Abstract
Background:
Recent studies have revealed the involvement of hedgehog (Hh) signaling component in proliferation and invasive behavior of many carcinomas.
Aim:
This study aims to identify the expression of sonic Hh (SHH) protein of SHH pathway in oral epithelial dysplasia and oral squamous cell carcinoma (OSCC) using SHH (H-160) (Santa Cruz, sc-9042) which could have therapeutic implication in future.
Materials and Methods:
A total of 250 cases comprising 50 normal oral mucosa, 50 cases of oral epithelial dysplasia, 50 well, 50 moderate and 50 poorly differentiated OSCCs were included in the study. Immunohistochemical evaluation of SHH protein expression was conducted using monoclonal antibody. Interpretation of the expression was done by immunoreactive score of Remmele and Stegner (IRS) scoring method.
Statistical Analysis:
Chi-Square test was used to analyze the results.
Results:
The study showed that SHH signaling molecules are highly expressed in OSCC, and their expression was mainly in the cytoplasm of epithelial cells.
Conclusion:
The SHH signaling component is associated with the pathological parameter in OSCC and oral epithelial dysplasia.
Keywords: Dysplasia, oral squamous cell carcinoma, sonic hedgehog
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common malignancy in the head and neck region, accounting for about 2,60,000 new cases and 1,24,000 OSCC-related deaths worldwide annually.[1] According to the study carried out by the National Institute of Public Health (Japan), 86% of the world's oral cancer victims reside in India. The scenario of oral cancer ranks number one among men and third among women.[2] Cancer that forms in tissues of the oral cavity or the oropharynx are referred as oral cancer and among all oral mucosal cancers, about 90% are squamous cell carcinomas (SCC).[3]
Symptoms may follow a white (leukoplakia) or red patch (erythroplakia), unhealable wounds, sores, tender lesions, characterized by painful chewing or swallowing. The major risk factor for these neoplasms is chronic exposure of oral mucosa to betel quid (paan) along with areca nuts chewing, a practice that is highly prevalent in different parts of India.[4] Infection by high-risk subtypes of human papillomavirus has also been established as an important etiologic factor that accounts for a trend for increasing the incidence of oropharyngeal cancers in men younger than age 50 years without a history of tobacco use.[5,6,7,8]
Three well-known pathways such as Wnt, Notch and Hedgehog (Hh) play an important role in the development and normal homeostasis. Conversely, deregulation of these pathways is shown in cancer stem cell (CSC) regulation and maintenance.[9,10,11]
The Hh pathway is one of the fundamental signal transduction pathways in animal development. The Hh ligands include Sonic, Indian and Desert Hh in vertebrates and Hh in Drosophila. These signal through binding to the membrane receptor Patched (Ptc)[12] and to reverse the Ptc-mediated inhibition of signaling they bind to trans-membrane protein Smoothened (Smo).[13] This allows Smo to activate the intracellular signaling components, resulting in stabilization of downstream transcriptional activator(s) resulting in activation of target genes.[14] Transcription activation is facilitated through the Gli family of transcription factors in vertebrates.[15] Hh signaling has a wide range of biological functions such as initiation of cellular growth, division, lineage specification, axon guidance and function as a survival factor.[16] Aberrant Hh signaling is associated with the development and progression of a wide range of human malignancies. Mutations such as PTCH-1 and Smo are associated with medulloblastoma, basal cell carcinoma and rhabdomyosarcoma. Aberrant activation of Hh signaling is also suggested to play a role in other cancers that have no known mutational basis, such as glioma, breast, esophageal, gastric, pancreatic, prostate, chondrosarcoma and small-cell lung carcinoma. In these tumors, the Hh pathway abnormalities are called ligand-dependent, which shows no mutation in Hh pathway genes but are characterized by upregulation of the expression of Hh ligand.[17] Interest in targeting this pathway for the treatment of cancer has arisen from recent evidence that Hh signaling is important for driving the self-renewal of CSCs, a small subset of cells in a tumor that can initiate tumor spread and which are typically resistant to chemotherapy, possibly contributing to tumor relapse.[18] Elimination of these CSC by targeting the Hh pathway in combination with chemotherapy has been shown to increase therapeutic efficacy in animal models of pancreatic cancer.[19,20] Image analysis in red, green, blue (RGB) mode provided objective evidence for over-expression of Hh signaling components in head and neck squamous cell carcinoma (HNSCC), particularly with regard to the transcription factors gli1 (10-fold) and sonic Hh (SHH) (5-fold) in comparison with healthy mucosa.[21]
Inhibitors of the Hh molecular signaling pathway have emerged in recent years as a promising new class of potential therapeutics for cancer treatment that target different members of this pathway, including Smo, SHH protein and gli1.[22] These reports have led to the emergence of Hh pathway inhibition either in tumor cells directly, or in surrounding non-malignant stromal cells that supply growth-promoting factors to the tumor.[23] Recent results from clinical trials using topical and systemic administration of Hh pathway inhibitors to basal cell carcinoma (BCC) patients provide the first evidence of the therapeutic benefit resulting from inhibition of this signaling pathway.[24,25]
Overexpression of the Hh signaling pathway has been described in several malignancies and is associated with a poor prognosis. Very few studies on the expression of SHH in OSCC have been done so far.[26,27,28] A comprehensive understanding of Hh signaling during development of OSCC will undoubtedly shed light into the mechanism of Hh in OSCC progression and to identify potential targets for therapeutic intervention. In view of identifying the expression of SHH molecule, this study was conducted. This is the pioneer study, evaluating the expression of SHH in normal buccal mucosa, dysplastic buccal mucosa and OSCC of buccal mucosa (well, moderate and poor) which will undoubtedly help further research for therapeutic intervention thus the effective intervention of the Hh pathway can be achieved at the level of ligand binding to its receptor itself using anti-Hh antibodies.
MATERIALS AND METHODS
The criteria used for inclusion into the study included the following: Adequate clinicopathologic data; histologically proven cases of oral epithelial dysplasia (moderate),[29] SCC (tumors were classified as well, moderately and poorly differentiated according to the WHO classification of histologic differentiation grade);[30] and no prior oncologic therapy.
For each case, the hematoxylin and eosin-stained sections were reviewed to confirm the diagnosis of oral epithelial dysplasia and different grades of OSCC. The study protocol was carried out with the approval of the Institutional Research Committee. The slides were verified twice by two pathologists in a blinded fashion without the knowledge of any patient's clinicopathologic information.
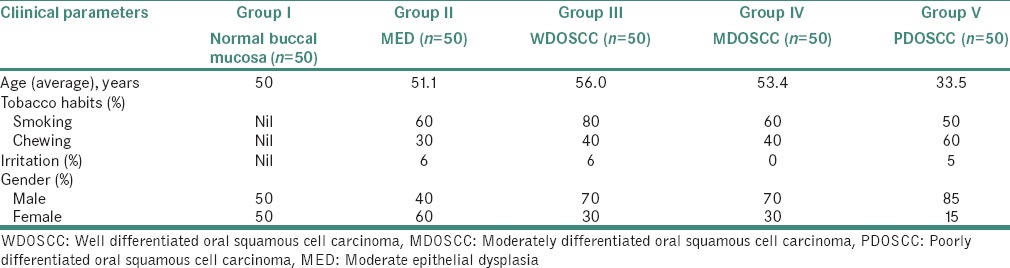
This study comprised a total of 250 biopsy specimens. Patient's details regarding age, gender, location of lesion, habits and irritation were recorded [Table 1]. The specimens were collected as follows, 50 specimens were of normal mucosa which was obtained from the volunteers without any tobacco smoking or chewing habits, or irritation after obtaining the informed consent from the patients, 50 from the diagnosed case of oral, moderate epithelial dysplasia (MED), 50 of well differentiated OSCC, 50 of moderately differentiated OSCC and 50 of poorly differentiated OSCC.
Table 1.
Distribution of study samples according to age, habits, irritation, and gender
Immunohistochemistry
Four-micrometer-thick tissue sections were cut and placed on positively charged glass slides. Immunohistochemical staining was performed using SHH (H-160) (Santa Cruz, sc-9042) according to manufacturer's protocol. SHH (1:100) were applied to the tissue sections and incubated overnight at 4°C. Secondary biotinylated antibody and streptavidin – HRP conjugated complex were applied for 60 and 30 min, respectively. After washing in buffer, the chromogen diaminobenzidine was applied for 5 min followed by counterstaining with Mayer's hematoxylin. Negative controls included substituting the primary antisera with preimmune sera from the same species and omitting the primary antibody. For positive control, carcinoma in lymphnode and carcinoma of breast were used.
Evaluation of immunohistochemical staining
The immunohistochemical score is based on IRS score. The IRS score is calculated by combining the quantity score (percentage of positively stained cells) with the staining intensity score. The quantity score ranges from 0 to 4 and the staining intensity score ranges from 0 to 3. The scoring method is described in Table 2.
Table 2.
IRS scoring method

RESULTS
Based on immunohistochemistry, the results showed very highly significant difference (P < 0.001) in the expression of SHH protein in the cancerous specimens compared with that of the noncancerous oral mucosa.
According to statistical evaluation, the normal oral epithelium [Figure 1] did not show any SHH expression. 70% of samples with MED [Figure 2] showed positive SHH expression mainly in the cytoplasm, out of which 60% was mild, and 10% was moderate. 90% of the samples with OSCC showed SHH expression [Tables 3–5] in the cytoplasm. On grade-wise evaluation, the samples with well-differentiated OSCCs [Figure 3] showed 40% positive expression in the cytoplasm out of which 30% was mild, and 10% was moderate. 100% of samples with moderately differentiated OSCCs [Figure 4] showed positive SHH expression in the cytoplasm, out of which 70% was mild, and 30% was moderate. 84% of samples with poorly differentiated OSCC [Figure 5] showed SHH-positive expression in the cytoplasm, out of which 62% showed moderate and 22% showed strong SHH expressions [Tables 3 and 5]. The results correlate with the aggressiveness of the lesion, but we found more SHH positivity with mild intensity in MED than in well-differentiated oral squamous cell carcinoma (WDOSCC).
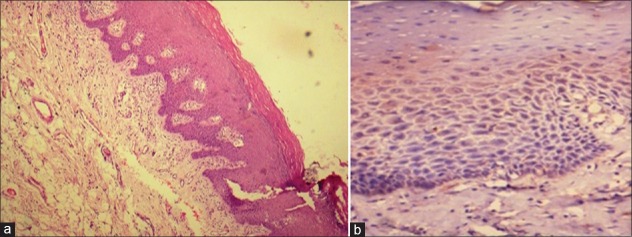
Figure 1.
Normal epithelium showing very mild expression of sonic hedgehog in the cytoplasm [(a) H&E stain, ×100, (b) IHC stain, ×200]
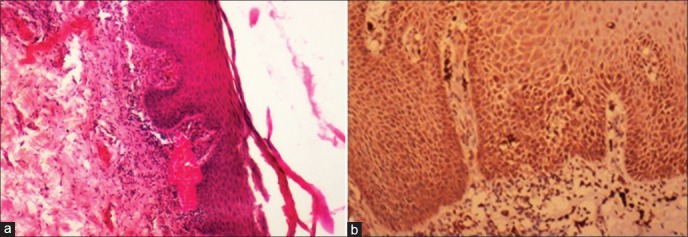
Figure 2.
Dysplastic epithelium showing mild expression of sonic hedgehog in the cytoplasm evenly in basal and parabasal areas [(a) H&E stain, ×100, (b) IHC stain, ×200]
Table 3.
Immunohistochemical expression of sonic hedgehog protein in each study group
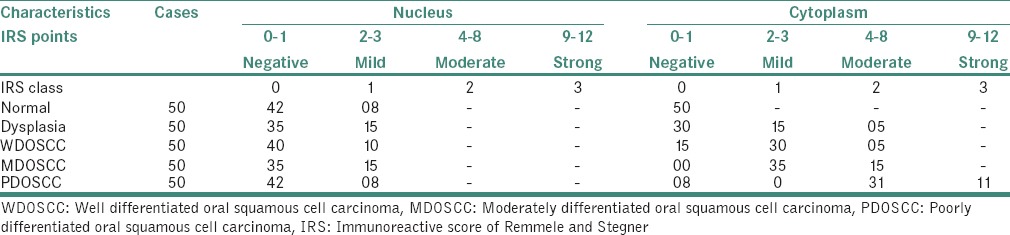
Table 5.
Chi-square test showing highly significant expression of sonic hedgehog protein in the cytoplasm of the cells of oral squamous cell carcinoma cases
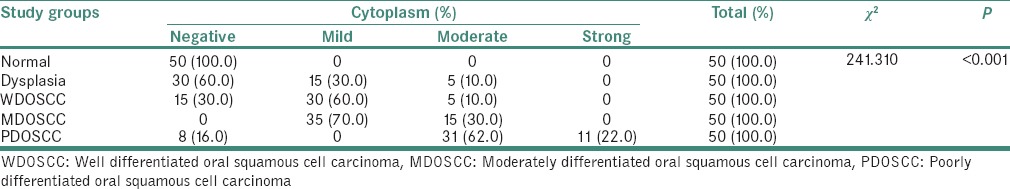
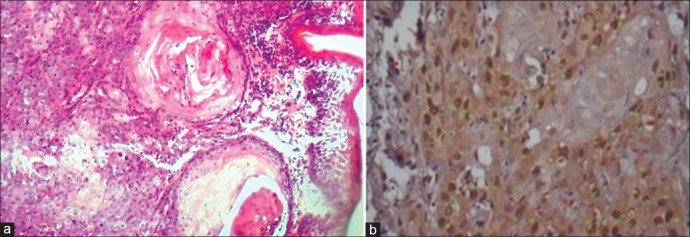
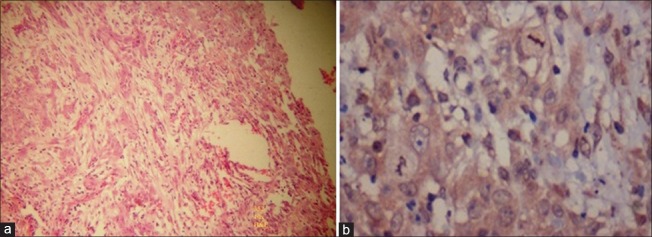
Figure 3.
Well differentiated oral squamous cell carcinoma showing mild to moderate expression of sonic hedgehog in cytoplasm, mainly in the periphery of the epithelial islands [(a) H&E stain, ×100, (b) IHC stain, ×400]
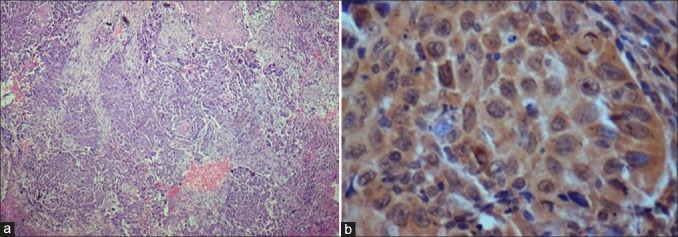
Figure 4.
Moderately differentiated oral squamous cell carcinoma showing moderate expression of sonic hedgehog in cytoplasm [(a) H&E stain, ×100, (b) IHC stain, ×400]
Figure 5.
Poorly differentiated oral squamous cell carcinoma showing moderate to strong expression of sonic hedgehog in cytoplasm [(a) H&E stain, ×100, (b) IHC stain, ×400]
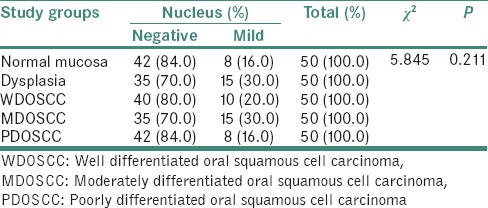
Table 4.
Chi-square test showing insignificant expression of sonic hedgehog protein in the nucleus of the cells in the study groups
DISCUSSION
HNSCC, including OSCC, is the sixth most common type of malignancy worldwide.[31] Although recent advances in the treatment have improved the quality of life, overall 5-year survival rates have not improved significantly.[32] HNSCC frequently shows local recurrence and metastasis after the initial treatment.[33] Increasing evidence indicates that the initiation, progression, recurrence and metastasis of HNSCC are related to the behavior of a small subpopulation of CSCs.[34,35,36] It has been postulated that CSCs within the bulk tumor may escape conventional therapies, thus leading to disease relapse. Therefore, an important goal of therapy could be to identify and kill this CSC population. If CSCs can be identified prospectively and isolated, then we should be able to identify new diagnostic markers and potential therapeutic targets.[37]
Some of the most important signals enumerated in maintaining stem cell proliferation in tumorigenesis are Oct-4, Notch, Wnt/Catenin, bone morphogenetic protein, SHH signaling pathway, Musashi-1 and so forth.[38] SHH signaling pathway is a major regulator of some of the fundamental processes including stem cell maintenance, cell differentiation, tissue polarity and cell proliferation.[39] SHH, are quiescent in adult tissues. When these pathways are activated aberrantly in adult tissues, they are frequently oncogenic.[28] In human and animal models, activation of the SHH pathway is associated with the development of tumors through diverse mechanisms. For example, in medulloblastoma and basal cell carcinomas, SHH signaling can be initiated because of PTCH-1 mutations, whereas in small cell lung cancer and intestinal adenocarcinoma, its activation is associated with high expression of the SHH ligand.[1] This study is undertaken to show the activation of Hh pathway in association with high expression of the SHH ligands.
The connection between Hh signaling and carcinogenesis was first detected in patients with basal cell nevus syndrome (Gorlin's syndrome). The enhanced expression of Hh signaling molecules has also been demonstrated in tumor tissues of the breast, stomach, endometrium, cervix, pancreas and colon.[27] Wang et al. in 2012, found overexpression of SHH in OSCC.[27] Schneider et al. assessed the expression of SHH signaling proteins in HNSCCs, he failed to note any expression of SHH signaling proteins in oral normal mucosa.[28] However, in this study, low levels of SHH were expressed in normal oral mucosa but was confined to the basilar area.
In this study, the aim was to find the levels of SHH proteins of the SHH pathway in human normal buccal mucosa, moderate oral epithelial dysplasia and different grades of OSCC. Based on immunohistochemistry, our results showed significant (<0.001) increase of SHH protein expression in the cancerous specimens compared with the noncancerous oral mucosa and between each category. The expression of SHH protein in dysplastic oral epithelium was found to be slightly higher than seen in WDOSCC, but the pattern of expression was evenly in basal and parabasal areas, whereas the pattern of expression of SHH in WDOSCC was mainly observed in the periphery of the tumor island which can be due to the increased activity of SHH in the invasive front. The IRS score was found to be moderate in moderately differentiated oral squamous cell carcinoma (MDOSCC) and strong in poorly differentiated oral squamous cell carcinoma (PDOSCC) and also IRS scoring of SHH was mainly in the cytoplasm. Yue et al. suggested that the SHH/Gli pathway may be critical for SCC recurrence, metastasis and resistance to chemotherapy. Inhibition of the SHH/Gli pathway activity/function is a potential therapeutic strategy for the treatment of SCC patients.[40]
CONCLUSION
The expression of SHH when studied in NOM, MED and OSCC showed that SHH was significantly expressed MED and OSCC. The expression of SHH in dysplastic oral epithelium was found to be comparatively more than in WDOSCC. The study will be carried out with increased number of samples to confirm these results, but the pattern of expression was mainly even in basal and parabasal areas where the metabolic activity is high. The pattern of expression of SHH in WDOSCC was mainly observed in the periphery of the tumor island. The IRS score was found to be higher in MDOSCC and strong in PDOSCC which correlates with the aggressiveness of the high-grade tumor. These findings show important implication in the grades, progression and aggressiveness of OSCC and further studies need to be conducted to find out the therapeutic implications. Thus, activation of Hh pathway which is an important signaling mechanism crucial in embryogenesis may have a link to carcinogenesis, and the aberrant regulation of this pathway can result in the development of tumors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Khan U Z. An overview of oral cancer in Indian subcontinent and recommendations to decrease its incidence. Webmedcentral Cancer [Internet] 2012;3(8) WMC003626. doi: 10.9754/journal.wmc.2012.003626. [Google Scholar]
- 3.Aruna DS, Prasad KV, Shavi GR, Ariga J, Rajesh G, Krishna M. Retrospective study on risk habits among oral cancer patients in Karnataka Cancer Therapy and Research Institute, Hubli, India. Asian Pac J Cancer Prev. 2011;12:1561–6. [PubMed] [Google Scholar]
- 4.Sinha N, Mukhopadhyay S, Das DN, Panda PK, Bhutia SK. Relevance of cancer initiating/stem cells in carcinogenesis and therapy resistance in oral cancer. Oral Oncol. 2013;49:854–62. doi: 10.1016/j.oraloncology.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marur S, D’souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nisa L, Aebersold DM, Giger R, Caversaccio MD, Borner U, Medová M, et al. Profiling invasiveness in head and neck cancer: Recent contributions of genomic and transcriptomic approaches. Cancers (Basel) 2015;7:585–97. doi: 10.3390/cancers7020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta. 2013;1830:2481–95. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Purow B. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol. 2012;727:305–19. doi: 10.1007/978-1-4614-0899-4_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dash S, Sunkara RR, Waghmare SK. Developmental signalling in maintenance and regulationof cancer stem cells. Biomed Res J. 2015;2:37–56. [Google Scholar]
- 12.Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–63. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 13.Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996;86:221–32. doi: 10.1016/s0092-8674(00)80094-x. [DOI] [PubMed] [Google Scholar]
- 14.Hooper JE, Scott MP. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989;59:751–65. doi: 10.1016/0092-8674(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 15.Ingham PW, McMahon AP. Hedgehog signaling in animal development: Paradigms and principles. Genes Dev. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MM., Jr The hedgehog signaling network. Am J Med Genet A. 2003;123A:5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- 17.Vanessa MV, Guadalupe AG, Silvia DP, Luis MA. Signalling Pathways Driving Cancer Stem Cells: Hedgehog Pathway. [Last accessed on 2016 Sep 05]. Available from: http://www.intechopen.com .
- 18.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 19.Jimeno A, Feldmann G, Suárez-Gauthier A, Rasheed Z, Solomon A, Zou GM, et al. A direct pancreatic cancer xenograft model as a platform for cancer stem cell therapeutic development. Mol Cancer Ther. 2009;8:310–4. doi: 10.1158/1535-7163.MCT-08-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–13. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 21.Dimitrova K, Stoehr M, Dehghani F, Dietz A, Wichmann G, Bertolini J, et al. Overexpression of the hedgehog signalling pathway in head and neck squamous cell carcinoma. Onkologie. 2013;36:279–86. doi: 10.1159/000350322. [DOI] [PubMed] [Google Scholar]
- 22.Peukert S, Miller-Moslin K. Small-molecule inhibitors of the hedgehog signaling pathway as cancer therapeutics. ChemMedChem. 2010;5:500–12. doi: 10.1002/cmdc.201000011. [DOI] [PubMed] [Google Scholar]
- 23.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 24.Tas S, Avci O. Rapid clearance of psoriatic skin lesions induced by topical cyclopamine. A preliminary proof of concept study. Dermatology. 2004;209:126–31. doi: 10.1159/000079596. [DOI] [PubMed] [Google Scholar]
- 25.Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164–72. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 26.Cavicchioli Buim ME, Gurgel CA, Gonçalves Ramos EA, Lourenço SV, Soares FA. Activation of sonic hedgehog signaling in oral squamous cell carcinomas: A preliminary study. Hum Pathol. 2011;42:1484–90. doi: 10.1016/j.humpath.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Wang YF, Chang CJ, Lin CP, Chang SY, Chu PY, Tai SK, et al. Expression of hedgehog signaling molecules as a prognostic indicator of oral squamous cell carcinoma. Head Neck. 2012;34:1556–61. doi: 10.1002/hed.21958. [DOI] [PubMed] [Google Scholar]
- 28.Schneider S, Thurnher D, Kloimstein P, Leitner V, Petzelbauer P, Pammer J, et al. Expression of the sonic hedgehog pathway in squamous cell carcinoma of the skin and the mucosa of the head and neck. Head Neck. 2011;33:244–50. doi: 10.1002/hed.21437. [DOI] [PubMed] [Google Scholar]
- 29.Speight PM. Update on oral epithelial dysplasia and progression to cancer. Head Neck Pathol. 2007;1:61–6. doi: 10.1007/s12105-007-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson N, Franceschi S, Ferlay J, Ramadas K, Schmid S, MacDonald DG. In: Squamous cell carcinoma. Pathology and Genetics: Head and Neck Tumours. Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Lyon: IARC Press; 2005. pp. 168–75. [Google Scholar]
- 31.Zini A, Czerninski R, Sgan-Cohen HD. Oral cancer over four decades: Epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299–305. doi: 10.1111/j.1600-0714.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- 32.Goon PK, Stanley MA, Ebmeyer J, Steinsträsser L, Upile T, Jerjes W, et al. HPV and head and neck cancer: A descriptive update. Head Neck Oncol. 2009;1:36. doi: 10.1186/1758-3284-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marur S, Forastiere AA. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Filho MS, Nör JE. The biology of head and neck cancer stem cells. Oral Oncol. 2012;48:1–9. doi: 10.1016/j.oraloncology.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayed SI, Dwivedi RC, Katna R, Garg A, Pathak KA, Nutting CM, et al. Implications of understanding cancer stem cell (CSC) biology in head and neck squamous cell cancer. Oral Oncol. 2011;47:237–43. doi: 10.1016/j.oraloncology.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Krishnamurthy S, Nör JE. Head and neck cancer stem cells. J Dent Res. 2012;91:334–40. doi: 10.1177/0022034511423393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell Mol Life Sci. 2011;68:4009–22. doi: 10.1007/s00018-011-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int 2013. 2013:319489. doi: 10.1155/2013/319489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jena RK, Kansurkar SS, Swain TR. Cancer stem cell? Essence of tumorigenesis. J Carcinog Mutagen S1[Internet] 2012;006:S1–006. doi: 10.4172/2157-2518. [Google Scholar]
- 40.Yue D, Li H, Che J, Zhang Y, Tseng HH, Jin JQ, et al. Hedgehog/Gli promotes epithelial-mesenchymal transition in lung squamous cell carcinomas. J Exp Clin Cancer Res. 2014;33:34. doi: 10.1186/1756-9966-33-34. [DOI] [PMC free article] [PubMed] [Google Scholar]