Abstract
Background:
Many studies have been carried out to study the role of extracellular matrix proteins, growth factors and matrix metalloproteinases on tumor invasion. However, literature related to the analysis of connective tissue fibers in varying grades of oral squamous cell carcinoma (OSCC) is very limited.
Aim:
To analyze the changes in collagen and elastic fibers in varying grades of (OSCC).
Settings and Design:
This retrospective study was carried out using a light and polarizing microscope.
Materials and Methods:
Three sections each were cut from fifty samples of varying grades of OSCC and ten samples of control followed by staining with H and E, Picrosirius-Red and Verhoeff–Van Gieson. Qualitative and quantitative analysis of collagen and elastic fibers were accomplished using set criteria.
Statistical Analysis:
Data were entered into the Statistical Package for Social Sciences (SPSS) version 13.5 for analysis.
Results:
A change in colors of collagen fibers was seen on progressing from well to poorly differentiated OSCC. Thin collagen fibers predominantly exhibited greenish yellow, but the thick fibers exhibited a variety of colors. As the grade of OSCC progressed, collagen fibers were loosely packed haphazardly arranged. Statistically insignificant results were obtained for quantitative analysis of collagen and qualitative analysis of elastic fibers.
Conclusion:
The collagen fibers undergo a change in color, orientation and packing in the stroma of varying grades of OSCC. The uniqueness of this study lies in the exploration of elastic fibers in OSCC which has not been done so far.
Keywords: Collagen, elastic fibers, oral squamous cell carcinoma, picrosirius red, polarization, Verhoeff
INTRODUCTION
Squamous cell carcinoma (SCC) consists of two interdependent components – the tumor epithelial cells and the stroma. These components interact on a regular basis and to an extent that discrepancy in one could affect the other.[1] Hence, for a better prediction of course and outcome of SCC, both should be given equal weightage in the evaluation/grading system.
Although the epithelial component of oral SCC (OSCC) has been studied extensively, the stroma is still a less explored arena. Among the stromal components, only the inflammatory component has been included in few of the grading systems for SCC.[2] The other stromal constituents, i.e., the collagen, elastic and reticulin fibers still await to receive a recognition as an important factor in the progression of this disease.
Various authors have studied the birefringence property of collagen fibers,[3,4,5] but studies which evaluated the other aspects of collagen fibers (orientation, arrangement, density, packing and area fraction) in OSCC are only a handful.[6,7,8,9,10] Apart from collagen fibers, the elastic fibers also are an integral component of the extracellular matrix (ECM). As per the literature, there is an interaction between the tumor cells and elastic fibers, but its effect on the progression of carcinoma is questionable. The number of studies conducted on elastic fibers in SCC are very limited, with studies on OSCC being even lesser.[3]
Hence, the present study was carried out with an aim to analyze the morphological changes of collagen and elastic fibers in varying grades of OSCC. The study is based on 5-fold objectives. First, to qualitatively observe and compare the color changes in picrosirius red (PSR)-stained collagen fibers in varying grades of OSCC. Second, to correlate the color change of collagen fiber in varying grades of OSCC with the thickness of collagen fibers. Third, to observe and compare the orientation and density of collagen fibers in the immediate vicinity of tumor islands. Fourth, to quantitatively measure and compare the area fraction of collagen fibers in varying grades of OSCC using a computer-based software. Finally, to qualitatively analyze the morphologic changes exhibited by the elastic fibers using Verhoeff's stain in varying grades of OSCC, if any.
MATERIALS AND METHODS
This retrospective study was conducted using a total of fifty formalin-fixed and paraffin-embedded soft tissue samples of varying grades of OSCC which were retrieved from the archives of the Department of Oral Pathology and Microbiology during 2012–2014 after obtaining the Institutional Ethical Committee Clearance. From these samples, three sections of 4 μ each were cut, and one section of each sample was stained with H and E using the standard protocol and was graded by two independent observers using the Broder's system (1920). The results showed that the samples consisted of 19 well, 20 moderate and 11 poorly differentiated OSCC. In addition, ten control tissues were taken (five tissues of normal oral mucosa as a control for collagen fibers and five tissues of the artery as a control for elastic fibers). This was followed by staining 1 section of each sample with PSR and another section with Verhoeff–Van Gieson stain using the standard protocols.[4,5] Based on the objectives, the assessment of PSR stained sections was carried out under a polarizing microscope (Olympus BX 41) in the following steps.
Color changes in collagen fibers
The colors were evaluated under a polarizing microscope and ranged from reddish orange (RO), yellowish orange (YO) and greenish yellow (GY).
Correlation of the color of collagen fiber with its thickness
The images were clicked and entered into image analysis software (Image J, version 1.47, NIH, United States). The thickness of fibers in the immediate vicinity of tumor islands was measured. The fibers measuring 0.8 μm or less were grouped under thin fibers, and a thickness between 1.6 and 2.4 μm was taken as thick fibers.[11] In each section, the color was observed for a total of fifty clearly discernible fibers (25 each of thick and thin fibers) in two separate fields.
Orientation and packing of collagen fibers in the immediate vicinity of tumor islands
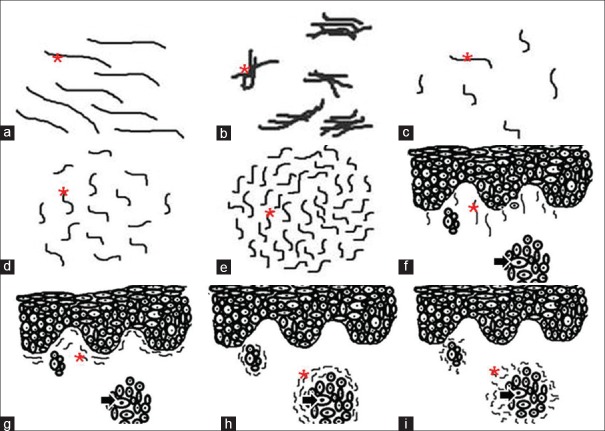
The orientation was categorized as parallel or haphazard, and the packing of collagen fibers was categorized as either loose or dense [Figure 1].
Figure 1.
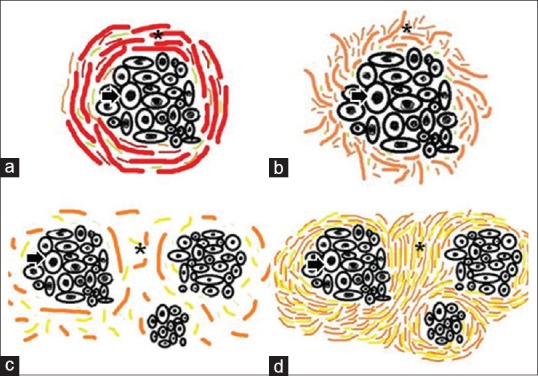
Arrangement and packing of collagen fibers. The figure depicts the line diagrams of arrangement and packing of collagen fibers around the tumor islands. The arrangement of collagen fibers (a) parallel (b) haphazard packing of collagen fibers, (c) loose (d) dense. Asterick = Collagen fibers, Arrow = Tumor island
Area fraction occupied by collagen fibers
The images of PSR stained sections at 40x magnification were fed into image analysis software (Image J software, version 1.47) and the percentage of area occupied by collagen fibers was calculated for each grade of OSCC.
Qualitative analysis of elastic fibers
The elastic fibers were analyzed at 10x and 40x magnifications for morphology, density, pattern of grouping, orientation in relation to tumor islands and overlying epithelium [Figure 2].
Figure 2.
Patterns of elastic fibers. The figure depicts line diagrams of various patterns exhibited by elastic fibers in varying grades of oral squamous cell carcinoma. (a) Long, thin and straight elastic fibers present singly, (b) short, thick and wavy elastic fibers present in bunches; density of elastic fibers, (c) scanty, (d) moderate, (e) abundant; orientation of elastic fibers in relation to overlying epithelium (f) perpendicular, (g) parallel; orientation of elastic fibers in relation to tumor islands (h)= parallel, (i) haphazard. Asterick = Elastic fiber, Arrow = Tumor island
Statistical analysis
Data were entered into the Statistical Package for Social Sciences (SPSS) version 13.5 (IBM Analytics) for analysis. It was subjected to descriptive and inferential statistics to generate frequencies and percentages. For comparison of categorical variables between groups, the Chi-square test was used at 95% confidence interval. Interobserver agreement was assessed using kappa statistics.
RESULTS
Color exhibited by collagen fibers
In majority of the samples of normal mucosa (control) and well-differentiated OSCCs, bundles of collagen fibers exhibited a predominantly RO birefringence (64% and 63.15%, respectively). The moderately differentiated OSCCs exhibited YO (47%) and the poorly differentiated OSCCs exhibited GY (74.54%) as the predominant color of birefringence [Figures 3 and 4]. Interobserver agreement was analyzed using kappa statistics and 91% agreement was found between the two observers.
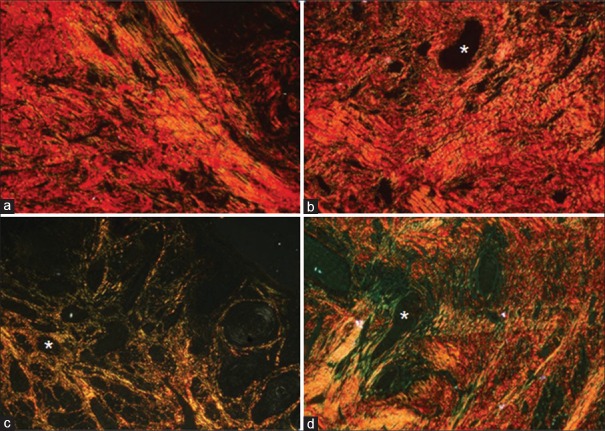
Figure 3.
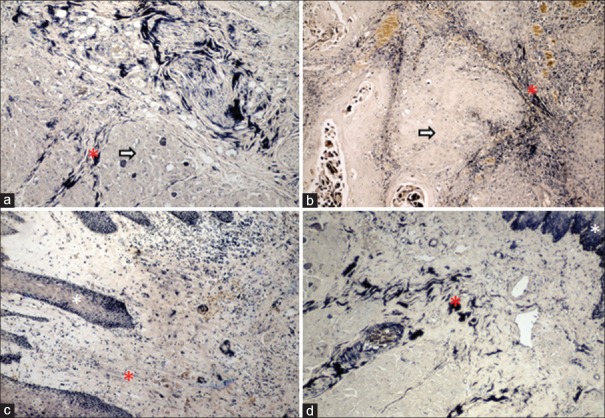
The histopathological image depicts colors exhibited by collagen fibers in stroma of varying grades of oral squamous cell carcinoma and its control tissue; (a) normal buccal mucosa exhibiting predominantly reddish orange birefringence; predominant color of birefringence around the tumor islands (b) well-differentiated oral squamous cell carcinoma exhibiting reddish orange birefringence, (c) moderately differentiated oral squamous cell carcinoma exhibiting yellowish orange birefringence, (d) poorly differentiated oral squamous cell carcinoma exhibiting greenish yellow birefringence. Asterick = tumor island (PSR stain, polarizing microscope, ×100)
Figure 4.
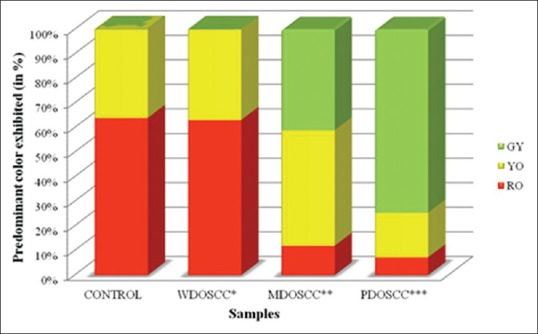
Colors exhibited by bundles of collagen fibers. The figure depicts a bar diagram showing comparison of the birefringence colors exhibited by picrosirius red-stained bundles of collagen fibers under a polarizing microscope in stroma of varying grades of oral squamous cell carcinoma and its control tissue; *WDOSCC: Well-differentiated oral squamous cell carcinoma; **MDOSCC: Moderately differentiated oral squamous cell carcinoma; ***PDOSCC: Poorly differentiated oral squamous cell carcinoma, GY: Greenish yellow, YO: Yellowish orange, RO: Reddish orange
Correlation of the color of collagen fiber with its thickness
Thick fibers
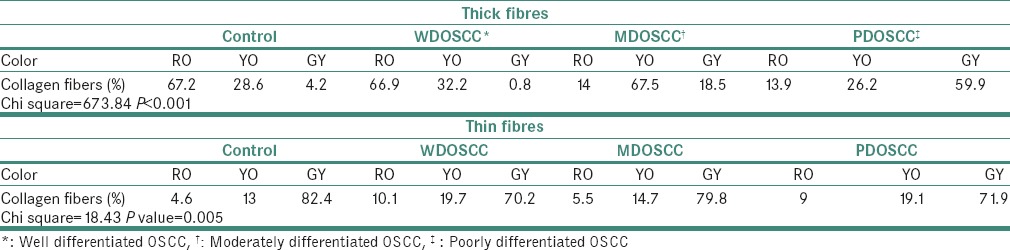
In control tissues and well-differentiated OSCCs, majority of the thick fibers exhibited RO color (67.2% and 66.9%, respectively). The color exhibited by majority of the thick fibers in moderately differentiated OSCCs was YO (67.5%) and in the case of poorly differentiated OSCCs, it was GY (59.9%) [P < 0.001, Table 1].
Table 1.
Consolidated table of colors exhibited by individual thick and thin collagen fibers in stroma of varying grades of OSCC and its control tissue (in %)
Thin fibers
In control tissues (82.4%), well (70.2%), moderate (79.8%) and poorly differentiated OSCCs (71.9%), majority of the thin fibers exhibited GY color [P = 0.005 Table 1].
Orientation of collagen fibers in relation to the tumor island
It changed from parallel in well-differentiated OSCC to a haphazard orientation in poorly differentiated OSCC [P = 0.03, Table 2 and Figure 5].
Table 2.
Pattern of arrangement of collagen fibers in control tissue and around the tumor islands in varying grades of OSCC.
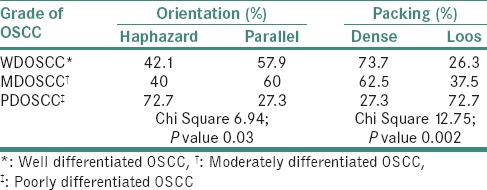
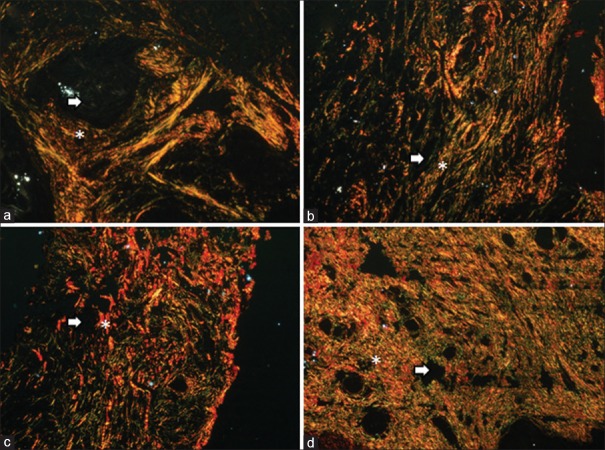
Figure 5.
Arrangement and packing of collagen fibers. Histopathologic image shows the arrangement and packing of collagen fibers around the tumor islands. Arrangement of collagen fibers (a) parallel, (b) haphazard; packing of collagen fibers (c) loose, (d) dense. Asterick = Collagen fibers, Arrow = Tumor island (PSR stain, polarizing microscopy, ×100)
Packing of collagen fibers in the immediate vicinity of tumor islands
It changed from densely packed to loosely packed on progressing from well-differentiated OSCC to poorly differentiated OSCC [P = 0.002, Table 2 and Figure 5].
Area fraction occupied by collagen fibers in a field of known dimensions
The mean area fraction was 40.283% in the normal mucosa samples, 14.005% in well-differentiated OSCCs, 25.781% in moderately differentiated OSCCs and 22.034% in poorly differentiated OSCCs. However, the results were statistically insignificant.
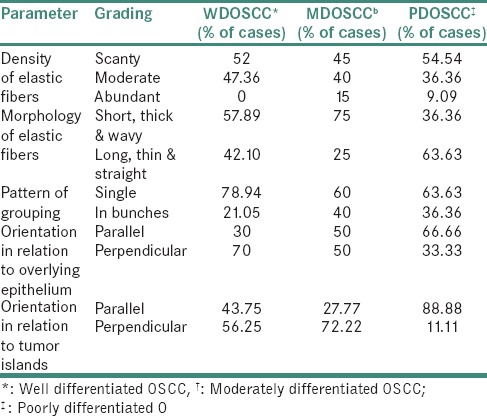
Assessment of elastic fibers
The analysis of elastic fibers revealed varying patterns on progressing from a lower grade of OSCC to the higher grade of OSCC [Figures 6 and 7]. The orientation of majority of the elastic fibers changed from perpendicular to parallel in relation to the overlying epithelium [Table 3].
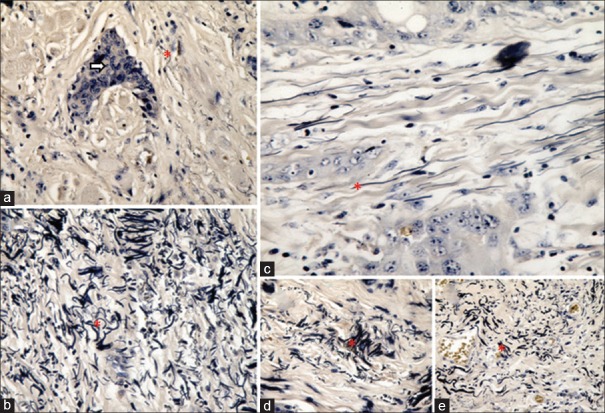
Figure 6.
Patterns of elastic fibers. Histopathologic image shows patterns of elastic fibers in varying grades of oral squamous cell carcinoma: Density of elastic fibers (a) scanty (VVG stain ×400), (b) abundant (VVG stain ×400), (c) = long, thin and straight elastic fibers, elastic fibers present singly, (VVG stain, ×100) (d) elastic fibers present in bunches, (e) short, thick and wavy elastic fibers (VVG stain ×400). Red asterick = Elastic fiber, Arrow: tumor island
Figure 7.
Orientation of elastic fibers. Histopathologic image shows the orientation of elastic fibers in varying grades of oral squamous cell carcinoma: Orientation of elastic fibers in relation to the tumor islands (a) haphazard, (b) parallel; orientation of elastic fibers in relation to the overlying epithelium (c) perpendicular, (d) parallel. Red asterick: Elastic fiber; White asterick: Overlying epithelium, Arrow: Tumor island (VVG stain, ×40)
Table 3.
Qualitative assessment of elastic fibers
DISCUSSION
The primary purpose of this study was to enlighten the readers regarding the role of stromal fibers during the progression of OSCC which has been underestimated till date. The stromal fibers, especially the collagen fibers, play a mixed role;[12] they can benefit the tumor by reducing access to the host lymphocytes. Alternatively, these fibers can benefit the host by walling off the invading tumor.[8]
The collagen fibers have been studied by various authors, and it has been revealed that they exhibit changes in the type, diameter, color (under a polarizing microscope), orientation, density and amount with tumor progression.[8] However, the studies revealing changes in elastic fibers present in the stroma of OSCC are sparse and not well documented.
Our study revealed a statistically significant (P < 0.001) change in the colors of bundles of collagen fibers which varied from RO in well-differentiated OSCC to YO in moderately differentiated OSCC to GY in poorly differentiated OSCC. However, the control samples predominantly exhibited RO color. These changes in the color of bundles of collagen fibers were seen predominantly around the tumor islands. The RO color of control tissue and well-differentiated OSCC appear to be related to the higher amount of thick/Type I collagen fibers. The GY color in poorly differentiated OSCCs could be either because of increase in the number of thinner fibers or it could be the result of abnormal/pathological collagen formed by the tumor cells or stroma. In accordance to our study, a similar change in color of collagen fibers was observed by Arun Gopinathan et al., Kalele et al. and Aparna and Charu in the stroma of various grades of OSCC mainly around the tumor islands using PSR stain under polarizing microscopy.[6,7,8]
The reason for this color change occurring predominantly in the immediate vicinity of tumor islands is still not clear. van den Hooff suggested that this difference in birefringence of color surrounding the tumor islands could be firstly due to the action of enzymes such as collagenases or the metalloproteinases secreted by tumor cells on the collagen in the immediate vicinity.[13] Second, there could be an abnormal disintegration of the matrix by the tumor cells. Third, the dedifferentiated tumor cells could be secreting an abnormal matrix. Fourth, there could be a formation of disorganized or abortive stroma around the tumor islands.[13]
In the present study, the birefringence colors exhibited by thick (1.6–2.4 μm) and thin (0.8 μm or less) individual collagen fibers were compared. The thin fibers predominantly exhibited a GY birefringence in all the samples (including control tissue and varying grades of OSCC), but the thick fibers exhibited a variety of colors. In control tissue and well-differentiated OSCC, the thick fibers predominantly exhibited an RO birefringence. However, as the grade of carcinoma progressed from moderate to poorly differentiated OSCC, thick fibers exhibited a change in color from YO to GY. In accordance to our study, Arun Gopinathan et al. observed a similar pattern of change in the color of thick collagen fibers which was also found to be statistically significant.[6] It is known that the birefringence color of a collagen fiber depends on its diameter and packing.[4] As the diameter was kept constant, the change in color could be attributed to an altered internal packing of collagen fiber. The other possible reasons could be the formation of new collagen by the stroma or tumor cells, or there could be a degeneration of the existing collagen.
The assessment of orientation and packing of collagen fibers in the immediate vicinity of tumor islands in our study revealed a statistically significant difference (P = 0.002 and P = 0.03, respectively) among various grades of OSCC. As the grade of OSCC progressed, the packing of collagen fibers decreased, and the orientation of collagen fibers changed from parallel to haphazard. This could possibly be due to some enzymatic degeneration of the existing collagen or due to the formation of new abnormal/pathologic collagen. Moreover, it could also be the result of increased amount of Type III collagen which occurs singly rather than in bundles like Type I collagen. Martins in their study using PSR-stained collagen fibers on benign and malignant mammary neoplasms in dogs observed that in comparison to benign neoplasms, carcinomas exhibited irregular and randomly distributed collagen fibers in their stroma which partially or discontinuously surrounded the neoplastic area.[14] Similarly, Martins et al. in their study on OSCC observed that in a lower grade of malignancy (i.e., well-differentiated OSCC), collagen fibers near invasive front were arranged parallel to the tumor islands. However, in a higher grade of malignancy, the ECM exhibited lower levels of collagen synthesis and the fibers were irregular, disorganized and dissociated.[10] Manjunatha et al. in their study on OSCC evaluated the arrangement of collagen fibers which they categorized into bundles, cross hatchet and swirl type. Collagen fibers were seen as swirls in well-differentiated OSCC and moderately differentiated OSCC. In poorly differentiated OSCC, collagen was seen in bundles along with cross hatchet arrangement. They concluded that the collagen fibers were more disorganized in moderately to poorly differentiated OSCC.[9]
In our study, the evaluation of area fraction occupied by collagen fibers in the stroma of OSCC did not reveal any statistically significant correlation in varying grades of OSCC. Ziober et al. in their study examined the production of matrix metalloproteinases (MMPs) in the invasive OSCC and observed that MMP-1 causes degradation of type I collagen, thus indicating a decrease in collagen content during the invasion.[15] Moreover, George et al. in their study evaluated the response of stroma in varying grades of OSCC and observed that as the grade of carcinoma progressed, the amount of collagen decreased.[12]
The role of elastic fibers in the progression of OSCC is still unknown. The present study attempts to provide a platform based on the qualitative assessment of elastic fibers in varying grades of OSCC. To the best of our knowledge, there is no published study done on OSCC in English language literature which can enlighten us regarding the changes in the elastic fibers, if any.
As per the literature, there is an interaction between the tumor cells and elastic fibers, but its effect on the progression of carcinoma is questionable. Tuxhorn et al. quoted that the cancer cells can interact specifically with elastin through two elastin-binding proteins and galectin-3.[16] Lapis and Tímár have suggested that there is a positive correlation between tumor progression and the presence of elastic fibers in the tumor stroma.[17] Although a chemotactic effect of elastin peptides on cancer cells has been demonstrated, there is still some controversy as to whether elastin can influence the proliferation of tumor cells.
Patricia et al. in their study on carcinomas of human prostate observed that the most aggressive and anaplastic tumor samples had lost the original pattern of elastic fiber distribution and showed degraded elastic fibers close to tumor cells.[3] George et al. conducted a study on OSCC and observed elastic fibers along with many other components of tumor stroma. They only commented that the amount of elastin in the stroma of OSCC was less as compared to collagen, and no details were given regarding the pattern and morphology of elastic fibers in various grades of OSCC.[12] Ma in their study reported a primary increase followed by a decrease in the amount of elastic fibers with progressing grades of carcinoma.[18] Whereas, another study conducted by Zhang et al. revealed a decrease in elastic fibers with neoplastic progression from epithelial atypia to early invasive carcinoma.[19]
Our study revealed that the elastic fibers were predominantly scanty and were present singly in all the grades of OSCC. Short, thick and wavy elastic fibers were more common among well and moderately differentiated OSCC as compared to long, thin and straight fibers observed in poorly differentiated OSCC. The orientation of elastic fibers in relation to the overlying epithelium changed from perpendicular in the case of well-differentiated OSCC to parallel in the case of poorly differentiated OSCC. The evaluation of orientation of elastic fibers in relation to the tumor islands revealed that the elastic fibers were predominantly haphazardly arranged in case of well and moderately differentiated OSCCs. However, poorly differentiated OSCCs exhibited a parallel orientation predominantly. None of the criteria used for the assessment of elastic fibers correlated with varying grades of OSCC except orientation of elastic fibers in relation to the overlying epithelium. The sparse literature, lesser number of elastic fibers in lamina propria and the masking effect of overlying inflammatory cells could be the limiting factors in the assessment of elastic fibers in OSCC. This calls for more sensitive labeling systems for assessing elastic fibers.
CONCLUSION
In OSCC, the changes occurring within the epithelial cells and their invasion into the connective tissue bring about a massive amount of alterations in the underlying stroma. The interaction of epithelium with connective tissue is often ignored while grading dysplasia and carcinomas. Thus, there is a need to conduct more elaborative studies on connective tissue changes occurring during the progression of a carcinoma and to integrate them into the present grading system to offer better prediction of the clinical course of carcinoma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawazaki-Calone I, Rangel A, Bueno AG, Morais CF, Nagai HM, Kunz RP, et al. The prognostic value of histopathological grading systems in oral squamous cell carcinomas. Oral Dis. 2015;21:755–61. doi: 10.1111/odi.12343. [DOI] [PubMed] [Google Scholar]
- 3.Patricia SL, Suzigan S, Carvalho HF, Taboga SR. Structural characterization and distribution of elastic system fibers in the human prostate and some prostatic lesions. Braz J Morphol Sci. 2003;20:101–7. [Google Scholar]
- 4.Dayan D, Hiss Y, Hirshberg A, Bubis JJ, Wolman M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93:27–9. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- 5.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. 6th ed. Philadelphia: Churchill Livingstone, Elsevier; 2008. [Google Scholar]
- 6.Arun Gopinathan P, Kokila G, Jyothi M, Ananjan C, Pradeep L, Humaira Nazir S. Study of collagen birefringence in different grades of oral squamous cell carcinoma using picrosirius red and polarized light microscopy. Scientifica (Cairo) 2015. 2015:802980. doi: 10.1155/2015/802980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalele KK, Managoli NA, Roopa NM, Kulkarni M, Bagul N, Kheur S. Assessment of collagen fiber nature, spatial distribution, hue and its correlation with invasion and metastasis in oral squamous cell carcinoma and surgical margins using picro sirius red and polarized microscope. J Dent Res Rev. 2014;1:14–7. [Google Scholar]
- 8.Aparna V, Charu S. Evaluation of collagen in different grades of oral squamous cell carcinoma by using the picro sirius red stain-A histochemical study. J Clin Diagn Res. 2010;4:3444–9. [Google Scholar]
- 9.Manjunatha BS, Agrawal A, Shah V. Histopathological evaluation of collagen fibers using picrosirius red stain and polarizing microscopy in oral squamous cell carcinoma. J Cancer Res Ther. 2015;11:272–6. doi: 10.4103/0973-1482.154061. [DOI] [PubMed] [Google Scholar]
- 10.Martins GB, Reis SR, Silva TM. Collagen type I expression in squamous cell carcinoma of the oral cavity. Pesqui Odontol Bras. 2003;17:82–8. doi: 10.1590/s1517-74912003000100016. [DOI] [PubMed] [Google Scholar]
- 11.Hirshberg A, Sherman S, Buchner A, Dayan D. Collagen fibres in the wall of odontogenic keratocysts: A study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999;28:410–2. doi: 10.1111/j.1600-0714.1999.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 12.George J, Narang RS, Rao NN. Stromal response in different histological grades of oral squamous cell carcinoma: A histochemical study. Indian J Dent Res. 2012;23:842. doi: 10.4103/0970-9290.111291. [DOI] [PubMed] [Google Scholar]
- 13.van den Hooff A. Stromal involvement in malignant growth. Adv Cancer Res. 1988;50:159–96. doi: 10.1016/s0065-230x(08)60437-6. [DOI] [PubMed] [Google Scholar]
- 14.Martins AM. Histochemical study of fibrillar proteins of the extracellular matrix in benign and malignant mammary neoplasms in dogs. Braz J Vet Res Anim. 2002;39:43–9. [Google Scholar]
- 15.Ziober BL, Turner MA, Palefsky JM, Banda MJ, Kramer RH. Type I collagen degradation by invasive oral squamous cell carcinoma. Oral Oncol. 2000;36:365–72. doi: 10.1016/s1368-8375(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 16.Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–83. [PubMed] [Google Scholar]
- 17.Lapis K, Tímár J. Role of elastin-matrix interactions in tumor progression. Semin Cancer Biol. 2002;12:209–17. doi: 10.1016/S1044-579X(02)00024-X. [DOI] [PubMed] [Google Scholar]
- 18.Ma CK. Morphological and chemical investigation of dermal, elastic, and collagenic tissue during epidermal carcinogenesis. Cancer Res. 1949;9:481–7. [PubMed] [Google Scholar]
- 19.Zhang P, Du YB, Yu M, Yin X, Lv YH, Gao ZX, et al. Changes of the elastic fibers and collagen fibers during the development and progression of experimentally induced tongue carcinoma in hamsters. Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:2696–8. [PubMed] [Google Scholar]