Abstract
Introduction:
Oral squamous cell carcinoma (OSCC) comprises one of the largest subsets of cancers with a tendency for regional metastasis. Nodal status is a key prognostic indicator in patients with OSCC, particularly with N0 neck. Occult metastasis in the form of micrometastasis (MM) and isolated tumor cells (ITCs), often goes undetected by routine hematoxylin and eosin (H&E) examination using 1–2 sections for analysis. This limitation could be overcome by combining serial sectioning (SS) with immunohistochemistry (IHC) for the detection of MM and ITC. Pan-cytokeratin (pan-CK) (AE1/AE3) is particularly a useful marker to detect these deposits as their presence has resulted in varied interpretations and different applications of the tumor-node-metastasis system.
Objectives:
The objective of the study was to identify a suitable method for detecting MM and ITC in lymph nodes (LNs) of OSCC by combining SS and IHC and to compare it with conventional H&E staining.
Materials and Methods:
This laboratory-based, prospective study was conducted on 133 LNs harnessed from ten patients treated with radical neck dissection for primary OSCC. The LNs were subjected to SS at 100 μm intervals. The sections were stained with routine H&E staining, pan-CK and analyzed for MM and ITC according to criteria laid by Hermanek et al.
Statistical Analysis:
The obtained data were subjected to statistical analysis using Chi-square test.
Results:
The application of combination of SS and IHC using pan-CK (AE1/AE3) in our study revealed the presence of MM and ITC in 2.25% of the LNs diagnosed as negative on routine H&E examination. The detection of these occult metastatic deposits resulted in upstaging of 33.33% of the patients.
Conclusion:
In the view of crucial role of occult LN metastasis in prognosis and survival of OSCC patients with N0 neck, diagnostic tools such as IHC staining, particularly with pan-CK (AE1/AE3), combined with SS should be preferred over conventional methods as they result in upstaging, thus sparing the low-risk patients the morbidity of unnecessary treatment.
Keywords: Cervical lymph nodes, isolated tumor cells, micrometastasis, oral squamous cell carcinoma, pan-cytokeratin (AE1/AE3), serial sectioning, tumor-node-metastasis staging
INTRODUCTION
Oral squamous cell carcinoma (OSCC) is one of the most common cancers today with high tendency for regional and distant metastases and thus remains one of the most difficult malignancies to control. The status of cervical lymph nodes (LNs) is the single most important prognostic factor for patients with OSCC and the presence of metastatic LNs decreases survival rate by more than 50%.[1]
Watchful waiting until OSCC patients with clinically negative (N0) neck develop detectable neck disease has been shown to significantly decrease survival. The basic rule for prophylactic treatment of N0 neck is to treat any patient whose risk of occult LN metastasis is 20% or higher. The challenge lies in identifying patients who are at risk for developing LN metastasis to treat them prophylactically and decrease the risk of poor prognosis.[2]
Over the period, many authors have shown that 15–20% of patients without LN metastases as assessed by light microscopy had a recurrence within 10 years which have led to interest in the detection of occult/subclinical metastasis.[3] The subclinical metastasis occurs in two forms which could be either micrometastasis (MM) or isolated tumor cells (ITCs).
MM is defined as proliferation within the node of tumor tissue measuring more than 0.2 mm but <2 mm in diameter, whereas ITC is defined as single cell or groups of tumor cells <0.2 mm in diameter.[4]
Traditional diagnostic methods such as clinical assessment, histopathological examination and imaging techniques are limited in their capacity to provide information on prognosis and treatment of choice for head and neck cancer.[3]
Thus, the technique of serial sectioning (SS) which involves cutting of the LN block at different intervals until the block is completely exhausted was introduced to detect occult metastatic deposits.[5]
Due to limitations of the conventional methods, newer methods such as immunohistochemistry (IHC) and molecular assays were introduced in 1980s for improved detection of disseminated tumor cells and MM within the lymph and blood circulation, thus presenting improved diagnosis of the disease.[6]
Developed by Coons in 1940s, IHC is the application of immunological principles and techniques to detect antigens in cells of a tissue section by exploiting the principle of antibody binding, specifically to antigens in biological tissues using various monoclonal antibodies such as vascular endothelial growth factor, cytokines, epithelial membrane antigen (EMA), matrix metalloproteinases and cytokeratins (CKs).[6]
Among these markers, CKs are applied most commonly for analysis of LN metastasis in OSCC patients. CKs are the keratin proteins containing intermediate filaments found in the intracytoplasmic cytoskeleton of the epithelial tissue and reflect distinct cellular properties and differentiation stages in epithelial organs.[7]
Pan-cytokeratin (pan-CK) (AE1/AE3) can be used to detect a wide spectrum of both acidic and basic CKs and is widely used as an important marker to differentiate epithelial tumors from the nonepithelial ones with a high degree of accuracy even under low-power standard light microscope.[8]
Due to limitations of hematoxylin and eosin (H&E) alone, the adjunctive use of IHC and SS has been suggested for enhanced tissue interpretation and proper diagnosis of N0 OSCC patients.[9] This combination has resulted in upstaging and hence improving diagnosis and prognosis of OSCC patients.[10]
The aim of the present study was to identify a suitable method for detecting LN metastasis in the form of MM and ITC in patients with primary OSCC. SS using H&E as well as IHC (pan-CK) was employed for detection of nodal metastasis, particularly MM and ITC in N0 OSCC patients.
MATERIALS AND METHODS
The study was conducted on ten patients diagnosed with primary OSCC who underwent radical neck dissection in the institute.
Patients diagnosed with primary OSCC, those with operable tumor as assessed by tumor-node-metastasis (TNM) stage, tumor location and general health status and patients in the N0 preoperative stage according to TNM staging were included in the study. On the contrary, patients with history of radiotherapy, preadjunctive chemotherapy, recurrent OSCC and active lesion of squamous cell carcinoma at sites other than oral cavity were excluded from the study.
Harnessing of lymph nodes
A total of 133 LNs were harnessed from all the cases with size ranging from 3 to 15 mm in maximum dimensions. All LNs were cut into sections not thicker than 2 mm through and parallel to the longest axis and were immediately fixed in 10% neutral buffered formalin for 24 h at room temperature to avoid rapid proteolytic degradation of target proteins within the tissue so as to reduce nonspecific immunoreactivity. After proper fixation, the sections were processed routinely and were embedded in paraffin wax separately. A section of the tumor tissue was also processed along with the LNs for the histopathological grading of the tumor.
Histopathological evaluation
Initially, a section was cut from each block and subjected to H&E staining for evaluation by light microscopy. All the cases taken up for the study were classified as pN0 after routine histopathological examination. The diagnosis of all the cases was reevaluated and confirmed by two independent pathologists after reviewing the microscopic slides and clinical histories of the patients, according to the criterion laid by the latest WHO Classification of Head and Neck Tumors (2005).
The grading of the tumor was done according to the criteria suggested by Broder in 1927.[11]
The pathologic reporting of LNs was done according to the criteria laid by Hermanek et al. 1999:[4]
Macrometastasis: Proliferation within the node of tumor tissue measuring more than 2 mm in diameter.
MM: Proliferation within the node of tumor tissue measuring more than 0.2 mm but <2 mm in diameter.
ITCs: Single cell or groups of tumor cells <0.2 mm in diameter.
Serial sectioning procedure
The LNs classified as negative after the initial histopathological examination were subjected to SS. Serial microsections, each measuring 3–5 μm, were retained every 100 μm throughout the block. The number of sections obtained varied according to the size of LNs. At each level, alternate sections were stained with H&E and pan-CK (AE1/AE3). Both sensitivity and clinical significance of IHC using monoclonal antibody against pan-CK (AE1/AE3) for detection of MM and ITC were evaluated and compared with routine H&E-stained slides. IHC was performed in the laboratory according to the standardized protocol.
Immunolabeling
All stained slides were examined under a light microscope (Nikon E200 Optical microscope) independently by two experienced pathologists for expression of the positive immunostaining. Only those cells that were defined by a strong, globoid cytoplasmic reactivity were considered as neoplastic. The positive sections were then observed under ×40 objective.
All the sections were scored by two independent observers and the values obtained were subjected to statistical analysis by Chi-square test. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 17.0 (IBM, Chicago Inc). P < 0.05 was considered statistically significant.
RESULTS
All the stained tissue sections showed a variable labeling pattern from one tissue to another and sometimes within the same tissue. The observations of positive and negative controls in concomitance with H&E-stained sections proved the validity of staining of pan-CK (AE1/AE3).
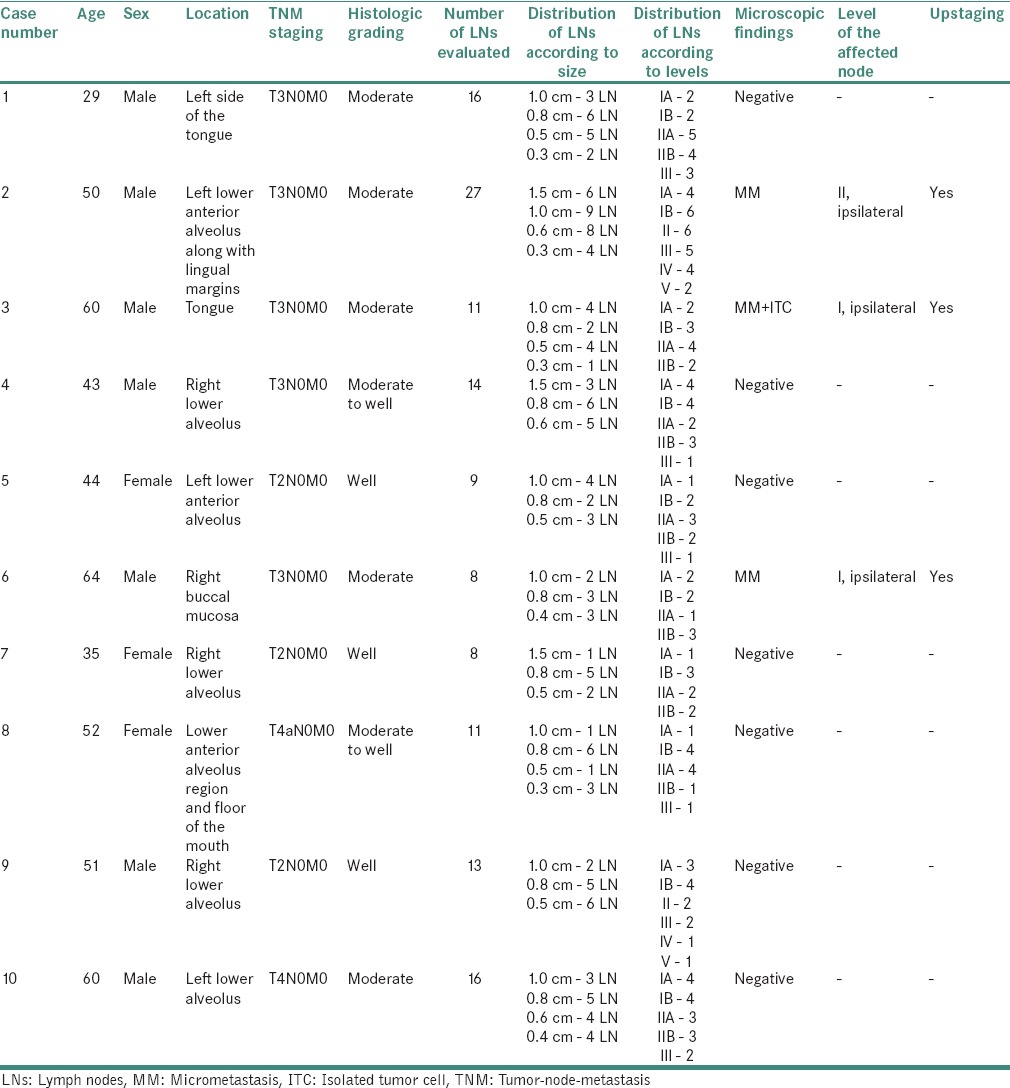
The age of the patients ranged from 29 to 64 years with the highest prevalence seen in the 5th and 6th decades. Of the total sample size, males showed higher predominance (70%) as compared to females (30%). Alveolus was the most common site of involvement, followed by tongue. Majority of the cases (50%) were found to be graded as moderately differentiated OSCC. The details of clinical data along with staging, grading and LN distribution were recorded [Table 1].
Table 1.
Clinical details, staging, grading, lymph node distribution and findings of the selected cases
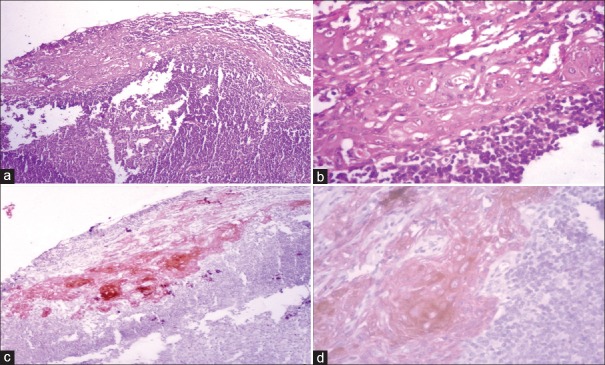
Comparative assessment of SS stained with H&E and IHC revealed MM in 1 and 3 LNs, respectively [Figures 1–3]. On SS, tumor-like cells were detected in the LNs initially diagnosed as reactive hyperplasia, which were further confirmed by positive pan-CK staining [Figure 2b and c]. Software analysis revealed the size of metastatic deposits <2 mm, thus establishing it as MM.
Figure 1.
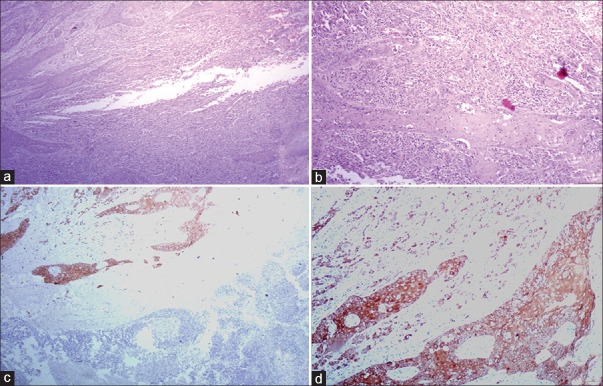
Frank metastasis was observed in lymph node of moderately differentiated squamous cell carcinoma at low and high-power view [H&E stain, (a) ×40, (b) ×100]. Immunostaining with pan-cytokeratin (AE1/AE3) confirmed the tumoral invasion [IHC stain, (c) ×40, (d) ×100]
Figure 3.
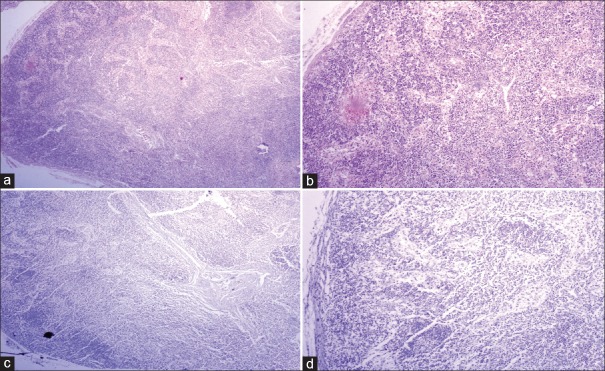
Lymph node of moderately differentiated squamous cell carcinoma showing absence of metastatic deposits [H&E stain, (a) ×40, (b) ×100]; the absence of immunopositivity for pan-cytokeratin (AE1/AE3) confirmed the absence of occult metastatic deposits [IHC stain, (c) ×40, (d) ×100]
Figure 2.
(a) Lymph node of moderately differentiated squamous cell carcinoma revealed reactive hyperplasia at low-power (H&E stain, ×100); (b) high-power view of the same area revealed epithelial-like cells (H&E stain, ×400); (c) immunostaining with pan-cytokeratin (AE1/AE3) depicted metastatic deposits at low power (IHC stain, ×100) which were confirmed to be micrometastasis at high power (IHC stain, ×400)
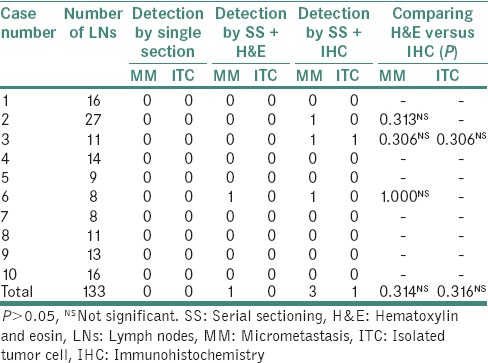
MM was found in the LNs of size ≥1.0 cm. ITCs were seen in only one LN measuring 0.8 cm by IHC. However, these values were found to be statistically nonsignificant when Chi-square test was applied. Although SS revealed 1 MM by H&E and 3 MM and 1 ITC by IHC, the statistical value of this comparative analysis was found to be nonsignificant [Tables 2–4].
Table 2.
Details of microscopic evaluation of lymph nodes according to size
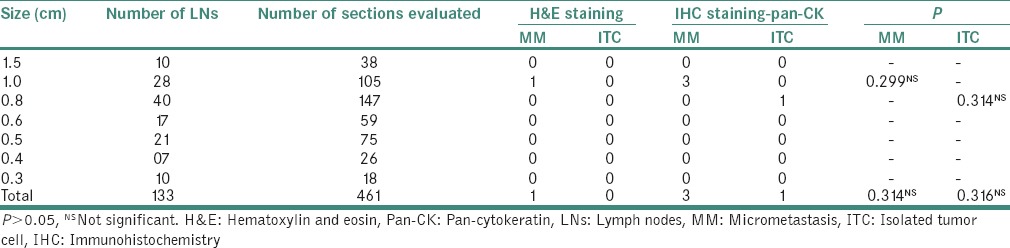
Table 4.
Comparison of micrometastasis and isolated tumor cell detected by hematoxylin and eosin and immunohistochemistry

Table 3.
Comparison of detection rate with hematoxylin and eosin and immunohistochemistry with sectioning method
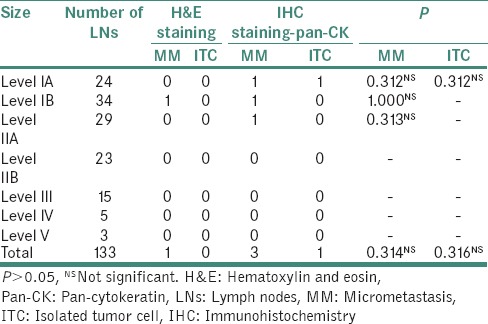
MM was detected in the LNs harvested from levels IA, IB and IIA while ITC was detected only in 1 LN from level IA. The Chi-square test did not reveal any significant value for this comparison [Table 5].
Table 5.
Details of microscopic evaluation of lymph nodes according to levels
DISCUSSION
OSCC frequently metastasizes to the regional LNs which are the first sites of arrest for tumor cells that have invaded the peritumoral lymphatics and hence the strongest predictor of disease prognosis and outcome.[12,13]
Clinical TNM staging system devised by Pierre Denoix is widely used by clinicians for the management of OSCC patients.[14] However, the size of surface tumor and cervical LNs does not correlate with the occurrence of metastasis in OSCC. Studies have shown that LNs measuring more than 20 mm might have reactive hyperplasia without metastasis on histopathological examination.[15] General policy of elective neck dissection based on clinical TNM staging exposes many OSCC patients to wide neck dissection that may not be necessary.[14,16]
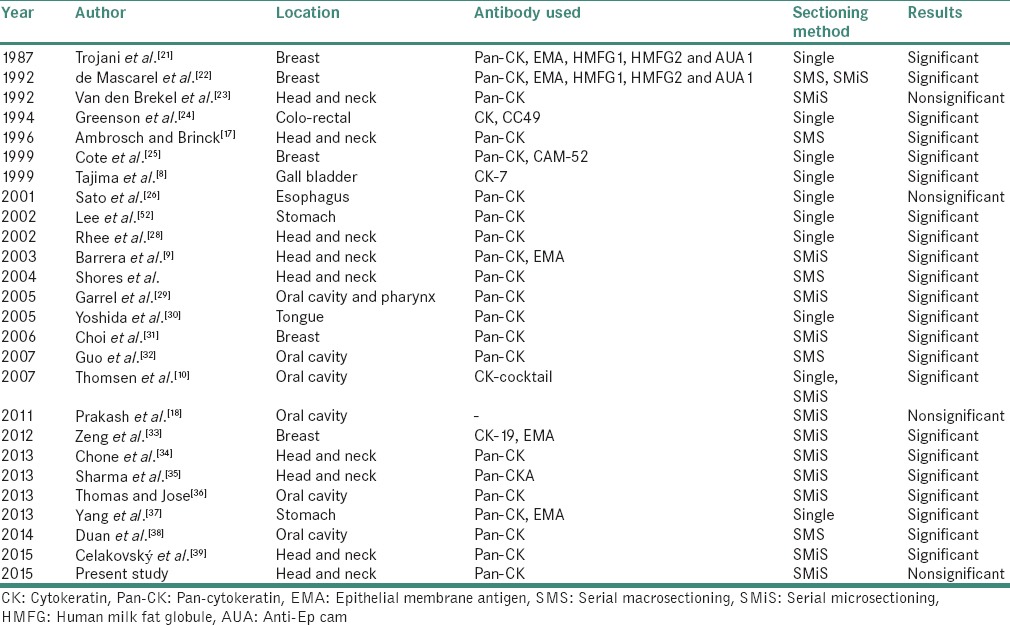
Histopathological examination of the neck dissection specimens provides basic information of diagnosis and staging.[17] Because of the impact of nodal status on treatment and survival in OSCC, accurate staging of cervical LNs is critical. Conventional techniques of pathologic analysis of neck dissections are potentially limited, hence leading to incomplete analysis.[5] The conventional method of obtaining few sections for histopathological examination misses out the small deposits resulting in high recurrence rate in patients diagnosed as N0 neck cases. In addition, resemblance of histiocytes and endothelial cells to malignant cells pose difficulty in their detection.[18] This led to obtaining serial sections from the blocks to detect the occult metastasis and thus improve prognosis.[19,20] A number of studies in the available English literature have compared the IHC analysis with H&E in detecting MM and ITC in regional LNs from primary carcinomas [Table 6].
Table 6.
Comparison of immunohistochemical analysis with hematoxylin and eosin in detecting micrometastasis and isolated tumor cell in regional lymph nodes from primary carcinoma
When compared to N0 neck disease, the presence of even a single MM in an LN is associated with a significant difference in recurrence and survival as cure rates for patients with pathologically metastatic LNs drop to one-half of the patients without nodal involvement.[17,40,41] The deleterious effect of cervical metastases on prognosis is so great that even a 20% chance of metastases in an otherwise clinically and radiographically negative neck pushes most clinician towards extensive treatment.[13,42]
Limitation of conventional H&E technique has been overcome with the introduction of newer methods such as IHC and molecular pathology methods.[43,44,45,46] These highly sensitive techniques in combination with SS have resulted in better detection of occult metastasis and improved prognosis of patients with N0 OSCC.
For identification of an epithelial component, a cocktail of antibody clones can be applied depending on its origin. The clones employed for detection of epithelial tumors include KL1, MNF116 and AE1/AE3. KL1 is recommended for detection of CKs 1, 2, 5, 6, 7, 8, 11, 14, 16, 17 and 18. This antibody stains with neoplastic cells of epithelial origin. Nonepithelial cells and skin basal cell layer do not stain with this antibody. MNF116 detects CKs 5, 6, 8, 17, 19 and shows reactivity for tissues from simple glandular epithelia to stratified squamous epithelia.[46,47,48]
AE1/AE3 is a broad-spectrum anti-pan-CK antibody cocktail which differentiates epithelial from nonepithelial tumors.[49] It recognizes the acidic and basic (type I-CKs 9, 10, 11, 16, 17, 18, 20 and type II-CKs 1, 2, 3, 4, 5, 6, 7, 8) subfamilies of CKs. Due to the broader spectrum of CKs labeled by AE1/AE3, this clone is preferred over the other two (KL1 and MNF116) for screening and diagnosis of tumors of epithelial origin.[27,29,49] Furthermore, pan-CK is highly advantageous for screening of nodal MM even when low-power standard light microscopic examination is employed.[8] Hence, this marker was chosen over other markers for detection of MM and ITC in this study.
EMA is widely used for identification of secretory epithelia and their malignant counterparts. However, the false-positive and false-negative rates of EMA staining are much higher. It has shown false positivity for germinal centers and also stains plasma cells. Some studies have reported that EMA misses detection of cancer cells. Furthermore, the visual field of EMA staining is not as clear-cut as in pan-CK (AE1/AE3) staining. Keeping the above limitations in mind, pan-CK (AE1/AE3) was preferred over EMA for detection of MM and ITC in LNs for identification of tumors according to their lineage in the present study.[46,47]
Extensive histopathological workup by SS has increased the detection of MM and ITC in determining both prognosis and diagnosis of OSCC. As histopathological analysis of neck dissection specimens is usually performed on few sections from each LN and as MM represents tumor deposits measuring <2 mm in diameter, they can be easily missed on routine histopathological examination. Therefore, our study evaluated SS for detection of MM and ITC for an improved diagnosis of OSCC.
The latest trends include the combined use of SS and IHC which has resulted in great improvement in diagnosis and prognosis of OSCC as they are especially useful in detecting metastasis in the nodes which were negative on routine histopathological staining, thus helping in upstaging of cancer.
According to the present study, MM/ITC was observed in 33% of the cases which were originally diagnosed as negative on histopathological examination, resulting in upstaging. Thus, the results support the significance of MM/ITC in prognosis and planning of therapeutic regimen. Although this method has the advantage of preserving cellular and tissue morphological features, it is expensive and time-consuming and therefore cannot be used for the most routine applications.[13,29,32,50]
Among the studies conducted on head and neck squamous cell carcinoma (HNSCC), Yoshida et al. in 2005 detected the presence of occult metastasis in LNs of the patients with N0 tongue carcinoma using pan-CK (AE1/AE3) and detected MM and ITC in 58% of the patients which was considerably higher as compared to the other studies and our study.[30] This could be attributed to the rich lymphatic network and highly muscularized structure of the tongue that makes it poorly equipped to protect itself from invasion and metastasis. Thus, it is more frequently associated with metastasis to draining LNs than any other cancer of the oral cavity.[51]
In the studies conducted in HNSCC, the detection rate ranged from 2 to 33% of the cases. The variations of results could be attributed to various clinical parameters, sample size, manual and manufacturer variations during the procedure of IHC in these studies.
A number of studies have been conducted over the last decade where both SS and IHC were applied to compare the results with routine single section technique using H&E. The studies conducted over time have evaluated the results based on either the number of patients or the number of LNs affected.
The significance of MM and ITC has been a topic of controversy. Many authors believe that the presence of a few tumor cells within LN or blood vessels might not be indicative of true MM or distant metastases because they may have been induced as artifacts during specimen handling and may not affect patient's outcome. However, recent studies have shown that the presence of these occult metastatic deposits could be correlated with a poor prognosis.[52]
There are certain pitfalls associated with IHC staining technique. The most commonly encountered problems with IHC staining are cross-reactivity with other antigens, false positivity and nonspecificity.[53] Hence, application of more sensitive molecular assays such as reverse transcriptase-polymerase chain reaction for comparing and improving the detection of occult metastasis can be considered.
CONCLUSION
On the basis of the above data, we support the view that earlier detection of MM and ITC in the cervical LNs improves the survival rate of patients with primary OSCC. Application of SS in combination with pan-CK (AE1/AE3) has proven to be efficient in upstaging the primary tumor and thus affects treatment and prognosis of patients with OSCC.
Keeping the advantages of above-mentioned techniques in mind, although more expensive and laborious than conventional methods, the combined application of these methods is justified for improved diagnosis and treatment planning of N0 OSCC patients. It should be recommended for diagnostic use in controlled studies of patients with negative LN metastasis on routine H&E stain, sparing low-risk patients the morbidity of unnecessary treatment while appropriately identifying aggressive tumors that warrant adjuvant therapy despite their early stage. Further studies with larger sample size should be conducted to validate the accuracy and application of this procedure in routine practice to provide improved staging, better prognosis and increase the survival rate of such patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: A review of the literature. Head Neck. 2005;27:1080–91. doi: 10.1002/hed.20275. [DOI] [PubMed] [Google Scholar]
- 2.Jalisi S. Management of the clinically negative neck in early squamous cell carcinoma of the oral cavity. Otolaryngol Clin North Am. 2005;38:37–46, viii. doi: 10.1016/j.otc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Saphir O, Amromin GD. Obscure axillary lymph-node metastasis in carcinoma of the breast. Cancer. 1948;1:238–41. doi: 10.1002/1097-0142(194807)1:2<238::aid-cncr2820010208>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer. 1999;86:2668–73. [PubMed] [Google Scholar]
- 5.Wilkinson EJ, Hause L. Probability in lymph node sectioning. Cancer. 1974;33:1269–74. doi: 10.1002/1097-0142(197405)33:5<1269::aid-cncr2820330512>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Matos LL, Trufelli DC, de Matos MG, da Silva Pinhal MA. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark Insights. 2010;5:9–20. doi: 10.4137/bmi.s2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke WW, Schmid E, Osborn M, Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979;81:570–80. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tajima Y, Tomioka T, Ikematsu Y, Ichinose K, Inoue K, Kanematsu T. Immunohistochemical demonstration of cytokeratin is useful for detecting micrometastatic foci from gallbladder carcinoma in regional lymph nodes. Jpn J Clin Oncol. 1999;29:425–8. doi: 10.1093/jjco/29.9.425. [DOI] [PubMed] [Google Scholar]
- 9.Barrera JE, Miller ME, Said S, Jafek BW, Campana JP, Shroyer KR. Detection of occult cervical micrometastases in patients with head and neck squamous cell cancer. Laryngoscope. 2003;113:892–6. doi: 10.1097/00005537-200305000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Thomsen JB, Christensen RK, Sørensen JA, Krogdahl A. Sentinel lymph nodes in cancer of the oral cavity: Is central step-sectioning enough? J Oral Pathol Med. 2007;36:425–9. doi: 10.1111/j.1600-0714.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 11.Akhter M, Hossain S, Rahman QB, Molla MR. A study on histological grading of oral squamous cell carcinoma and its co-relationship with regional metastasis. J Oral Maxillofac Pathol. 2011;15:168–76. doi: 10.4103/0973-029X.84485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker MT, Shores CG, Yu KK, Yarbrough WG. Molecular assay to detect metastatic head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:21–7. doi: 10.1001/archotol.130.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Ferris RL, Xi L, Raja S, Hunt JL, Wang J, Gooding WE, et al. Molecular staging of cervical lymph nodes in squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:2147–56. doi: 10.1158/0008-5472.CAN-04-3717. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH. TNM, sixth edition: New developments in general concepts and rules. Semin Surg Oncol. 2003;21:19–22. doi: 10.1002/ssu.10017. [DOI] [PubMed] [Google Scholar]
- 15.Mesquita JA, Cavalvanti AL, Nonaka CF, Godoy GP, Alves PM. Clinical and histopathological evidence of oral squamous cell carcinoma in young patients: Systematized review. Braz J Pathol Lab Med. 2014;50:67–74. [Google Scholar]
- 16.Patel SG, Shah JP. TNM staging of cancers of the head and neck: Striving for uniformity among diversity. CA Cancer J Clin. 2005;55:242–58. doi: 10.3322/canjclin.55.4.242. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosch P, Brinck U. Detection of nodal micrometastases in head and neck cancer by serial sectioning and immunostaining. Oncology (Williston Park) 1996;10:1221–6. [PubMed] [Google Scholar]
- 18.Prakash AR, Kumar GS, Shetty P. A study on detection of micrometastasis in the cervical lymph nodes of oral squamous cell carcinoma by serial sectioning. J Clin Diagn Res. 2011;5:78–81. [Google Scholar]
- 19.Fisher ER, Swamidoss S, Lee CH, Rockette H, Redmond C, Fisher B. Detection and significance of occult axillary node metastases in patients with invasive breast cancer. Cancer. 1978;42:2025–31. doi: 10.1002/1097-0142(197810)42:4<2025::aid-cncr2820420452>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson EJ, Hause LL, Kuzma JF, Rothwell DJ, Donegan WL, Clowry LJ, et al. Occult axillary lymph-node metastasis in patients with invasive breast-carcinoma. Lab Invest. 1981;44:A83. [PubMed] [Google Scholar]
- 21.Trojani M, de Mascarel I, Bonichon F, Coindre JM, Delsol G. Micrometastases to axillary lymph nodes from carcinoma of breast: Detection by immunohistochemistry and prognostic significance. Br J Cancer. 1987;55:303–6. doi: 10.1038/bjc.1987.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Mascarel I, Bonichon F, Coindre JM, Trojani M. Prognostic significance of breast cancer axillary lymph node micrometastases assessed by two special techniques: Reevaluation with longer follow-up. Br J Cancer. 1992;66:523–7. doi: 10.1038/bjc.1992.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Brekel MW, Stel HV, van der Valk P, van der Waal I, Meyer CJ, Snow GB. Micrometastases from squamous cell carcinoma in neck dissection specimens. Eur Arch Otorhinolaryngol. 1992;249:349–53. doi: 10.1007/BF00179388. [DOI] [PubMed] [Google Scholar]
- 24.Greenson JK, Isenhart CE, Rice R, Mojzisik C, Houchens D, Martin EW., Jr Identification of occult micrometastases in pericolic lymph nodes of Duke's B colorectal cancer patients using monoclonal antibodies against cytokeratin and CC49. Correlation with long-term survival. Cancer. 1994;73:563–9. doi: 10.1002/1097-0142(19940201)73:3<563::aid-cncr2820730311>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 25.Cote RJ, Peterson HF, Chaiwun B, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet. 1999;354:896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 26.Sato F, Shimada Y, Li Z, Watanabe G, Maeda M, Imamura M. Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg. 2001;88:426–32. doi: 10.1046/j.1365-2168.2001.01687.x. [DOI] [PubMed] [Google Scholar]
- 27.Bahrami A, Truong LD, Ro JY. Undifferentiated tumor: True identity by immunohistochemistry. Arch Pathol Lab Med. 2008;132:326–48. doi: 10.5858/2008-132-326-UTTIBI. [DOI] [PubMed] [Google Scholar]
- 28.Rhee D, Wenig BM, Smith RV. The significance of immunohistochemically demonstrated nodal micrometastases in patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2002;112:1970–4. doi: 10.1097/00005537-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Garrel R, Makeieff M, Alocisetti C, Valerie C, Frederic C, Cramppette L, et al. Sentinel lymph nodes in oropharyngeal and oral carcinomas. Otorhinolaryngologie. 2005;88:108–15. [Google Scholar]
- 30.Yoshida K, Kashima K, Suenaga S, Nomi N, Shuto J, Suzuki M. Immunohistochemical detection of cervical lymph node micrometastases from T2N0 tongue cancer. Acta Otolaryngol. 2005;125:654–8. doi: 10.1080/00016480410025252. [DOI] [PubMed] [Google Scholar]
- 31.Choi YJ, Yun HR, Yoo KE, Kim JH, Nam SJ, Choi YL, et al. Intraoperative examination of sentinel lymph nodes by ultrarapid immunohistochemistry in breast cancer. Jpn J Clin Oncol. 2006;36:489–93. doi: 10.1093/jjco/hyl045. [DOI] [PubMed] [Google Scholar]
- 32.Guo CB, Li YA, Gao Y. Immunohistochemical staining with cytokeratin combining semi-serial sections for detection of cervical lymph node metastases of oral squamous cell carcinoma. Auris Nasus Larynx. 2007;34:347–51. doi: 10.1016/j.anl.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Zeng J, Xie H, Lu Y, Feng Z, Li F. Clinical implications of micrometastasis detection in internal mammary nodes of breast cancer patients. Breast Care (Basel) 2012;7:216–9. doi: 10.1159/000339686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chone CT, Aniteli MB, Magalhães RS, Freitas LL, Altemani A, Ramos CD, et al. Impact of immunohistochemistry in sentinel lymph node biopsy in head and neck cancer. Eur Arch Otorhinolaryngol. 2013;270:313–7. doi: 10.1007/s00405-012-2032-5. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AK, Mishra P, Gupta S. Immunohistochemistry, a valuable tool in detection of cervical lymph node micrometastases in head and neck squamous cell carcinoma: A prospective study. Indian J Otolaryngol Head Neck Surg. 2013;65(Suppl 1):89–94. doi: 10.1007/s12070-012-0551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas J, Jose M. Immunohistochemistry over routine histopathology to trace micrometastasis in lymph nodes in patients with oral squamous cell carcinomas. Health Sci. 2013;4:1–12. [Google Scholar]
- 37.Yang Y, Li J, Mao S, Zhu H. Comparison of immunohistology using pan-CK and EMA in the diagnosis of lymph node metastasis of gastric cancer, particularly micrometastasis and isolated tumor cells. Oncol Lett. 2013;5:768–72. doi: 10.3892/ol.2012.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan Q, Jia M, Yue K, Zhang X, Chen J, Zhang C. Cytokeratin expression in cervical lymph nodes of patients with mandibular gingival squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 2014;49:652–6. [PubMed] [Google Scholar]
- 39.Celakovský P, Kalfert D, Smatanová K, Chrobok V, Laco J. Detection of cervical lymph node micrometastases in patients with squamous cell carcinoma of the oral cavity, pharynx and larynx. Acta Medica (Hradec Kralove) 2015;58:62–5. doi: 10.14712/18059694.2015.95. [DOI] [PubMed] [Google Scholar]
- 40.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29:1446–9. doi: 10.1002/1097-0142(197206)29:6<1446::aid-cncr2820290604>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 41.Shear M, Hawkins DM, Farr HW. The prediction of lymph node metastases from oral squamous carcinoma. Cancer. 1976;37:1901–7. doi: 10.1002/1097-0142(197604)37:4<1901::aid-cncr2820370440>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Hamakawa H, Takemura K, Sumida T, Kayahara H, Tanioka H, Sogawa K. Histological study on pN upgrading of oral cancer. Virchows Arch. 2000;437:116–21. doi: 10.1007/s004280000199. [DOI] [PubMed] [Google Scholar]
- 43.Schoenfeld A, Luqmani Y, Smith D, O’Reilly S, Shousha S, Sinnett HD, et al. Detection of breast cancer micrometastases in axillary lymph nodes by using polymerase chain reaction. Cancer Res. 1994;54:2986–90. [PubMed] [Google Scholar]
- 44.Mori M, Mimori K, Inoue H, Barnard GF, Tsuji K, Nanbara S, et al. Detection of cancer micrometastases in lymph nodes by reverse transcriptase-polymerase chain reaction. Cancer Res. 1995;55:3417–20. [PubMed] [Google Scholar]
- 45.Yamamoto N, Kato Y, Yanagisawa A, Ohta H, Takahashi T, Kitagawa T. Predictive value of genetic diagnosis for cancer micrometastasis: Histologic and experimental appraisal. Cancer. 1997;80:1393–8. doi: 10.1002/(sici)1097-0142(19971015)80:8<1393::aid-cncr5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 46.Leong CF, Raudhawati O, Cheong SK, Sivagengei K, Noor Hamidah H. Epithelial membrane antigen (EMA) or MUC1 expression in monocytes and monoblasts. Pathology. 2003;35:422–7. doi: 10.1080/00313020310001602576. [DOI] [PubMed] [Google Scholar]
- 47.Khan NR, Khan AN, Waris E, Suleman BA, Khan AA. Ck AE1/AE3 tumor marker expression in SCC of head and neck. Cell Biol Res Ther. 2014;3:1–6. [Google Scholar]
- 48.Vaidya MM, Sawant SS, Borges AM, Ogale SB, Bhisey AN. Cytokeratin expression in precancerous lesions of the human oral cavity. Oral Oncol. 1998;34:261–4. [PubMed] [Google Scholar]
- 49.Sun SS, Hsieh JF, Tsai SC, Ho YJ, Lee JK, Kao CH. Cytokeratin fragment 19 and squamous cell carcinoma antigen for early prediction of recurrence of squamous cell lung carcinoma. Am J Clin Oncol. 2000;23:241–3. doi: 10.1097/00000421-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Ensani F, Enayati L, Rajabiani A, Omranipour R, Alavi N, Mosahebi S. Improved detection of metastases by step sectioning and immuno-histochemical staining of axillary sentinel nodes in patients with breast carcinoma. Asian Pac J Cancer Prev. 2013;14:5731–4. doi: 10.7314/apjcp.2013.14.10.5731. [DOI] [PubMed] [Google Scholar]
- 51.Bello IO, Soini Y, Salo T. Prognostic evaluation of oral tongue cancer: Means, markers and perspectives (I) Oral Oncol. 2010;46:630–5. doi: 10.1016/j.oraloncology.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Lee E, Chae Y, Kim I, Choi J, Yeom B, Leong AS. Prognostic relevance of immunohistochemically detected lymph node micrometastasis in patients with gastric carcinoma. Cancer. 2002;94:2867–73. doi: 10.1002/cncr.10562. [DOI] [PubMed] [Google Scholar]
- 53.Fritschy JM. Is my antibody-staining specific. How to deal with pitfalls of immunohistochemistry? Eur J Neurosci. 2008;28:2365–70. doi: 10.1111/j.1460-9568.2008.06552.x. [DOI] [PubMed] [Google Scholar]