in China showed increased fruit and vegetable intake to be protective against NPC [48]. In another Chinese case-control study, exploring the association between various components of traditional Cantonese diet and NPC consumption of Canton-style salted fish, preserved vegetables and preserved/cured meat were significantly associated with increased risk, while consumption of fresh fruit, Canton-style herbal tea and herbal slow-cooked soup was associated with decreased risk of NPC [49]. Habitual consumption of soy products had no significant effect on the risk of NPC in Chinese adults [50]. A case-control study in Taiwan showed an inverse association with intake of fresh fish, green tea, coffee and vitamin A intake from plant sources, whereas no association was observed with the intake of meats, salted fish, fresh vegetables, fruits and milk [51].
The mechanism for the observed association between DII and NPC is not known. A possible explanation is through the attenuating effect of dietary components on STAT 3 activation, which is an oncogenic transcription factor responsible for NPC [52]. Additionally, diet plays a role in the expression of transcription factors; nuclear factor (NF)-κB and Nuclear factor (erythroid-derived 2)-like 2 (Nrf-2), which are regulators of inflammatory responses [53,54]. Accordingly, a pro-inflammatory diet enhances the production of the tumor-promoting cytokines IL-6 and TNF, which cause nasopharyngeal inflammation and activation of STAT3, which then results in increased NPC risk.
Because of the rarity of NPC in Western populations, studies of NPC in Europe are feasible only using case-control designs. In this case-control study both cases and controls came from comparable catchment areas and were interviewed by uniformly trained interviewers in their respective hospital settings. To limit possible sources of bias, we included in the control group subjects admitted for a wide spectrum of acute, non-neoplastic conditions, unrelated to the major risk factors for NPC. The comparable catchment areas of cases and controls contributed to reduce selection bias. Furthermore the uniformity of the hospital setting would have served to improve the comparability of information, as cases and controls are interviewed under similar conditions. Subjects were unaware of any particular study-related hypothesis in relation to diet, thereby reducing potential selection and information bias [55]. The FFQ was satisfactorily reliable [33] and valid [34]. Still, some degree of misclassification may exist in the dietary assessment.
Participation among eligible cases and controls was almost complete, and we excluded from the controls patients admitted for diseases likely to be related to tobacco smoking, alcohol consumption and long-term dietary changes. Our results were adjusted for the major known risk factors for NPC, including education, smoking, and alcohol drinking. In addition, analyses were adjusted for energy intake. Possible limitations are the non-availability of the remaining 14 food parameters for the DII calculation. DII calculated from these 31 food parameters has not been validated with inflammatory markers. However, in previous validation studies, the DII has been calculated from food parameters ranging from 17 to 44 [38,13]. Moreover, there could be a possible overestimation due to the inclusion of food items in the DII calculation that also are a source of important nutrients. However, each of these food items has an inflammatory effect score, which is derived from an extensive review of literature looking at the association between these foods and inflammation. In the sensitivity analyses conducted excluding food parameters that were strongly correlated, we did not observe any significant change in the results. Another limitation could be the absence of evidence of DII being associated with inflammatory markers in this study; though the case-control nature of the study is not amenable to adding such measures during the etiologically relevant period in any event. The large majority of cases were EBV positive, as recognized for nasopharyngeal carcinoma. The effect of DII was independent from EBV status. Genetic susceptibility was considered by Turati et al, 2013 [56]. Family history of nasopharyngeal cancer in first degree relatives was reported by 3 cases and 6 controls, giving an OR of 1.2 (95%%CI: 0.30–5.7). We did not include genetic susceptibility in the analyses since there were just 9 participants with family history of NPC.
In conclusion, Italian men and women who consumed a more pro-inflammatory diet, high in carbohydrate and fat and characterized by low intake of fruit and vegetables had an increased risk of NPC compared to those who consumed a more anti-inflammatory diet. This indicates that encouraging intake of more anti-inflammatory dietary factors, such as omega-3 fatty acid rich foods, white meat in place of red meat, and plant-based foods rich in vitamin E and phytochemicals, and reducing intake of pro-inflammatory factors, such as red meat rich in saturated fat, may be a strategy for reducing the risk of NPC.
Acknowledgments
Funding: This study was supported by the Italian Foundation for Research on Cancer (FIRC) by the Italian Ministry of Health, General Directorate of European and International Relations. Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases.
This study was supported by the Italian Institute of Health, Italian Ministry of Health, General Directorate of European and International Relations and the Italian Foundation for Research On Cancer.
Footnotes
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
Author contribution: AZ, MM, CLV, ML, WG and DS designed and conducted the case-control study, NS conducted the analyses and wrote the first draft of the manuscript, MR, JRH, CLV and AZ provided suggestions and revised the manuscript. All authors approved the final version of the manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.GLOBOCAN. [Accessed 30 August 2015];2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide. 2012 www.globocan.iarc.fr.
- 3.Curado MP, BE, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P. Cancer Incidence in Five Continents. Vol. 9. Lyon, France: 2007. IARC Sci Publ No, 160. [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. Epub 22011 Feb 20104. [DOI] [PubMed] [Google Scholar]
- 5.Vokes EE, Liebowitz DN, Weichselbaum RR. Nasopharyngeal carcinoma. Lancet. 1997;350(9084):1087–1091. doi: 10.1016/S0140-6736(97)07269-3. [DOI] [PubMed] [Google Scholar]
- 6.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(10):1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 7.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15(17):1949–1955. doi: 10.2174/138161209788453167. [DOI] [PubMed] [Google Scholar]
- 8.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009;57(11):4467–4477. doi: 10.1021/jf900612n. [DOI] [PubMed] [Google Scholar]
- 9.Qin X, Peng Q, Lao X, Chen Z, Lu Y, Lao X, Mo C, Sui J, Wu J, Zhai L, Yang S, Li S, Zhao J. The association of interleukin-16 gene polymorphisms with IL-16 serum levels and risk of nasopharyngeal carcinoma in a Chinese population. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35(3):1917–1924. doi: 10.1007/s13277-013-1257-2. [DOI] [PubMed] [Google Scholar]
- 10.Qu YL, Yu H, Chen YZ, Zhao YX, Chen GJ, Bai L, Liu D, Su HX, Wang HT. Relationships between genetic polymorphisms in inflammation-related factor gene and the pathogenesis of nasopharyngeal cancer. Tumour Biology. 2014;35(9):9411–9418. doi: 10.1007/s13277-014-2123-6. [DOI] [PubMed] [Google Scholar]
- 11.de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkanen H, Uusitupa M. A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia. 2011;54(11):2755–2767. doi: 10.1007/s00125-011-2285-3. [DOI] [PubMed] [Google Scholar]
- 12.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8(10):2868–2875. doi: 10.1111/j.1743-6109.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- 13.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, Mavroeidi A, Simpson WG, Duthie GG, Macdonald HM. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. The British journal of nutrition. 2014;112(8):1341–1352. doi: 10.1017/S0007114514001962. [DOI] [PubMed] [Google Scholar]
- 15.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public health nutrition. 2014;17(8):1825–1833. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood LG, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2015;45(1):177–183. doi: 10.1111/cea.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I. Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. The British journal of nutrition. 2015;113(4):665–671. doi: 10.1017/S000711451400395X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, Park HL, Rosal MC, Shikany JM, Shivappa N, Ockene JK, Hebert JR. Construct validation of the dietary inflammatory index among postmenopausal women. Annals of epidemiology. 2015;25(6):398–405. doi: 10.1016/j.annepidem.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkerwi Aa, Shivappa N, Crichton G, Hébert JR. No significant independent relationships with cardiometabolic biomarkers were detected in the Observation of Cardiovascular Risk Factors in Luxembourg study population. Nutrition Research. doi: 10.1016/j.nutres.2014.07.017. http://dx.doi.org/10.1016/j.nutres.2014.07.017. [DOI] [PMC free article] [PubMed]
- 21.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D, Salas-Salvado J, Fito M, Lamuela-Raventos RM, Rekondo J, Fernandez-Crehuet J, Fiol M, Santos-Lozano JM, Serra-Majem L, Pinto X, Martinez JA, Ros E, Estruch R, Martinez-Gonzalez MA. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. The British journal of nutrition. 2015;113(6):984–995. doi: 10.1017/S0007114514004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivappa N, Hebert JR, Karamati M, Shariati-Bafghi SE, Rashidkhani B. Increased inflammatory potential of diet is associated with bone mineral density among postmenopausal women in Iran. European journal of nutrition. 2015 doi: 10.1007/s00394-015-0875-4. [DOI] [PubMed] [Google Scholar]
- 24.Zamora-Ros R, Shivappa N, Steck SE, Canzian F, Landi S, Alonso MH, Hebert JR, Moreno V. Dietary inflammatory index and inflammatory gene interactions in relation to colorectal cancer risk in the Bellvitge colorectal cancer case-control study. Genes & nutrition. 2015;10(1):447. doi: 10.1007/s12263-014-0447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, Hebert JR. Inflammatory potential of diet and risk of colorectal cancer: a case-control study from Italy. The British journal of nutrition. 2015;114(1):152–158. doi: 10.1017/S0007114515001828. [DOI] [PubMed] [Google Scholar]
- 26.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, Wactawski-Wende J, Ockene JK, Hebert JR. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer causes & control : CCC. 2015;26(3):399–408. doi: 10.1007/s10552-014-0515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR. The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. The British journal of nutrition. 2015;113(11):1819–1827. doi: 10.1017/S000711451500104X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR. Association between dietary inflammatory index and prostate cancer among Italian men. The British journal of nutrition. 2014:1–6. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. The British journal of nutrition. 2014:1–7. doi: 10.1017/S0007114514003626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polesel J, Negri E, Serraino D, Parpinel M, Barzan L, Libra M, Bosetti C, Garavello W, Montella M, La Vecchia C, Franceschi S, Talamini R. Dietary intakes of carotenoids and other nutrients in the risk of nasopharyngeal carcinoma: a case-control study in Italy. British journal of cancer. 2012;107(9):1580–1583. doi: 10.1038/bjc.2012.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bidoli E, Pelucchi C, Polesel J, Negri E, Barzan L, Franchin G, Franceschi S, Serraino D, De Paoli P, La Vecchia C, Talamini R. Fiber intake and risk of nasopharyngeal carcinoma: a case-control study. Nutrition and cancer. 2013;65(8):1157–1163. doi: 10.1080/01635581.2013.828088. [DOI] [PubMed] [Google Scholar]
- 32.Shanmugaratnam K, Sobin LH. The World Health Organization histological classification of tumours of the upper respiratory tract and ear. A commentary on the second edition. Cancer. 1993;71(8):2689–2697. doi: 10.1002/1097-0142(19930415)71:8<2689::aid-cncr2820710843>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 33.Franceschi S, Negri E, Salvini S, Decarli A, Ferraroni M, Filiberti R, Giacosa A, Talamini R, Nanni O, Panarello G, et al. Reproducibility of an Italian food frequency questionnaire for cancer studies: results for specific food items. Eur J Cancer. 1993;29A(16):2298–2305. doi: 10.1016/0959-8049(93)90225-5. [DOI] [PubMed] [Google Scholar]
- 34.Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, Negri E, Salvini S, Falcini F, Giacosa A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Annals of epidemiology. 1996;6(2):110–118. doi: 10.1016/1047-2797(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 35.Istituto Nazionale di Statistica. Indagine Multiscopo sulle famiglie: stili di vita e condizioni di salute. 5. ISTAT; Rome, Italy: 2005. [Google Scholar]
- 36.Gnagnarella P, Parpinel M, Salvini S, Franceschi S, Palli D, Boyle P. The update of the Italian Food Composition Database. Journal of Food Composition and Analysis. 2004;17(3–4):509–522. doi: http://dx.doi.org/10.1016/j.jfca.2004.02.009. [Google Scholar]
- 37.Maisonneuve P, Shivappa N, Hebert JR, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. European journal of nutrition. 2015 doi: 10.1007/s00394-015-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Canela M, Zazpe I, Shivappa N, Hebert JR, Sanchez-Tainta A, Corella D, Salas-Salvado J, Fito M, Lamuela-Raventos RM, Rekondo J, Fernandez-Crehuet J, Fiol M, Santos-Lozano JM, Serra-Majem L, Pinto X, Martinez JA, Ros E, Estruch R, Martinez-Gonzalez MA. Dietary inflammatory index and anthropometric measures of obesity in a population sample at high cardiovascular risk from the PREDIMED (PREvencion con DIeta MEDiterranea) trial. The British journal of nutrition. 2015:1–12. doi: 10.1017/S0007114514004401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner-McGrievy GM, Wirth MD, Shivappa N, Wingard EE, Fayad R, Wilcox S, Frongillo EA, Hebert JR. Randomization to plant-based dietary approaches leads to larger short-term improvements in Dietary Inflammatory Index scores and macronutrient intake compared with diets that contain meat. Nutrition research. 2015;35(2):97–106. doi: 10.1016/j.nutres.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hébert J. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case–control study from Italy. Cancer Causes & Control. 2015:1–9. doi: 10.1007/s10552-015-0636-y. [DOI] [PubMed] [Google Scholar]
- 41.Maisonneuve P, Shivappa N, Hébert J, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G, Gnagnarella P. Dietary inflammatory index and risk of lung cancer and other respiratory conditions among heavy smokers in the COSMOS screening study. Eur J Nutr. 2015:1–11. doi: 10.1007/s00394-015-0920-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. American Journal of Epidemiology. 1986;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 43.Polesel J, Serraino D, Negri E, Barzan L, Vaccher E, Montella M, Zucchetto A, Garavello W, Franceschi S, La Vecchia C, Talamini R. Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer causes & control : CCC. 2013;24(6):1157–1165. doi: 10.1007/s10552-013-0195-z. [DOI] [PubMed] [Google Scholar]
- 44.Edefonti V, Nicolussi F, Polesel J, Bravi F, Bosetti C, Garavello W, La Vecchia C, Bidoli E, Decarli A, Serraino D, Calza S, Ferraroni M. Nutrient-based dietary patterns and nasopharyngeal cancer: evidence from an exploratory factor analysis. British journal of cancer. 2015;112(3):446–454. doi: 10.1038/bjc.2014.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farrow DC, Vaughan TL, Berwick M, Lynch CF, Swanson GM, Lyon JL. Diet and nasopharyngeal cancer in a low-risk population. International journal of cancer Journal international du cancer. 1998;78(6):675–679. doi: 10.1002/(sici)1097-0215(19981209)78:6<675::aid-ijc2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 46.Polesel J, Franceschi S, Talamini R, Negri E, Barzan L, Montella M, Libra M, Vaccher E, Franchin G, La Vecchia C, Serraino D. Tobacco smoking, alcohol drinking, and the risk of different histological types of nasopharyngeal cancer in a low-risk population. Oral oncology. 2011;47(6):541–545. doi: 10.1016/j.oraloncology.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 47.Gallicchio L, Matanoski G, Tao XG, Chen L, Lam TK, Boyd K, Robinson KA, Balick L, Mickelson S, Caulfield LE, Herman JG, Guallar E, Alberg AJ. Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. International journal of cancer Journal international du cancer. 2006;119(5):1125–1135. doi: 10.1002/ijc.21946. [DOI] [PubMed] [Google Scholar]
- 48.Liu YT, Dai JJ, Xu CH, Lu YK, Fan YY, Zhang XL, Zhang CX, Chen YM. Greater intake of fruit and vegetables is associated with lower risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. Cancer Cause Control. 2012;23(4):589–599. doi: 10.1007/s10552-012-9923-z. [DOI] [PubMed] [Google Scholar]
- 49.Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX, Liu WS, Qin HD, Feng QS, Chen LZ, Yao SY, Zeng YX. Traditional Cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer. 2010;10:446. doi: 10.1186/1471-2407-10-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YT, Fan YY, Xu CH, Lin XL, Lu YK, Zhang XL, Zhang CX, Chen YM. Habitual consumption of soy products and risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. PLoS ONE. 2013;8(10):e77822. doi: 10.1371/journal.pone.0077822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu WL, Pan WH, Chien YC, Yu KJ, Cheng YJ, Chen JY, Liu MY, Hsu MM, Lou PJ, Chen IH, Yang CS, Hildesheim A, Chen CJ. Lowered risk of nasopharyngeal carcinoma and intake of plant vitamin, fresh fish, green tea and coffee: a case-control study in Taiwan. PLoS ONE. 2012;7(7):e41779. doi: 10.1371/journal.pone.0041779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin CH, Chao LK, Hung PH, Chen YJ. EGCG inhibits the growth and tumorigenicity of nasopharyngeal tumor-initiating cells through attenuation of STAT3 activation. Int J Clin Exp Pathol. 2014;7(5):2372–2381. [PMC free article] [PubMed] [Google Scholar]
- 53.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes & nutrition. 2009;4(3):215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription Factor Nrf2 Regulates Inflammation by Mediating the Effect of 15-Deoxy-Δ12,14-Prostaglandin J2. Molecular and Cellular Biology. 2004;24(1):36–45. doi: 10.1128/mcb.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D. An assessment, and reproducibility of food frequency data provided by hospital controls. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 1997;6(3):288–293. doi: 10.1097/00008469-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 56.Turati F, Edefonti V, Bosetti C, Ferraroni M, Malvezzi M, Franceschi S, Talamini R, Montella M, Levi F, Dal Maso L, Serraino D, Polesel J, Negri E, Decarli A, La Vecchia C. Family history of cancer and the risk of cancer: a network of case-control studies. Ann Oncol. 2013;24(10):2651–2656. doi: 10.1093/annonc/mdt280. [DOI] [PubMed] [Google Scholar]