Abstract
Introduction:
Periodontal diseases, if left untreated, can lead to tooth loss and affect at least one tooth in 80% of adults worldwide, with the main cause being a bacterial plaque. Among subgingival plaque bacterial species, Porphyromonas gingivalis has been implicated as a major etiological agent causing tooth loss. Diabetics and smokers are two patient groups at high risk for periodontal disease. The increase in the number of this organism with the coexistence of other pathogenic microbes leads to rapid destruction of the periodontium, premature loss of teeth and also because of its virulence has implications in systemic pathology. Our aim was to observe the involvement of P. gingivalis in diabetes mellitus (DM) patients associated with periodontitis with and without tobacco-associated habits and to compare them with periodontitis patients having no other systemic pathologies.
Materials and Methods:
Subgingival plaque samples from a total of seventy subjects were included in the study. DNA was isolated from the collected sample and was quantified using spectrophotometer for standardizing the polymerase chain reaction. The quantity of the isolated DNA was checked in a ultraviolet-visible spectrophotomer.
Statistics:
One-way ANOVA and Tukey's multiple post hoc procedures were carried out.
Results:
The maximum score of P. gingivalis was seen in periodontitis patients having DM, whereas the least score was seen in periodontitis patients having DM with tobacco smoking habit compared to the other groups.
Conclusion:
P. gingivalis count is significantly reduced in periodontitis patients having DM with smoking habit; it is concluded that P. gingivalis might not be a key causative organism responsible for the periodontal destruction in case of smokers despite the DM condition. The decrease in counts may be attributed to change in the local environment like chemical (tobacco nitrosamines) and physical changes preventing the growth of P. gingivalis.
Keywords: Chronic periodontitis, diabetes mellitus, Porphyromonas gingivalis, smokers
INTRODUCTION
Periodontal diseases are among the most prevalent dental diseases affecting people worldwide as well as in the Indian community.[1] Periodontal diseases cause loss of teeth and also affect systemic health with the oro-hematogenous spread of bacteria. The related systemic diseases include cardiovascular diseases, preterm delivery of low birth weight, osteoporosis, diabetes mellitus (DM) and respiratory infections.[2]
Periodontal diseases are infectious in nature, and bacteria play a major role in its initiation and progression.[3] It occurs as a result of complex interactions between periodontopathic microorganisms and the host tissues.[4] This process is modified by the status of the host immune system, genetic factors and a complex array of environmental exposures.[5]
Chronic periodontitis is associated with accumulation of plaque and calculus and has a slow to moderate rate of disease progression; however, periods of rapid destruction are also observed. The increase in the rate of disease progression is caused by the impact of local, systemic or environmental factors that influence the normal host-bacterial interaction.[3,4,6]
As periodontitis is an infectious disease, it is mainly associated with a group of Gram-negative bacteria such as Actinobacillus actinomycetemcomitans (AA), Porphyromonas gingivalis and Bacteroides forsythus. Of these, P. gingivalis and B. forsythus are found in chronic periodontitis, whereas AA is found in cases of aggressive periodontitis.[3,7]
P. gingivalis is an anaerobic, Gram-negative, rod-shaped and highly virulent organism implicated as a major pathogen in destructive periodontal disease with its ability to adhere and invade oral epithelium.[8] It is also implicated to be involved in the development of systemic diseases due to systemic inflammation with increased circulating cytokines and mediators, direct infection and cross-reactivity/molecular mimicry between bacterial antigens and self-antigens. It has been detected in heart valve lesions and atheromatous plaque, amniotic fluid of pregnant women with threatened premature labor and placentas from cases of preterm delivery, DM, respiratory diseases and osteoporosis subjects.[5]
DM is a metabolic disorder characterized by hyperglycemia due to defective secretion or activity of insulin. Diabetes increases the glucose concentration in the gingival crevicular fluid and decreases the salivary levels of epidermal growth factor which plays an important role in wound healing. These modifications in GCF affect plaque composition which is supported by an increased amount of plaque and increased numbers of Gram-negative anaerobes. The consequences include impaired cellular functions, impaired host defense, vascular alterations, prolonged inflammation, impaired bone formation or repair ultimately resulting in tooth mobility and premature loss of teeth.[9]
There is a two-way relationship between DM and periodontitis, where local periodontal infection can exacerbate and help in the progression of systemic complications. The systemic exposure to periodontal pathogens in the periodontium worsens the low-grade systemic inflammation present in diabetes. The consequences are alterations in glucose metabolism and regulation resulting in difficulties in maintaining optimal glycemic control, which in turn increase the development and progression of diabetic-related complications such as periodontitis.[9]
P. gingivalis is frequently detected in various distant organs such as the liver, cardiovascular tissue, cerebrospinal fluid and tubal-ovarian locations. P. gingivalis is also able to invade intracellularly without the signs of apoptosis and necrosis. It has virulence factors such as collagenase, aminopeptidase and trypsin-like enzyme activity. The structural components of P. gingivalis include lipopolysaccharide and fimbriae that trigger intracellular signaling events.[10] P. gingivalis in the oral cavity moves to the liver and is responsible for glycogen synthesis through Akt/GSK-3 β signaling in the liver cells. Hence, P. gingivalis in the oral cavity contributes to the pathogenesis of DM by affecting hepatic glycogenesis through Akt/GSK-3 β signaling.[11]
Local factors such as plaque with a complex array of environmental exposures also play an important role in the periodontal destruction. This involves cigarette smokers who develop severe periodontitis up to five times more than nonsmokers.[2,12] Smokers have been associated with deeper pockets and greater attachment loss, more pronounced furcation involvement and increased alveolar bone loss.[2,13]
As both DM and smoking are important and significant risk factors for periodontitis, it is very important to know the role of P. gingivalis and its implications. Hence, from our study, we intend to quantify the P. gingivalis count using real-time polymerase chain reaction (PCR). Accurate quantification of P. gingivalis is needed to understand bacterial etiology of periodontitis. Hence, we used real-time PCR which provides a sensitive, efficient and reliable method for quantification of P. gingivalis.[14,15,16]
Our aim is to observe the relative involvement of P. gingivalis in DM patients having periodontitis with tobacco smoking habit and to compare them with Group IA (no periodontal diseases) and Group IB (periodontal disease with no systemic pathology).
MATERIALS AND METHODS
The study was conducted on the subgingival plaque of patients with chronic periodontitis, with and without type II DM associated with smoking habit from our institution. A total of seventy cases were considered.
Group I (Control): (a) Fifteen healthy individuals without periodontal and systemic pathologies. (b) Fifteen chronic periodontitis patients without systemic pathology. Group II (Study): (a) Twenty newly diagnosed cases of DM having chronic periodontitis without tobacco smoking habit. (b) Twenty newly diagnosed cases of DM having chronic periodontitis with tobacco smoking habit.
The random blood sugar levels of all the patients were taken. The subgingival plaque was collected under aseptic and sterile conditions using standard universal curettes. The sample was placed in a vial containing phosphate buffer at –4°C and transported to the laboratory for further procedures. DNA was isolated from samples by modified cetyltrimethylammonium bromide method by centrifuging at 10,000 rpm for 10 min to pellet down the bacterial cells. The quantity of the isolated DNA was checked in ultraviolet-visible spectrophotomer (Vivaspec Biophotometer, Germany). From the stock, 1 μl DNA was mixed with 49-μl sterile distilled water to get fifty times dilution. The A260/A280 ratio was recorded to check the purity of DNA preparation.
The primers for quantification analysis were designed using Perkin-Elmer Primer Express® software. The melting temperature was calculated, and the synthesized primers were purified by high-performance liquid chromatography. The quantified DNA was used to detect the presence of P. gingivalis using the specific primers, and primer optimization was done in a gradient PCR at the annealing temperature of 55°C [Tables 1 and 2]. Amplified products were resolved on 2% ethidium bromide-stained agarose gel along with a 100 bp DNA ladder
Table 1.
Polymerase chain reaction Design and synthesis

Table 2.
Polymerase chain reaction Temperature profile

Electrophoresis results were analyzed in gel documentation system and photographed.
RESULTS AND OBSERVATIONS
Of 70 samples, 46 were male (65.71%) and 24 were female (34.28%) [Table 3].
Table 3.
Distribution of study subjects by gender
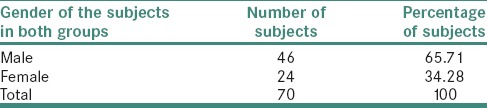
The study subjects were found to be in the age group between 2nd to 5th decades [Table 4].
Table 4.
Distribution of study subjects in each group by age
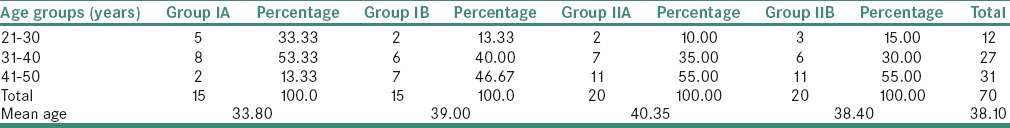
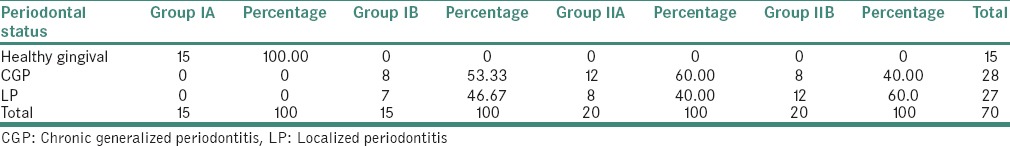
The maximum chronic generalized periodontitis were seen in Group IIA and localized periodontitis in Group IIB [Table 5].
Table 5.
Distribution of study subjects based on their periodontal status
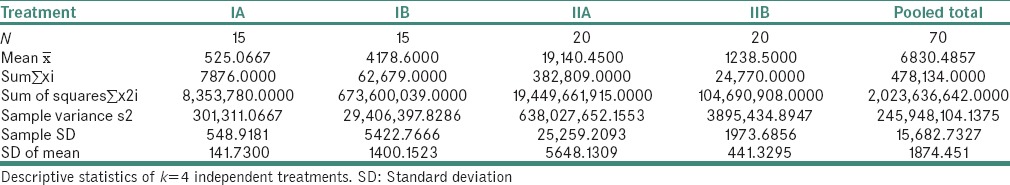
The maximum P. gingivalis count were observed in Group IIA and minimum count in Group IA [Table 6 and Graph 1]. It was also observed that the P. gingivalis count was significantly reduced in Group IIB compared to nonsmokers.
Table 6.
Comparison of Porphyromonas gingivalis scores in all the study groups
Graph 1.
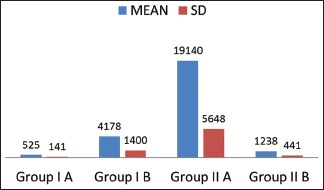
Comparison of Porphyromonas gingivalis scores in all the study groups (Groups IA, IB, IIA and IIB)
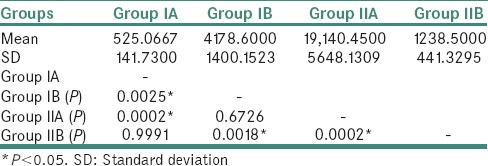
Pair-wise comparison of all the groups with respect to P. gingivalis scores as given by Tukey's multiple post hoc procedures is presented in Table 7.
Table 7.
Pair-wise comparison of four groups with respect to log Porphyromonas gingivalis scores by Tukey's multiple post hoc procedures
DISCUSSION
Periodontitis is a disease that affects the periodontium which is characterized by the loss of periodontal attachment including the alveolar bone. The bacterial etiology of periodontal disease is complex, with a variety of organisms responsible for the initiation and progression of the disease. The microbe which causes periodontitis contains primarily Gram-negative rods and cocci, filaments, flagellated rods and spirochetes. Many of these organisms are also present in periodontally healthy individuals and can exist in commensal harmony with the host.[14] The mere presence of pathogen alone is not sufficient to cause disease but should be above a determined threshold to cause periodontal disease.
P. gingivalis has been implicated to be associated with many systemic diseases, where multiple fold increase is seen. These pathogens gain entry into circulation through the ulcerated epithelium and exposed capillaries during periodontal inflammation and may induce systemic symptoms.[5]
DM is commonly associated with periodontal diseases. The interrelationship between periodontitis and DM provides an example of systemic disease predisposing to oral infection, and once that infection is established, the oral infection exacerbates systemic disease.[9]
The effects of smoking in oral cavity include bad breath, tooth discoloration, inflammation of the salivary gland openings, increased plaque and tartar on the teeth, risk of leukoplakia and gum diseases leading to tooth loss.[17] Nicotine, a major smoke component, induces periodontal collagen degradation by increasing expression of the collagenase matrix metalloproteases which results in detachment of the periodontal ligaments. P. gingivalis persistence in the oral cavity of smokers is attributed to a compromised immune response and/or increased bacterial virulence. Despite increased P. gingivalis, smokers consistently display reduced clinical inflammation due to lower levels of proinflammatory cytokines at diseased sites.[17,18]
As periodontal diseases are polymicrobial infections with various etiologic factors, accurate quantitation of the number of cells of individual bacterial species in dental plaque samples is needed for understanding the bacterial etiology of periodontitis. Detection and quantification of this microorganism is relevant for diagnosis and treatment planning.[19] The real-time PCR provides precise counts through direct monitoring of the increasing amount of PCR product throughout the enzymatic assay and is the most sensitive method with detection limits of 10[2] genome copies.[20]
Hence, we used real-time PCR method for quantification of P. gingivalis.
In Group IA, P. gingivalis counts ranged from 0 to 1561 with a mean of 525. It implies that P. gingivalis exists in commensal harmony with other organisms present in the periodontally healthy individuals. The study is supported by other studies, where Lyons et al.[19] and Van Winkelhoff et al.[21] showed the presence of P. gingivalis in healthy subjects by PCR assay and concluded that this organism may also be a normal inhabitant of a periodontally healthy dentition.
In Group IB, P. gingivalis counts ranged from 288 to 21697 with a mean of 4178. It implies that P. gingivalis is one of the major pathogens implicated in the development and progression of the periodontal disease. Hence, we can observe the substantial increase in the P. gingivalis count when compared to healthy individuals from our results. Our study results are supported by Kirakodu et al.[20] and Socransky et al.,[22] who conducted a study on quantification of P. gingivalis from subgingival plaque by quantitative PCR assay which showed an increased prevalence of P. gingivalis organisms ranging from 6 × 102 – 8.6 × 104 in chronic periodontitis patients.
In Group IIA, P. gingivalis count ranged from 204 to 81691 with a mean of 19140. It shows that high glucose levels in type II DM patients lead to the development of periodontogenic flora due to reduced oxygen production and defense cells. Hence, diabetic patients have an increased susceptibility for more severe periodontal disease due to increased prevalence of P. gingivalis. In our study, Group IIA had statistically significant increase in the P. gingivalis counts by 40 folds than Group IA. However, it was increased only by 4 folds when compared to Group IB. Our study results are supported by Campus et al.[23] and Ebersole et al.,[24] who conducted a study using PCR assay and concluded that these patients have an increased susceptibility for severe periodontal disease and increased prevalence of P. gingivalis.
In Group IIB, the P. gingivalis counts ranged from 0 to 8684 with a mean of 1238. The counts of P. gingivalis have significantly decreased by 16-fold (P = 0.0002) when compared to Group IIA. This is because the organism cannot thrive in the altered environment that is attributed to heat and tobacco smoke extracts despite increased blood glucose levels. Hence, the destruction of periodontium might be caused by other virulent periodontal pathogens. The studies done by Bagaitkar[25] and Zeller et al.[26] showed increased prevalence for P. gingivalis as compared to nonsmokers; whereas studies done by Zambon[27] and Kinane and Chestnutt[28] gave inconclusive results about the prevalence of P. gingivalis in smokers, where they concluded that no statistically significant difference was found between smokers and nonsmokers. Although the previous studies have shown increased prevalence of this organism in smokers compared to nonsmokers, our study showed conflicting results where the count of this organism was significantly reduced. However, none of the previous studies have included both the parameters of diabetes and smoking together for assessing P. gingivalis count in periodontitis subjects. Hence, further studies need to be done with a large sample size. We chose quantitative real-time PCR as it is more efficient and sensitive compared to other methods and provides precise counts through direct monitoring of the increasing amount of PCR product throughout the enzymatic assay. Subgingival plaque samples were chosen over other samples such as saliva and gingival crevicular fluid for better yield and to understand bacterial etiology of periodontitis.
CONCLUSION
P. gingivalis is one of the major etiological agents in periodontal destruction and in recent years, it is gaining importance because of its implications in the systemic pathologies such as DM. It is also considered a risk factor associated with tobacco smoking habit. Thus, by assessing its quantity, we can analyze its role as a pathogen and the risk associated with this organism as studies have shown that it exacerbates the systemic pathology. A quantitative real-time PCR is an efficient and sensitive method which allows us to study specific organisms and their role in various pathologies. Larger studies of this kind can throw light on a variety of etiological microorganisms. Further studies with larger sample size should be conducted to confirm the findings of our study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agarwal V, Khatri M, Singh G. Prevalence of periodontal diseases in India. J Oral Health Community Dent. 2010;4:7–16. [Google Scholar]
- 2.Kinane DF, Lindhe J. Clinical Periodontology and Implant Dentistry. 4th ed. Oxford (UK): Munksgaard, Blackwell Publishing Company; 2003. [Google Scholar]
- 3.Albandar JM, Rams TE. Global epidemiology of periodontal diseases: An overview. Periodontol 2000. 2002;29:7–10. doi: 10.1034/j.1600-0757.2002.290101.x. [DOI] [PubMed] [Google Scholar]
- 4.Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: Assembling the players. Periodontol 2000. 1997;14:33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 5.Inaba H, Amano A. Roles of oral bacteria in cardiovascular diseases – From molecular mechanisms to clinical cases: Implication of periodontal diseases in development of systemic diseases. J Pharmacol Sci. 2010;113:103–9. doi: 10.1254/jphs.09r23fm. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Periodontology. The pathogenesis of periodontal diseases. J Periodontol. 1999;70:457–70. doi: 10.1902/jop.1999.70.4.457. [DOI] [PubMed] [Google Scholar]
- 7.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 8.Kesic L, Milasin J. Microbial etiology of periodontal disease. A mini review. Med Biol. 2008;15:1–6. [Google Scholar]
- 9.Page RC. The pathobiology of periodontal diseases may affect systemic diseases: Inversion of a paradigm. Ann Periodontol. 1998;3:108–20. doi: 10.1902/annals.1998.3.1.108. [DOI] [PubMed] [Google Scholar]
- 10.Marigo L, Cerreto R, Giuliani M, Somma F, Lajolo C, Cordaro M. Diabetes mellitus: Biochemical, histological and microbiological aspects in periodontal disease. Eur Rev Med Pharmacol Sci. 2011;15:751–8. [PubMed] [Google Scholar]
- 11.Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N, et al. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3ß signaling pathway. Biochim Biophys Acta. 2013;1832:2035–43. doi: 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12.AlJehani YA. Risk factors of periodontal disease: Review of the literature. Int J Dent 2014. 2014:182513. doi: 10.1155/2014/182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Berezow AB, Darveau RP. Microbial shift and periodontitis. Periodontol 2000. 2011;55:36–47. doi: 10.1111/j.1600-0757.2010.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonnenmacher C, Mutters R, de Jacoby LF. Microbiological characteristics of subgingival microbiota in adult periodontitis, localized juvenile periodontitis and rapidly progressive periodontitis subjects. Clin Microbiol Infect. 2001;7:213–7. doi: 10.1046/j.1469-0691.2001.00210.x. [DOI] [PubMed] [Google Scholar]
- 15.Boutaga K, Savelkoul PH, Winkel EG, van Winkelhoff AJ. Comparison of subgingival bacterial sampling with oral lavage for detection and quantification of periodontal pathogens by real-time polymerase chain reaction. J Periodontol. 2007;78:79–86. doi: 10.1902/jop.2007.060078. [DOI] [PubMed] [Google Scholar]
- 16.Clais S, Boulet G, Van Kerckhoven M, Lanckacker E, Delputte P, Maes L, et al. Comparison of viable plate count, turbidity measurement and real-time PCR for quantification of Porphyromonas gingivalis. Lett Appl Microbiol. 2015;60:79–84. doi: 10.1111/lam.12341. [DOI] [PubMed] [Google Scholar]
- 17.Bergström J, Eliasson S, Dock J. Exposure to tobacco smoking and periodontal health. J Clin Periodontol. 2000;27:61–8. doi: 10.1034/j.1600-051x.2000.027001061.x. [DOI] [PubMed] [Google Scholar]
- 18.Mahmoud AT. The effects of smoking on periodontal disease: An evidence-based comprehensive literature review. Open J Stomatol. 2014;4:33–41. [Google Scholar]
- 19.Lyons SR, Griffen AL, Leys EJ. Quantitative real-time PCR for Porphyromonas gingivalis and total bacteria. J Clin Microbiol. 2000;38:2362–5. doi: 10.1128/jcm.38.6.2362-2365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirakodu SS, Govindaswami M, Novak MJ, Ebersole JL, Novak KF. Optimizing qPCR for the quantification of periodontal pathogens in a complex plaque biofilm. Open Dent J. 2008;2:49–55. doi: 10.2174/1874210600802010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002;29:1023–8. doi: 10.1034/j.1600-051x.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- 22.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL. Microbial complexes in subgingival plaque. J Clin Periodontol. 2005;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 23.Campus G, Salem A, Uzzau S, Baldoni E, Tonolo G. Diabetes and periodontal disease: A case-control study. J Periodontol. 2005;76:418–25. doi: 10.1902/jop.2005.76.3.418. [DOI] [PubMed] [Google Scholar]
- 24.Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in Hispanic Americans with type 2 diabetes. J Periodontol. 2008;79:637–46. doi: 10.1902/jop.2008.070455. [DOI] [PubMed] [Google Scholar]
- 25.Bagaitkar J. Tobacco-induced Changes to Porphyromonas Gingivalis Gene Expression, Phenotype and Host-pathogen Interactions. Louisville University of Louisville; 2010. [Google Scholar]
- 26.Zeller I, Hutcherson JA, Lamont RJ, Demuth DR, Gumus P, Nizam N, et al. Altered antigenic profiling and infectivity of Porphyromonas gingivalis in smokers and non-smokers with periodontitis. J Periodontol. 2014;85:837–44. doi: 10.1902/jop.2013.130336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambon JJ. Periodontal diseases: Microbial factors. Ann Periodontol. 1996;1:879–925. doi: 10.1902/annals.1996.1.1.879. [DOI] [PubMed] [Google Scholar]
- 28.Kinane DF, Chestnutt IG. Smoking and periodontal disease. Crit Rev Oral Biol Med. 2000;11:356–65. doi: 10.1177/10454411000110030501. [DOI] [PubMed] [Google Scholar]


